Abstract
Platelets express glycoproteins (IIb/IIIa, Ib/IX, Ia/IIa, IV, and HLA-1) that are polymorphic and can become targets for antibody responses. Patients at threat are those who received multiple platelet transfusions. Modified antigen capture elisa (MACE) is a qualitative solid phase Elisa designed to detect IgG antibodies against platelet specific antigens. The study has been carried out over a period of 2 years. A total of 100 patients were selected, who had been transfused with at least 15 units of platelet concentrate. All patients were having either hematological malignancies or bone marrow failure syndromes. Platelet antibodies were identified using MACE-1&2. Data was analysed statistically, using odds ratio (OR) with 95 % confidence interval. 39 % of the patients were found to be alloimmunized against platelet antigens, of which eleven showed refractoriness. Six patients (54.5 %) with HLA-1, two patients (9.5 %) with GPIb/IX, two patients (40 %) with both HLA-1 and GPIIb/IIIa, and one patient with GPIIb/IIIa antibodies showed refractoriness. Production of HLA-1 antibody and the development of refractoriness was found to be significant with OR 14.05 and P value 0.0025. MACE-1&2 enabled specific detection and identification of platelet antibodies, which in turn correlated well with the development of refractoriness in multi transfused patients. GPIb/IX was detected as the commonest antibody in our patient population, which is in variance with Europian studies where it is GPIa/IIIa (HPA-1a/5b). This technique should be utilised in patients who are at an increased risk of developing alloimmunisation due to repeated platelet transfusions.
Keywords: Platelet, Alloimmunization, Refractoriness, MACE
Introduction
Platelets express a variety of polymorphic glycoproteins (GPs) like GPIIb/IIIa, GPIb/IX, GPIa/IIa, GPIV, and class I Human Leukocyte Antigen (HLA). GPIIb/IIIa, GPIb/IX, GPIa/IIa, and GPIV are grouped as platelet specific antigens (PSA). In the platelet transfusion setting, alloimmunization involves the production of antibodies against these GPs. Patients transfused with multiple units of platelet concentrates over a longer periods of time, such as those suffering from haemato-oncological diseases and bone marrow failure syndromes, are the main victims for platelet alloimmunization. Platelet refractoriness is defined as an increase in the platelet count after transfusion that is significantly lower than the expected response [14]. Approximately 20–85 % of patients who receive multiple platelet transfusions become immunized against one or the other platelet antigens (HLA and/or PSA) and approximately 30 % of patients who are alloimmunized develop refractoriness to platelet transfusions. In a study by Kerkhoffs et al. approximately 67 % of the cases of platelet refractoriness were due to nonimmune factors alone, whereas platelet alloimmunization was responsible for the remaining cases [10]. HLA class I antibodies are involved in most alloimmunization cases, whereas PSA may be involved in approximately 10–20 % of refractory cases. Combinations of both types of antibodies are involved in approximately 5 % of cases [3]. Patients without prior sensitization, develop antiplatelet antibodies approximately 10–26 days after the transfusion while patients previously sensitized due to transfusion, pregnancy or organ transplantation develop antiplatelet antibodies as early as 4 days after transfusion Sanz et al. [17]. The platelet antibody detection assays appeared later as compared to red cell serologic assays for diagnosis of immunologic disorders. This might be because of two reasons: firstly due to difficulty in separating platelets from whole blood specimens, and secondly, the detection of platelet antibodies would require to have an advanced technique to measure release of platelet contents or direct interaction of immunoglobulin with their corresponding platelet surface antigens [16].
There are three groups of assays currently available to detect platelet antibodies. The earliest were Phase I assays that involved mixing of patient serum with normal platelets and used platelet function-dependent endpoints such as alpha granule release, aggregation, or agglutination. These were followed by Phase II assays that measured either surface or total platelet associated immunoglobulin on patient or normal platelets after sensitization with patient serum. Recently Phase III assays have been developed which require the binding and detection of antibodies to isolated platelet surface GPs and or HLA class-1 antigens.
MACE technique is a Phase III assay, in which the elisa plate microwells are coated with platelet-specific monoclonal antibodies which capture epitope carrying molecules from a platelet lysate onto a solid matrix, and their corresponding immunoglobulins, if present in the test sera are identified.
MACE-1&2 are qualitative solid phase enzyme linked immunosorbent assay (ELISA) designed to detect IgG antibodies to HLA class-1 antigens and to epitopes on the platelet glycoprotein IIb/IIIa, Ib/IX, Ia/IIa and IV. Confirming the presence of these antibodies in patient serum, would help in the search for potentially compatible platelet products.
This study has been done to detect the development of anti-platelet antibodies in patients who are transfused with multiple units of platelet concentrate by utilizing MACE-1&2 techniques. Platelet refractoriness was assessed by measuring the corrected count increment (CCI) at 1 and 24 h after transfusion.
Materials and Methods
This is a prospective, analytical study carried out in the Department of Immunohaematology and Blood Transfusion, of a tertiary care hospital in Pune, Western India, over a period of 2 years from June 2010 to May 2012. The study was carried out after taking approval of Institutional Ethical committee, and informed consent from the patients.
Patient’s Selection
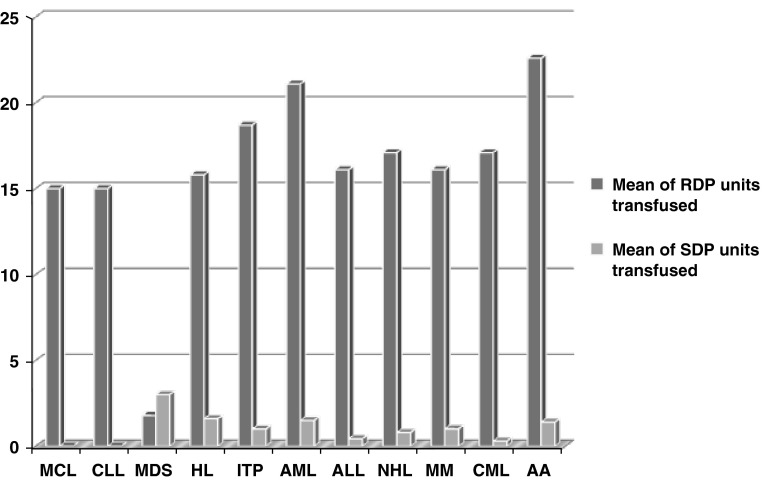
A total of 100 patients were selected, who had been transfused with at least 15 U of random donor platelets (RDP) and/or single donor platelets (SDP). All the patients were having either hematological malignancies or bone marrow failure syndromes. The disease conditions amongst study population requiring RDP/SDP transfusions are shown in Fig. 1.
Fig. 1.
Disease conditions amongst study population requiring RDP/SDP transfusions. AA aplastic anemia, ALL acute lymphoblastic leukemia, AML acute myeloblastic leukemia, CLL chronic lymphocytic leukemia, CML chronic myelocytic leukemia, HL Hodgkin’s lymphoma, ITP immune thrombocytopenic purpura, MCL mantle cell lymphoma, MDS myelodysplastic syndrome, MM multiple myeloma, NHL non Hodgkin’s lymphoma, RDP random donor platelet, SDP single donor platelet
Sample Collection and Storage
Whole blood samples from 100 patients who had been transfused with more than 15 units of platelet concentrate were collected in EDTA vacutainer using aseptic technique. Plasma was separated by centrifugation, aliquoted in small volumes (1.5 mL) in plastic eppendorf tubes and stored frozen at −24 °C. Frozen samples were thawed just prior to the testing.
Exclusion Criteria
Patients transfused with <15 units of platelet concentrate, patient with microbial infection and/or hemolysis are excluded from the study.
Methodology
Whole-blood measurements of platelet count before and after (60 min and 24 h) transfusions had been used to assess the effectiveness of platelet transfusions, which was assessed by corrected count increment (CCI).
Formula used to calculate CCI
Presence of platelet antibodies was tested by utilizing Modified Antigen Capture ELISA (MACE) technique. Each of these 100 patients would have received multiple transfusions (>15 units of platelet concentrate). The patient’s serum is mixed with pooled platelets & is incubated for 30 min. Antibody, if present will bind to the platelet GPs. Unbound antibodies are washed from the platelets. The antibody-sensitized platelets are solubilized by the addition of a lysis buffer solution. The platelet lysate containing soluble GPs is transferred to microwells for ELISA testing. During ELISA, platelet and HLA class-1 GPs was captured with immobilized monoclonal antibodies coated on microwells. After incubation, unbound GPs are washed off. An alkaline phosphatase labelled anti-human globulin reagent, anti-IgG is added and incubated. Unbound anti-IgG is washed off. Substrate PNPP (p-nitrophenyl phosphate) is added. After 30 min of incubation (by using sodium hydroxide solution) reaction is stopped. The optical density of the color that develops is measured by spectrophotometer. In each batch of tests, positive & negative controls were also run.
Data Analysis
Data was analyzed statistically by using odds ratio (OR) with confidence interval (CI) of 95 %. Data entry software Epi-Info (Version-7) was used to determine the association of anti HLA-1 & anti GPIIb/IIIa antibody with the development of platelet refractoriness. P value of <0.05 was considered statistically significant.
Results
Hundred samples from multiple platelet concentrate transfused patient were processed by modified antigen capture ELISA (MACE-1&2) technique. Eleven patients (11 %) were found to be positive for anti HLA-1 antibody. Anti-GPIIb/IIIa antibody was detected in two patients (2 %), and twenty-one patients developed anti GPIb/IX antibody (21 %). Five patients (5 %) showed both anti HLA-1 & anti-GPIIb/IIIa antibodies. Total 39 % of the patients were found to be alloimmunized against platelet antigens, of which eleven showed refractoriness. Six patients (54.5 %) who developed anti HLA-1, two patients (9.5 %) with anti GPIb/IX, two patients (40 %) with both anti HLA-1 & anti-GPIIb/IIIa antibodies, and one patient with anti-GPIIb/IIIa antibodies showed refractoriness (CCI < 5,000 on two consecutive occasions). Of eleven patients who showed refractoriness seven (63 %) were females. Eight patients showed CCI < 5,000, both 1 and 24 h after two consecutive ABO matched platelet concentrate (PC) transfusions. Remaining, two patients showed CCI < 5,000 after 24 h and one patient showed after 1 h of two consecutive ABO matched PC transfusions (Table 1). Two patients who did not show any antiplatelet antibodies, showed refractoriness. Relationship between the production of anti HLA-1 antibody and the development of refractoriness was found to be significant with OR 14.05 and P value 0.0025, which is shown in Fig. 2.
Table 1.
Modified antigen capture elisa (MACE1&2) results
| Patients Sr. no. | Age/Sex | Diagnosis | No of PC (RDP and SDP) transfused | Pre-transfusion plt count | CCI at 60 min after two consecutive ABO matched PC transfusion | CCI at 24 h after two consecutive ABO matched PC transfusion | MACE-1 result | MACE-2 result | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-HLA-1 | Anti- GP IIb/IIIa | Anti- GP Ia/IIa | Anti- GP Ib/IX | Anti- GP IV | |||||||
| 1 | 56/F | AML | 24 and 03 | 4,500 | 4,325 and 4,052 | 2,595 and 2,304 | −VE | +VE | −VE | −VE | −VE |
| 2 | 54/F | NHL | 12 and 02 | 58,000 | 16,723 | 14,705 | −VE | +VE | −VE | −VE | −VE |
| 3 | 45/F | AML | 18 and 03 | 11,000 | 4,786 and 4,703 | 2,450 and 2,700 | +VE | −VE | −VE | −VE | −VE |
| 4 | 21/F | ITP | 24 and 01 | 7,000 | 3,546 and 3,298 | 4,348 and 4,709 | +VE | −VE | −VE | −VE | −VE |
| 5 | 25/F | ITP | 15 | 11,000 | 4,993 and 3,476 | 4,380 and 3,864 | +VE | −VE | −VE | −VE | −VE |
| 6 | 58/F | AA | 20 | 19,000 | 15,570 | 4,980 and 4,423 | +VE | −VE | −VE | −VE | −VE |
| 7 | 48/F | ALL | 18 and 01 | 30,000 | 4,414 and 4,001 | 5,437 | +VE | −VE | −VE | −VE | −VE |
| 8 | 50/M | MDS | 16 | 39,000 | 10,956 | 4,036 and 4,179 | +VE | −VE | −VE | −VE | −VE |
| 9 | 43/M | MM | 15 and 02 | 40,000 | 24,508 | 18,741 | +VE | −VE | −VE | −VE | −VE |
| 10 | 66/M | CML | 16 | 48,000 | 18,143 | 16,034 | +VE | −VE | −VE | −VE | −VE |
| 11 | 59/M | NHL | 18 | 37,000 | 49,120 | 44,320 | +VE | −VE | −VE | −VE | −VE |
| 12 | 35/M | NHL | 23 | 22,000 | 44,897 | 37,862 | +VE | −VE | −VE | −VE | −VE |
| 13 | 56/M | NHL | 15 and 01 | 17,000 | 23,738 | 3,311 and 3,561 | +VE | −VE | −VE | +VE | −VE |
| 14 | 40/F | HL | 15 and 01 | 16,000 | 35,570 | 3,840 and 4,453 | +VE | −VE | −VE | +VE | −VE |
| 15 | 61/F | MM | 15 | 48,000 | 21,336 | 17,300 | +VE | −VE | −VE | +VE | −VE |
| 16 | 55/M | AML | 20 and 2 | 8,800 | 6,617 | 6,098 | +VE | −VE | −VE | +VE | −VE |
| 17 | 66/M | AA | 20 and 1 | 17,000 | 14,935 | 7,958 | +VE | −VE | −VE | +VE | −VE |
| 18 | 58/M | AA | 24 and 2 | 16,000 | 20,760 | 19,606 | +VE | −VE | −VE | −VE | −VE |
| 19 | 59/M | CML | 18 | 23,000 | 2,306 and 2,225 | 4,036 and 3,295 | −VE | −VE | −VE | +VE | −VE |
| 20 | 55/F | MDS | 15 | 19,000 | 40,913 | 38,076 | −VE | −VE | −VE | +VE | −VE |
| 21 | 48/F | MM | 12 and 1 | 33,000 | 16,338 | 12,013 | −VE | −VE | −VE | +VE | −VE |
| 22 | 56/M | CML | 17 | 27,000 | 20,003 | 16,218 | −VE | −VE | −VE | +VE | −VE |
| 23 | 65/F | MM | 16 | 31,000 | 21,913 | 15,570 | −VE | −VE | −VE | +VE | −VE |
| 24 | 44/M | AA | 24 and 1 | 37,000 | 8,650 | 7,785 | −VE | −VE | −VE | +VE | −VE |
| 25 | 37/M | CML | 20 | 24,000 | 21,084 | 20,003 | −VE | −VE | −VE | +VE | −VE |
| 26 | 42/M | AML | 20 | 9,000 | 11,702 | 6,614 | −VE | −VE | −VE | +VE | −VE |
| 27 | 75/M | MM | 12 and 2 | 4,000 | 15,897 | 12,624 | −VE | −VE | −VE | +VE | −VE |
| 28 | 41/M | HL | 20 and 1 | 15,000 | 9,158 | 9,158 | −VE | −VE | −VE | +VE | −VE |
| 29 | 66/M | CLL | 15 | 29,000 | 13,559 | 6,545 | −VE | −VE | −VE | +VE | −VE |
| 30 | 61/M | CML | 24 | 19,000 | 7,908 | 5,931 | −VE | −VE | −VE | +VE | −VE |
| 31 | 62/M | MM | 16 and 1 | 18,000 | 13,515 | 12,434 | −VE | −VE | −VE | +VE | −VE |
| 32 | 46/M | CML | 18 and 1 | 13,000 | 24,796 | 19,606 | −VE | −VE | −VE | +VE | −VE |
| 33 | 62/M | AA | 20 and 2 | 9,000 | 10,874 | 8,897 | −VE | −VE | −VE | +VE | −VE |
| 34 | 34/F | NHL | 20 and 1 | 11,000 | 23,066 | 20,969 | −VE | −VE | −VE | +VE | −VE |
| 35 | 51/M | MM | 20 and 4 | 13,000 | 15,858 | 13,936 | −VE | −VE | −VE | +VE | −VE |
| 36 | 57/M | MDS | 35 and 2 | 4,000 | 4,718 and 3,371 | 2,621 and 3,085 | −VE | −VE | −VE | +VE | −VE |
| 37 | 75/M | MM | 12 and 2 | 4,000 | 15,897 | 12,624 | −VE | −VE | −VE | +VE | −VE |
| 38 | 57/M | CML | 35 and 2 | 14,000 | 44,718 | 42,621 | −VE | −VE | −VE | +VE | −VE |
| 39 | 36/F | ITP | 30 and 1 | 6,000 | 6,425 | 6,494 | −VE | −VE | −VE | +VE | −VE |
PC platelet concentrate, CCI corrected count increment, GP Glycoprotein, RDP random donor platelet, plt platelet and SDP single donor platelet
Fig. 2.
Relationship between anti HLA-1 Ab positivity and the development of refractoriness
Details of the positive results are shown in the Table 1, and Table 2 shows the characteristics of MACE positive and MACE negative patients. Statistical analyses of the results are shown in the Table 3.
Table 2.
Characteristics of MACE positive and MACE negative patients
| Features | MACE-positives | MACE-negatives |
|---|---|---|
| No. | 39 | 61 |
| Males/females | 25/14 | 42/19 |
| Median age (years, range) | 51(21–75) | 56(19–76) |
| Mean Pre transfusion platelet count × 109/L | 20.802 | 23.564 |
| Mean CCI at 60 min after transfusion × 109/L | 17.53 | 21.96 |
| Mean CCI at 24 h after transfusion × 109/L | 13.14 | 15.64 |
| Patients showed refractoriness (13) | 11 | 02 |
Table 3.
Statistical analysis of results
| Platelet antibodies against | No. of patients developed refractoriness/no. of patients produced platelet antibodies | Odds ratio (OR) | P value |
|---|---|---|---|
| HLA-1 | 6/11 | 14.05 | 0.0025 |
| GPIb/IX | 2/21 | 0.757 | 0.391 |
| GPIIb/IIIa | 1/2 | 7.16 | 0.130 |
| HLA-1 + GPIb/IX | 2/5 | 5.60 | 0.057 |
Discussion
Platelet refractoriness is a clinical condition in which patients do not achieve the anticipated platelet count increment from a platelet transfusion. Refractoriness is usually defined as the occurrence of two consecutive post-transfusion platelet count increments, corrected for the patient’s size and number of administered platelets, at 10–60 min and at 18–24 h post-transfusion below 4,500–5,000 and 2,500 platelets per microliter respectively (Haematologica 2005; 90:247–253). Refractoriness does not necessarily imply alloimmunization. Indeed, the major cause of refractoriness to platelet transfusion are nonimmune factors that result in shortened platelet survival and markedly decreased platelet recoveries in patients who receive multiple transfusions [1, 2, 6, 19].
Drug-dependent platelet antibodies should be suspected in refractory patients with no evidence of alloimmunization or when they fail to respond to HLA-matched platelets, and the refractory responses are temporally related to drug therapy [21].
Non-immunological factors are responsible in 72–88 % of cases of refractoriness in patients with haematological diseases, while HLA antibodies in 25–39 % [6, 12]. Presence of HLA antibodies in the transfusion recipient are strongly associated with platelet refractoriness, but the relationship between PSA and refractoriness is weaker. About 45–70 % of chronically transfused patients developed anti-HLA class I antibody [11] while antibodies to PSA are found in 2–17 % [18].
In addition to platelets, transfusion of other blood products contaminated with leukocytes, and pregnancy also enhances the risk of HLA alloimmunization. 14 % of women who have had one or two pregnancies, and 26 % with three or more pregnancies can develop anti-HLA-I antibody [5].
There is a dose–response relationship between the number of exposures and the rate of alloimmunization i.e. more the donor exposure more would be the chance of alloimmunization and vice versa. Donor exposure can be minimized by using apheresis platelets, which provide adequate doses of platelets from a single donor rather than pooled platelet concentrates from multiple donors [8]. The contaminating donor leukocytes plays a major role in HLA alloimmunization in multiplatelet transfused patients, as suggested by various studies. One such study conducted by Murphy et al. showed that when leucodepleted platelets are transfused, primary immunization to HLA is delayed or does not occur at all, whereas unmodified platelet concentrates are associated with a rate of HLA alloimmunization ranging from 19 to 71 % [7, 15].
Antibodies to Class I HLA antigens can significantly affect the recovery and survival of transfused platelets. The risk of HLA alloimmunization is influenced by several patient and blood component factors. Transfused patients who were exposed previously to allogeneic HLA via transfusion or pregnancy developed HLA antibodies sooner. The underlying disease for which patients require platelet transfusion also influences the rate of HLA alloimmunization.
In our study, we found that 39 out of 100 (i.e. 39 %) of multiply platelet transfused patients have developed antiplatelet antibodies detected by MACE-1&2 tests, of which 11 patients showed refractoriness. Two patients did not develop antiplatelet antibody but they showed refractoriness. The rate of refractoriness amongst our multiple transfused patients population are similar to other studies, which showed an incidence of refractoriness of 5–15 % [20].
Eleven cases of refractoriness were because of immunological causes (i.e. antibody against HLA-1 and PSA), and two cases were due to some non immunological causes including history of drugs intake, fever, sepsis and bleeding etc. Of the eleven cases of immune etiology, two had acute myeloid leukaemia, two were diagnosed as immune thrombocytopenic purpura (ITP) and two had myelodysplastic syndrome. Aplastic anemias, acute lymphoblastic leukaemia, chronic myeloid leukemia, Hodgkin’s Lymphoma and Non Hodgkins Lymphoma (NHL) were diagnosed in remaining five cases (Table 1).
Two of three ITP patients had refractoriness and the antibody detected was HLA class-1. Transfusions in these situations should be made cautiously, with appropriate testing and cross matching if required, so that the risk of alloimmunization can be minimized and the patients can be transfused safely.
The first international study to compare methods for the detection of GP-specific PA-IgG was reported in [4]. Crossley et al. compared the Monoclonal Antibody-specific Immobilization of Platelet Antigens (MAIPA) and immunobead assays for the detection of GP IIb/IIIa-specific PA-IgG. The study showed MAIPA assay to be more reproducible than the immunobead assay, and the sensitivities were similar [4]. Recently, MACE and MAIPA were compared for the detection of GP IIb/IIIa-specific PA-IgG of 81 samples from thrombocytopenic patients. The GP-specific assays were found to be alike in sensitivity (39 %) and specificity (91 %) [22]. Although the sensitivity and specificity of the two methods (MAIPA and MACE) are almost similar, the procedure of MAIPA is quite tedious and time consuming. MAIPA takes around 10–12 h to complete the procedure while MACE takes only 4–5 h. MAIPA involves the use of anti-goat and anti-mouse antibodies and thus enhances the risk of getting false positive results [9]. In contrast, the MACE assay separates the sensitization and immobilization steps and thus human antibody has no opportunity to bind to immobilizing murine MoAb, minimizing the risk of false positivity to a large extent. The advantage of each of these antigen capture techniques is the ability to exclude non-platelet specific reactivity, since the MoAbs used to immobilize the immune complexes are specific for platelet GPs. With such assays, it is possible to detect antibodies specific for each of the recognized alloepitopes that have been described on the platelet surface GPs. Use of panels of platelets with known alloantigen phenotypes allows the identification of platelet antibody specificities in the evaluation of platelet alloimmune disorders. The drawbacks of these Phase III tests are that they are technically demanding to perform, requiring experienced technologists, and panels of typed platelets with which to perform the tests. Special attention has to be paid in performing the test, as the reaction wells in microtitre plate is small and specimen carryover and contamination has to be avoided. A further potential disadvantage of antigen capture assays is the possible disruption of alloantibody epitopes by detergent solubilization [13].
The risk of alloimmunization among multiple platelet concentrate transfused patients is one of the major issues of current haemotherapy. MACE is an efficient technique for detection and identification of anti-platelet antibody. It is effective in detection of major types of anti-platelet antibody such as anti-HLA-1, GPIIb/IIIa, GPIb/IX, GPIa/IIa and GPIV. The solid phase GP specific assays (MACE) appear to have improved specificity in distinguishing immune from non-immune thrombocytopenia.
HLA sensitization is the most common immune cause of platelet refractoriness in multiple transfused patient and can be diagnosed by demonstration of significant levels of HLA Class I antibodies in the refractory patient’s serum by MACE-1. When antibodies to HLA antigens are demonstrated, a widely used approach is to supply apheresis platelets from donors whose Class I HLA antigens are similar to those of the patient. HLA-matched platelets should be leukodepleted to reduce the risk of alloimmunization. MACE-2 testing has an added advantage of selecting platelets when the antibodies involved are directed at platelet specific, rather than HLA antigens.
An important observation of this study is that, in our patient population the most common platelet specific antibody detected was GP Ib/IX, unlike in the west, where it is GPIa/IIIa (HPA-1a/5b). This is the first study of this kind in India.
In conclusion, MACE-1&2 enabled specific detection and identification of platelet antibodies, which in turn correlated well with the development of refractoriness in multiple platelet concentrate transfused patients. This technique should specifically be utilised in the patients who are at an increased risk of developing alloimmunization due to repeated platelet transfusions, thereby permitting us to provide matched platelet units which will help in reducing the risk of refractoriness.
Acknowledgments
Dr J Philip and Dr R S Sarkar conceptualised coordinated and designed the research. Dr Neelesh Jain performed the research and analysed the data. Dr Neelesh Jain and Dr J Philip drafted and revised the manuscript.
Conflict of interest
The authors have no competing interest.
Contributor Information
R. S. Sarkar, Email: phaadu@yahoo.com
J. Philip, Email: eoj_in@yahoo.com
Neelesh Jain, Phone: +91-9874592738, Email: drneeleshjain@gmail.com.
References
- 1.Bishop JF, McGrath K, Wolf MM. Clinical factors influencing the efficacy of pooled platelet transfusions. Blood. 1988;71:383–387. [PubMed] [Google Scholar]
- 2.Bock M, Muggenthaler KH, Schmidt U, Heim MU. Influence of antibiotics on posttransfusion platelet increment. Transfusion. 1996;36:952–954. doi: 10.1046/j.1537-2995.1996.36111297091736.x. [DOI] [PubMed] [Google Scholar]
- 3.Buetens O, Shirey RS, Goble-Lee M, Houp J, Zachary A, King KE. Prevalence of HLA antibodies in transfused patients with and without red cell antibodies. Transfusion. 2006;46(5):754–756. doi: 10.1111/j.1537-2995.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 4.Crossley A, Calvert JE, Taylor PRA and Dickinson AM (1997) A comparison of monoclonal antibody immobilization of platelet antigen (MAIPA) and immunobead methods for detection of GPIIb/IIIa antiplatelet antibodies in immune thrombocytopenic purpura. Transfusion Medicine pp 127–134 [DOI] [PubMed]
- 5.Doughty HA, Murphy MF, Metcalfe P. Relative importance of immune and non-immune causes of platelet refractoriness. Vox Sang. 1994;66:200–205. doi: 10.1111/j.1423-0410.1994.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 6.Densmore TL, Goodnough LT, Ali S, Dynis M, Chaplin H. Prevalence of HLA sensitization in female apheresis donors. Transfusion. 1999;39:103–106. doi: 10.1046/j.1537-2995.1999.39199116901.x. [DOI] [PubMed] [Google Scholar]
- 7.Eernisse JG, Brand A. Prevention of platelet refractoriness due to HLA antibodies by administration of leukocyte-poor blood components. Exp Hematol. 1981;9:77–83. [PubMed] [Google Scholar]
- 8.Gmur J, von Felten A, Osterwalder B, et al. Delayed alloimmunization using random single donor platelet transfusions: a prospective study in thrombocytopenic patients with acute leukemia. Blood. 1983;62:473–479. [PubMed] [Google Scholar]
- 9.Hewitt J, Burton IE. Incidence of autoantibodies to GPIIb/IIIa chronic autoimmune thrombocytopenic purpura may be overestimated by the MAIPA. Eur J Haematol. 1994;86:418–420. doi: 10.1111/j.1365-2141.1994.tb04758.x. [DOI] [PubMed] [Google Scholar]
- 10.McFarland JG. Detection and identification of platelet antibodies in clinical disorders. Transfus Apheres Sci. 2003;28:297–305. doi: 10.1016/S1473-0502(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 11.Kerkhoffs JL, Eikenboom JC, van de Watering LM, van Wordragen-Vlaswinkel RJ, Wijermans PW, Brand A. The clinical impact of platelet refractoriness: correlation with bleeding and survival. Transfusion. 2008;48(9):1959–1965. doi: 10.1111/j.1537-2995.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- 12.Laundy GJ, Bradley BA, Rees BM, Younie M, Hows JM. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814–825. doi: 10.1111/j.1537-2995.2004.03387.x. [DOI] [PubMed] [Google Scholar]
- 13.Legler TJ, Fischer I, Dittmann J. Frequency and causes of refractoriness in multiply transfused patients. Ann Hematol. 1997;74:185–189. doi: 10.1007/s002770050280. [DOI] [PubMed] [Google Scholar]
- 14.Lin M, Shieh S, Liang D, Yang T, Shibata Y. Neonatal alloimmune thrombocytopenia in Taiwan due to an antibody against a labile component of HPA-3a. Vox Sang. 1996;69:336–340. doi: 10.1111/j.1423-0410.1995.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 15.Murphy MF, Metcalfe P, Thomas H. Use of leucocyte-poor blood components and HLA-matched-platelet donors to prevent HLA alloimmunization. Br J Haematol. 1986;62:529–534. doi: 10.1111/j.1365-2141.1986.tb02965.x. [DOI] [PubMed] [Google Scholar]
- 16.Nomura S, Yanabu M, Soga T. Analysis of idiopathic thrombocytopenic purpura patients with anti-glycoprotein IIb/IIIa or Ib autoantibodies. Actahaematol. 1991;86:25–30. doi: 10.1159/000204794. [DOI] [PubMed] [Google Scholar]
- 17.Sanz C, Freire C, Alcorta I, Ordinas A, Pereira A. Platelet-specific antibodies in HLA-immunized pts with chronic platelet support. Transfusion. 2001;41:762–765. doi: 10.1046/j.1537-2995.2001.41060762.x. [DOI] [PubMed] [Google Scholar]
- 18.Schnaidt M, Northoff H, Wernet D. Frequency and specificity of platelet-specific alloantibodies in HLA-immunized haematologic-oncologic patients. Transfusion Medicine. 1996;6:111–114. doi: 10.1046/j.1365-3148.1996.d01-58.x. [DOI] [PubMed] [Google Scholar]
- 19.Slichter SJ, Davis K, Enright H. Factors affecting post-transfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–4114. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebulla P. A mini-review on platelet refractoriness. Haematologica. 2005;90(2):47–253. [PubMed] [Google Scholar]
- 21.Von Drygalski A, Curtis BR, Bougie DW. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007;356:904–910. doi: 10.1056/NEJMoa065066. [DOI] [PubMed] [Google Scholar]
- 22.Warner MN, Moore JC, Warkentin TE, Santos AV, Kelton JG. A prospective study of protein-specific assays used to investigate idiopathic thrombocytopenic purpura. Br J Haematol. 1999;104:442–447. doi: 10.1046/j.1365-2141.1999.01218.x. [DOI] [PubMed] [Google Scholar]