Abstract
Bone marrow involvement in neuroblastoma indicates advanced stage of disease. The recent use of autologous bone marrow “rescue”, has provided an additional important reason for accurate assessment of bone marrow status in newly diagnosed patients. In this study, we analyzed 44 cases of neuroblastoma for bone marrow infiltration status and their hematological parameters. Eighty-eight bone marrow aspirate and trephine touch imprint smears and 44 trephine biopsy sections were examined in these 44 patients. Of these, 24 cases (54.5 %) showed marrow infiltration. Leucopenia and bicytopenia were significantly (p < 0.05) associated with marrow infiltration. Both bone marrow aspirate and biopsy were positive for infiltration in 16 out of 24 positive cases. Only aspirate smears were positive in 4 and only trephine biopsy in another 4 cases. The pattern of infiltration consisted of rosette formation in 40.7 % cases on aspirate smears and 22.2 % cases in trephine biopsies. Remaining cases showed diffuse and interstitial presence of tumor cells and cases positive only on trephine biopsy, showed marked stromal reaction. Bilateral trephine biopsies combined with aspirate smears picked up all positive cases compared to when they were assessed alone.
Keywords: Bone marrow, Cytopenia, Children, Infiltration, Neuroblastoma
Introduction
Neuroblastoma is an embryonal tumor arising from developing and incompletely committed precursor cells derived from the neural-crest tissue [1]. It characteristically arises in tissues of sympathetic nervous system, mostly in adrenal medulla or paraspinal ganglia and manifests as mass in neck, chest, abdomen or pelvis. It is the most common malignant neoplasm of infancy. Patients with advanced disease often have bone marrow involvement which is being categorised as stage 4 and 4S of the International Neuroblastoma Staging System (INSS) for staging this neoplasm. Bone marrow infiltration is being considered under intermediate and high-risk categories respectively for infants and children >1 year of age [1].
The usefulness of autologous bone marrow “rescue” for high-risk patients makes it important to assess the bone marrow status in newly diagnosed patients. Bone marrow aspiration and biopsy examination is a routinely employed procedure for this purpose, as it is an easy, cost-effective and quick method. Bilateral trephine biopsies have been found to be more accurate procedure for reporting the metastasis. However, they can also be frequently reported on examination of only aspirates [2]. Previously many studies have been conducted to evaluate bone marrow infiltration by solid tumors [3–5]. However, there are few studies which have analysed cases of bone marrow involvement in neuroblastoma with regards to its correlation with hematological profile and usefulness of bone marrow examination procedures [6–8]. We therefore planned this study to determine the prevalence of bone marrow involvement in neuroblastoma, the utility of bone marrow procedures and correlation with hematological profile in our group of patients.
Materials and Methods
The study was conducted in Department of Hematology, Postgraduate Institute of Medical Education and Research, Chandigarh. All cases of neuroblastoma referred for bone marrow examination from January 2009 to July 2012 were included in the study. Primary diagnosis of neuroblastoma was made on histopathology/fine needle aspiration cytology combined with clinico-radiological features. Bone marrow procedure was done from posterior superior iliac spine. Subsequently, aspirate smears and biopsy sections were stained by May-Grunwald Giemsa and hematoxylin and eosin respectively as per standard protocols [9, 10]. In all cases, routine morphological bone marrow details and counts, presence/absence of neuroblastoma infiltration and if present, its pattern were recorded. In addition peripheral blood counts, including, hemoglobin, total leukocyte count, platelet count and peripheral blood film morphology were also evaluated.
Results
During the study period, 55 cases of neuroblastoma underwent bone marrow examination. Of these 55 cases, 44 were newly diagnosed and 11 were on chemotherapy and bone marrow examination was done for residual disease. Three of the 11 cases (27.3 %) on chemotherapy showed residual disease; however, these 11 cases were excluded from the final analysis as data of bone marrow status at the time of diagnosis was not available.
Final analysis was done on 44 newly diagnosed cases, age range = 6 months to 11 years (median = 4 years), with male: female ratio of 2.6:1. Eighty-eight bone marrow aspirate and trephine touch imprint smears were examined. In addition, bilateral trephine biopsy sections for all 44 cases were also examined.
Twenty-four cases (54.5 %) showed marrow infiltration. Of these, 20 cases (83.3 %) were less than 5 years of age. There was a male preponderance with male:female ratio of 5:1 in involved cases.
Peripheral Blood
Complete blood counts in involved cases showed that anemia was present in 87.5 % cases, hemoglobin range = 47–137 g/L (median = 82 g/L); total leucocyte count below 4 × 109/L (leucopenia) was seen in 20.8 % cases, range = 2.3 × 109/L to 52.8 × 109/L (median = 9.8 × 109/L); and platelet count below 150 × 109/L (thrombocytopenia) was seen in 8.3 % cases, range = 21 × 109/L to 663 × 109/L (median = 338 × 109/L). In the uninvolved cases anemia was seen in 75 %, leucopenia in none and thrombocytopenia in 10 % cases. The presence of leucopenia was found to be significantly associated with bone marrow involvement (p < 0.05). On evaluating overall cytopenias, bicytopenia was present in 20.8 % of involved cases whereas none of the uninvolved cases harboured it. Only one involved case showed pancytopenia (Table 1). No circulating atypical cell was noted in involved cases.
Table 1.
Frequency of cytopenias in neuroblastoma cases with and without bone marrow infiltration
| Cytopenia | Involved cases (n = 24) | Uninvolved cases (n = 20) | p Value |
|---|---|---|---|
| Anemia | 21 (87.5 %) | 15 (75 %) | Not significant |
| Leucopenia | 5 (20.8 %) | 0 | <0.05 |
| Thrombocytopenia | 2 (8.3 %) | 2 (10 %) | Not significant |
| Bicytopenia | 5 (20.8 %) | 0 | <0.05 |
| Pancytopenia | 1 (4.2 %) | 0 | Not significant |
Bone Marrow
In all cases, bilateral trephine biopsies and aspirate smears were examined. Both aspirate smears and trephine biopsy showed infiltration in 16 out of 24 positive cases. Only aspirate smears were positive in 4 cases and only trephine biopsy in another 4 cases. Four cases with only aspirate showing presence of infiltration, biopsy sections showed predominantly fibrosis and in cases with only biopsy showing infiltration, there was focal involvement and was not seen in aspirate as a result of sampling error. The most common pattern of infiltration was in the form of rosette formation, seen in 40.7 % cases on aspirate smears (Fig. 1) and 22.2 % cases in trephine biopsies (Fig. 2). Remaining cases showed diffuse and interstitial pattern of infiltration. Cases positive only on trephine biopsy showed marked stromal reaction in the form of fibrosis. In addition, megaloblastosis was present in 37.5 and 47.5 % of involved and uninvolved cases respectively, however, it was not statistically significant (p = 0.313).
Fig. 1.
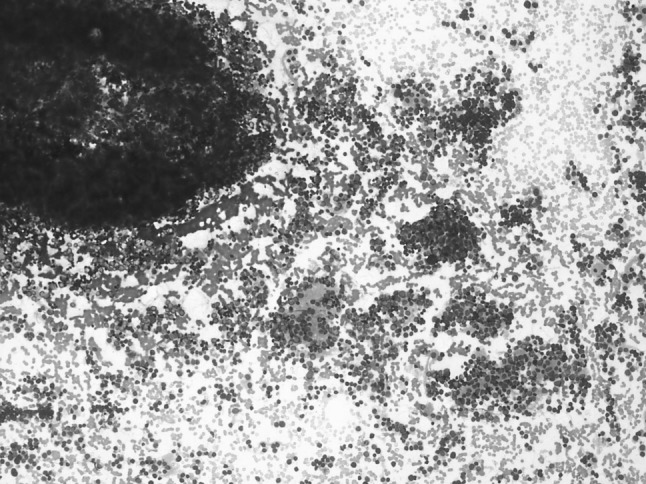
Bone marrow aspirate—showing infiltration by neuroblastoma, rossette and neurofibrillary is seen (May–Grunwald–Giemsa × 200X)
Fig. 2.
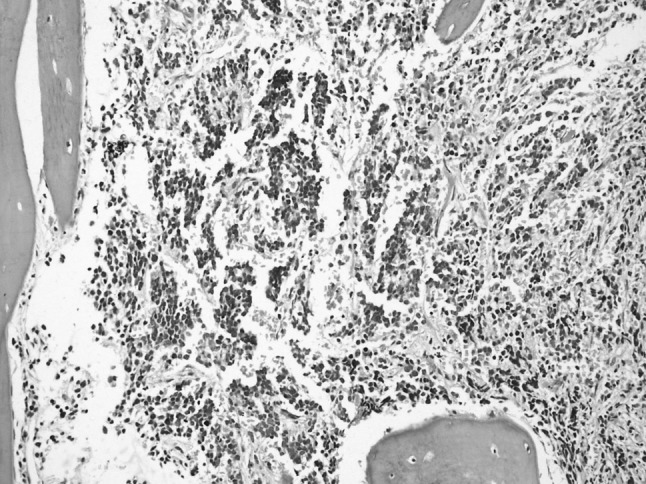
Bone marrow trephine biopsy—neuroblastoma infiltration—rossettes are seen (Hematoxylin and Eosin × 200X)
Discussion
Neuroblastoma is overall the third most common solid malignancy of childhood (with median age of 17 months) [11]. It arises from the neuroblasts which are undifferentiated precursor cells of sympathetic nervous system. Majority of neuroblastomas (~70 %) occur in retroperitoneum involving the adrenal medulla. They may locally invade the surrounding tissues like kidney or involve distant sites like bone marrow, liver, skull, orbit, lymph nodes, ovaries, testes and paratesticular region and central nervous system [12]. As described on routine fine needle aspirate cytology smears, the neuroblastoma cells may be seen singly scattered or arranged in small clusters that may be separated by pale blue to light purple fibrillar matrix, what are termed as Homer-Wright rosettes [11]. Tumors along the spinal column can enlarge through the intraforaminal spaces and can cause cord compression resulting in paralysis. However, lower-stage neuroblastomas are encapsulated and can be completely surgically excised.
Bone marrow infiltration needs to be evaluated in all cases of neuroblastoma for staging the disease and if found to be involved, is categorised as high-risk group requiring a course of aggressive chemotherapy [1]. In the present study we evaluated the hematological profile, bone marrow morphology and pattern of infiltration in neuroblastoma patients at initial presentation.
Bone marrow infiltration was seen in 54.5 % cases of neuroblastoma, predominantly in children under 5 years of age with a male preponderance. A similar incidence was found by Cozzutto et al. [13] who reported it in 58.3 % cases (in 7 out of 12 cases) and Franklin et al. [14] who reported it in 48.9 % cases (in 24 out of 49 cases). In a recent study which evaluated the incidence of bone marrow involvement at presentation in pediatric non-hematological small round cell tumours in children, found that neuroblastoma was the most common non-hematopoietic tumor to metastasize to bone marrow seen in 48.8 % cases followed by retinoblastoma (11.1 %), Ewing’s sarcoma/PNET (8.6 %) and rhabdomyosarcoma (3.2 %) [15].
With infiltration of bone marrow by metastatic tumor, the normal hematopoiesis gets suppressed resulting it peripheral cytopenias, we reported statistically significant incidence of leucopenia and bicytopenia in cases showing marrow infiltration versus those not showing infiltration in the present study (p < 0.05). These observations have also been recorded in previous studies [3, 16]. Anemia, though was common in both group of patients with and without infiltration, it is thought to be due to nutritional deficiency rather than due to tumor infiltration in marrow.
It is recommended that bone marrow should be assessed by bilateral posterior iliac crest marrow aspirates and trephine (core) bone marrow biopsies to exclude bone marrow involvement. To be considered adequate, core biopsy specimens must contain at least 1 cm of marrow, excluding cartilage. Bone marrow sampling may not be necessary for tumors that are otherwise stage 1 [17, 18]. In the present study also, with regards to the procedure selection, it was found that bone marrow aspirate along with bilateral trephine biopsies should be opted to get a maximum yield of results. Otherwise, the infiltration may be missed, as, in our study isolated aspirates showed positivity in 4 (9 %) cases and isolated biopsy in another 4 (9 %) cases, because of presence of a marked stromal reaction and focal marrow infiltration respectively. This strategy has also been advocated by Franklin et al. [14]. They evaluated 208 serial bone marrow samples from 49 consecutively diagnosed children with neuroblastoma. They found that trephine biopsies were more effective than aspirates for tumour detection in 20 % of the 154 paired aspirate/trephine procedures, whilst the reverse was the case in 7 %. Bilateral sampling (aspirates and trephines) improved the tumour detection rate by 10 % over that attained by sampling a single site. They concluded that bilateral iliac crest bone marrow aspirates and trephine biopsies should be done in children with neuroblastoma, both for initial staging and for monitoring of progress.
Neuroblastoma cells displayed a morphological pattern of infiltrate in the form of rosettes in about 40.7 % of cases on aspirate smears and 22.2 % on trephine biopsy. This is in contrast to that reported by Franklin et al., who found rosettes only in 2 % of marrow aspirate smears. This difference might be attributed to a relatively smaller number of patients (44 and 49 respectively) in these studies. Presence of rosettes ranging from as low as 18 % to as high as 72 % have previously also been reported in studies with fine needle aspirate cytology from primary or other metastatic sites [19, 20].
The other patterns we found in our study were diffuse and interstitial infiltration and stromal reaction in the form of fibrosis. Similar patterns have been reported in previous studies. Mills et al. [7] found myelofibrosis secondary to metastatic neuroblastoma as a frequent finding, being the predominant feature in 6 of the 48 cases evaluated.
Presence of marrow infiltration is associated with higher stage of disease and is a significant prognostic factor at diagnosis. Therefore in addition to bone marrow examination, newer sensitive methods for assessing marrow infiltration are now being used, including nuclear scans, reverse transcription-polymerase chain reaction (RT-PCR) for tyrosine hydroxylase and magnetic resonance imaging (MRI) for its detection [21]. However, bone marrow examination is a simple and cost effective method to stage and monitor cases in a setup with limited resources. Nuclear scan and MRI, though sensitive for metastasis detection, are available only at tertiary care centres and are expensive modalities.
Conclusion
In the present study we found presence of bone marrow involvement in 54.5 % cases and hence, these got upstaged to 4/4S INSS category. Median age of cases was 4 years and there was a male preponderance. Cases showing infiltration had statistically significant leucopenia and bicytopenia when compared to cases without infiltration. Most common morphological pattern of infiltrate was in the form of rosettes in 40.7 % of cases on aspirate smears and 22.2 % on trephine biopsy sections. Bilateral trephine biopsies combined with aspirate smears picked up all positive cases compared to when they were done alone.
References
- 1.Maris JM. Recent advances in neuroblastoma. New Eng J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valdes-Sanchez M, Nava-Ocampo AA, Palacios-Gonzalez RV, Perales-Arroyo A, Medina-Sanson A, Martinez-Avalos A. Diagnosis of bone marrow metastases in children with solid tumors and lymphomas. Aspiration, or unilateral or bilateral biopsy? Arch Med Res. 2000;31:58–61. doi: 10.1016/S0188-4409(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 3.Mohanty SK, Dash S. Bone marrow metastasis in solid tumors. Indian J Pathol Microbiol. 2003;46:613–616. [PubMed] [Google Scholar]
- 4.Tasleem RA, Chowdhary ND, Kadri SM, Chowdhary QA. Metastasis of solid tumours in bone marrow: a study from Kashmir, India. J Clin Pathol. 2003;56:803. doi: 10.1136/jcp.56.10.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finklestein JZ, Ekert H, Isaacs H, Jr, Higgins G. Bone marrow metastases in children with solid tumors. Am J Dis Child. 1970;119:49–52. doi: 10.1001/archpedi.1970.02100050051010. [DOI] [PubMed] [Google Scholar]
- 6.Aronica PA, Pirrotta VT, Yunis EJ, Penchansky L. Detection of neuroblastoma in the bone marrow: biopsy versus aspiration. J Pediatr Hematol Oncol. 1998;20:330–334. doi: 10.1097/00043426-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Mills AE, Bird AR. Bone marrow changes in neuroblastoma. Pediatr Pathol. 1986;5:225–234. doi: 10.3109/15513818609041204. [DOI] [PubMed] [Google Scholar]
- 8.Sorrentino S, Rosanda C, Parodi S, Rita Gigliotti A, Pasino M, Defferrari R, et al. Cyto-morphologic evaluation of bone marrow in infants with disseminated neuroblastoma. J Pediatr Hematol Oncol. 2012;34:154–158. doi: 10.1097/MPH.0b013e3182281dc3. [DOI] [PubMed] [Google Scholar]
- 9.Bates I. Bone marrow biopsy. In: Lewis SM, Bain BJ, Bates I, editors. Dacie and Lewis practical haematology. 10. Philadelphia: Churchill Livingstone; 2006. pp. 115–130. [Google Scholar]
- 10.Bain BJ, Lewis SM. Preparation and staining methods for blood and bone marrow films. In: Lewis SM, Bain BJ, Bates I, editors. Dacie and Lewis practical haematology. 10. Philadelphia: Churchill Livingstone; 2006. pp. 59–78. [Google Scholar]
- 11.Rajwanshi A, Srinivas R, Upasana G. Malignant small round cell tumors. J Cytol. 2009;26:1–10. doi: 10.4103/0970-9371.54861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H, Honzumi M, Funada M, Tomiyama H. Metachronous bilateral adrenal neuroblastoma. Cancer. 1985;56:1490–1492. doi: 10.1002/1097-0142(19850915)56:6<1490::AID-CNCR2820560645>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Cozzutto C, De Bernardi B, Comelli A, Guarino M. Bone marrow biopsy in children: a study of 111 patients. Med Pediatr Oncol. 1979;6:57–64. doi: 10.1002/mpo.2950060109. [DOI] [PubMed] [Google Scholar]
- 14.Franklin IM, Pritchard J. Detection of bone marrow invasion by neuroblastoma is improved by sampling at two sites with both aspirates and trephine biopsies. J Clin Pathol. 1983;36:1215–1218. doi: 10.1136/jcp.36.11.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhumati DS, Premlata CS, Devi VL, Appaii L, Kumari AB, Padma M, et al. Bone marrow involvement at presentation in pediatric non-haematological small round cell tumours. Indian J Pathol Microbiol. 2007;50:886–889. [PubMed] [Google Scholar]
- 16.Mehdi SR, Bhatt ML. Metastasis of solid tumors in bone marrow: a study from northern India. Indian J Hematol Blood Transfus. 2011;27:93–95. doi: 10.1007/s12288-011-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 18.Russell HV, Golding LA, Suell MN, et al. The role of bone marrow evaluation in the staging of patients with otherwise localized, low-risk neuroblastoma. Pediatr Blood Cancer. 2005;45:916–919. doi: 10.1002/pbc.20520. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar M, Ali MA, Sabbah RS, Bakry M, Sackey K, Nash EJ. Aspiration cytology of neuroblastoma. Light and electron microscopic correlations. Cancer. 1986;57:797–803. doi: 10.1002/1097-0142(19860215)57:4<797::AID-CNCR2820570419>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 20.Silverman JF, Dabbs DJ, Ganick DJ, Holbrook CT, Geisinger KR. Fine needle aspiration cytology of neuroblastoma, including peripheral neuroectodermal tumor, with immunocytochemical and ultrastructural confirmation. Acta Cytol. 1988;32:367–376. [PubMed] [Google Scholar]
- 21.Takemoto C, Nishiuchi R, Endo C, Oda M, Seino Y. Comparison of two methods for evaluating bone marrow metastasis of neuroblastoma: reverse transcription-polymerase chain reaction for tyrosine hydroxylase and magnetic resonance imaging. Pediatr Int. 2004;46:387–393. doi: 10.1111/j.1442-200x.2004.01921.x. [DOI] [PubMed] [Google Scholar]


