Abstract
Congenital macrothrombocytopenia is being increasingly recognised because of the increasing availability of automated platelet counts during routine complete blood count. If not recognised, these patients may be unnecessarily investigated or treated. The study was done to assess the occurrence of macrothrombocytopenia in the North Indian population and the role of automated platelet parameters in its detection. This prospective study was done on patients whose blood samples were sent for CBC to the hematology laboratory of a tertiary care hospital. Samples were run on Advia-120, a 5-part differential automated analyzer. Routine blood parameters including platelet count, mean platelet volume (MPV), platelet cytogram pattern and platelet flagging was studied along with peripheral blood smear examination. ANOVA was used to compare difference in mean MPV in patients with macrothrombocytopenia, and those with secondary thrombocytopenia and ITP. Seventy five (0.6 %) patients with CBC evaluation were detected to have macrothrombocytopenia, majority (96 %) of North Indian origin. The MPV (fl) in the 75 patients ranged from 10.9 to 23.3 (mean 15.1 ± 3.1 fl) with a dispersed cytogram pattern distinct from that seen in patients with normal platelet count, raised platelet count or low platelets due to secondary thrombocytopenia (MPV-10.9 ± 2.6) or ITP (10.8 ± 3.5). The difference in mean MPV in these patients was statistically significant (p < 0.00001). Macrothrombocytopenia is an under diagnosed condition and may be initially suspected on automated blood counts. Along with a blood smear examination, automated data (MPV and platelet cytogram pattern) aids the diagnosis and can avoid unnecessary investigations and interventions for these patients.
Keywords: Inherited, Macrothrombocytopenia, MPV, Platelet cytogram, Thrombocytopenia
Introduction
Thrombocytopenia raises concern in patients and clinicians alike and usually triggers off investigations to ascertain its cause. Acquired conditions causing thrombocytopenia are more frequently seen in clinical practice. Earlier considered very rare, congenital macrothrombocytopenia is now being increasingly recognised because of the increasing availability of automated platelet counts and related parameters on hematology analyzers during routine complete blood count processing [1, 2].
Congenital macrothrombocytopenia is an under diagnosed entity and is seen in a group of largely inherited disorders characterised by large sized platelets seen on the blood smear associated with raised mean platelet volume (MPV) and low to near normal platelet counts. Patients may be asymptomatic or may have bleeding of varying severity depending on the inherent genetic defect responsible [3].
If not recognised, some of these patients may be unnecessarily investigated or treated. Bone marrow examination, splenectomy and steroid therapy has been reported in these patients [4, 5]. Hence differentiating a non threatening thrombocytopenia from a symptomatic thrombocytopenia in clinical practice is vital. Macrothrombocytopenia has earlier been reported from India among the North Eastern population [6–8]. However it still remains a largely under diagnosed entity, even with the mushrooming of many small laboratories using automated hematology analysers.
This study highlights the role of automated platelet data: MPV and platelet cytogram patterns in the diagnosis of macrothrombocytopenia.
Materials and Methods
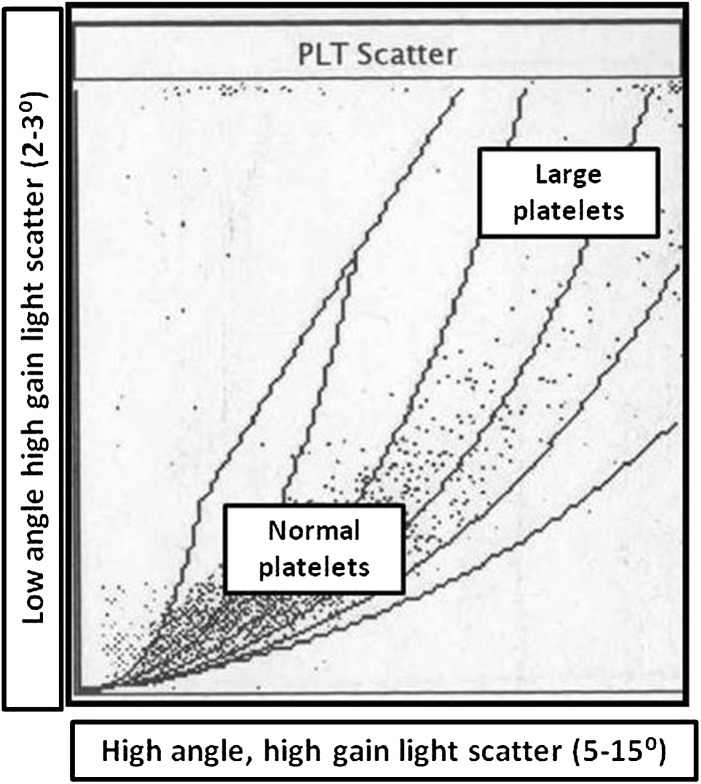
This prospective study was done on patients whose blood samples were sent for complete blood count to the hematology laboratory of a tertiary care hospital over a 2 year period. The aim of the study was to study the occurrence of macrothrombocytopenia in the North Indian population and the role of automated platelet parameters in its detection. Blood samples (collected in dipotassium EDTA) were run on Advia-120 automated analyzer (Siemens) within 2 h of collection. Advia-120 (Technicon H1 series) is a five-part differential system that analyzes blood cells by flow scatter and enumerates the differential count using myeloperoxidase staining. Platelet count is done by flow cytometry and the volume is plotted on the platelet cytogram. This cytogram is a graphical representation of two light scatter measurements: high angle (5°–15°) high gain scatter on the X axis and the low angle (2°–3°) high gain light scatter on the Y-axis (Fig. 1).
Fig. 1.
The normal platelet cytogram in the Advia-120 analyzer. Compared to normal sized platelets, large platelets have a wide angle scatter and are plotted in the high volume zone
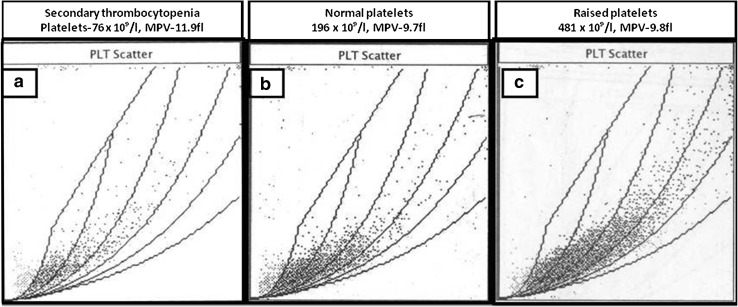
The cytogram encompasses platelet volumes between 0 and 30 fl. The platelet count includes normal sized platelets and large platelets (LPT). LPT have a wide angle scatter and are plotted in the high volume zone. The LPT flag is triggered if more than 10 % of all platelets are of a large volume. The platelet cytogram in patients with normal (Fig. 2b) or high platelet counts (Fig. 2c) shows a density pattern in the normal sized scatter zone signifying normal platelet volumes in majority of platelets for a particular sample. The same pattern is also maintained in patients with acquired thrombocytopenia (Fig. 2a). In all patients, routine numerical hematology data along with MPV was also recorded. Peripheral blood smear examination after Leishman staining was also done in all patients. Clinical data on all patients were obtained from the test request forms, case records and in consultation with the referring clinician.
Fig. 2.
The platelet cytogram in patients with normal (b) or high platelet counts (c) which shows a density pattern in the normal sized scatter zone signifying normal platelet volumes in majority of platelets for a particular sample. The same pattern is also maintained in patients with acquired (secondary) thrombocytopenia (a)
ANOVA was used to compare difference in mean MPV in patients with secondary thrombocytopenia, ITP and macrothrombocytopenia.
Results
Demographic Data
Seventy five (0.6 %) patients out of a total of 12,556 samples received for complete blood count during the study period were detected to have macrothrombocytopenia. Of the total of 12,556 blood samples, 7,407 were from males and 46 (0.62 %) of them had macrothrombocytopenia. There were 5,149 blood samples from female patients of which 29 (0.56 %) had macrothrombocytopenia. Hence no gender difference was noted. The age of the patients ranged from 7 to 73 years (mean 41.2 ± 14.2 years). Majority (96 %) of the patients were of North Indian origin while three patients belonged to Eastern India.
Indication for Complete Blood Count
In 62 (82.7 %) of the patients, complete blood count was done to investigate for unexplained thrombocytopenia. In 11 (14.7 %), the thrombocytopenia was incidental, the CBC having being done for an unrelated cause. In 2 (2.6 %) of the patients, the clinical diagnosis of ITP was considered. None of the patients had bleeding manifestations. Bleeding time was done in six patients and was normal.
In seven of the 75 patients, elective surgeries or invasive procedures had been deferred due to the detection of thrombocytopenia during the pre-operative and pre-procedure work up. In six patients, incidental thrombocytopenia was discovered in the antenatal period. In one patient, an emergency appendectomy was temporarily deferred till the thrombocytopenia was being investigated.
Treatment, Past and Family History
In two patients in whom ITP was clinically suspected, bone marrow examination had been done elsewhere to investigate for the cause of thrombocytopenia. These were reported to be normal. None of the patients had a history of recent hemato-toxic medication or bleeding from any site. There was no family history of bleeding. In 62 patients, low platelet counts had been documented on previous occasions also and only three patients had a peripheral blood smear report with LPT reported. The history of consanguinity was taken in 57 of the 75 patients and was negative. The mother of one of the patients also had thrombocytopenia (platelet count-65 × 109/l; MPV and a blood smear report was not available).
Hematological Parameters and Peripheral Blood Smear Findings
The haematological parameters in the 75 patients studied are shown in Table 1.
Table 1.
The haematological parameters in patients with Macrothrombocytopenia (n = 75)
| Parameter | Range | Mean ± SD | 95 % CI |
|---|---|---|---|
| Hemoglobin (×109g/l) | 74–155 | 125 ± 16 | 121–128 |
| Total leucocyte count (×109/l) | 3.08–17.23 | 7.05 ± 2.54 | 6.46–7.63 |
| Platelet count (×109/l) | 40–140 | 92 ± 25 | 86–98 |
| Mean platelet volume (MPV) (fl) | 10.9–23.3 | 15.1 ± 3.1 | 14.4–15.8 |
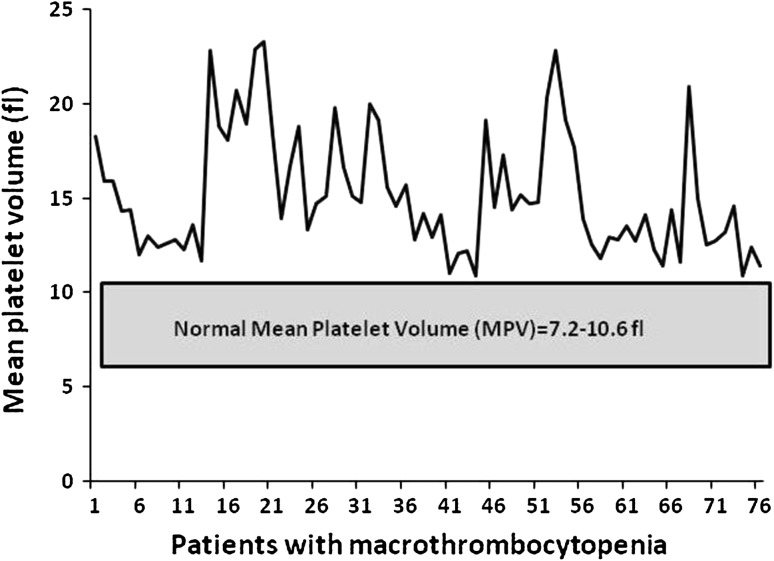
The MPV ranged from 10.9 to 23.3 fl (laboratory reference range 7.2–10.6 fl) Fig. 3.
Fig. 3.
The mean platelet volume (fl) in 75 patients with macrothrombocytopenia
Peripheral blood examination in all 75 patients showed variable degree of thrombocytopenia with frequent giant platelets. Figure 4 shows a peripheral blood smear in a normal individual. Figure 5 shows the blood smear in a patient with macrothrombocytopenia. Note that most of the platelets in the latter case are large sized. No leucocyte inclusions or platelet clumps were seen in any patients. No evidence of hemolysis or stomatocytic red cell morphology was seen in the peripheral blood smear of any patient.
Fig. 4.
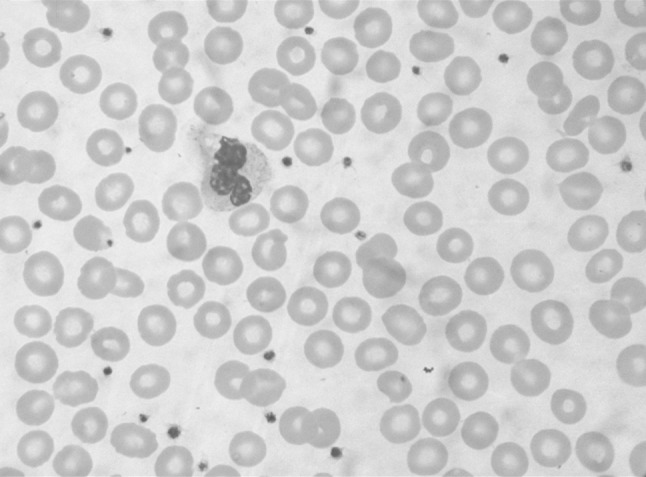
The peripheral blood smear from a normal individual with normal sized platelets. (Leishman ×1000)
Fig. 5.
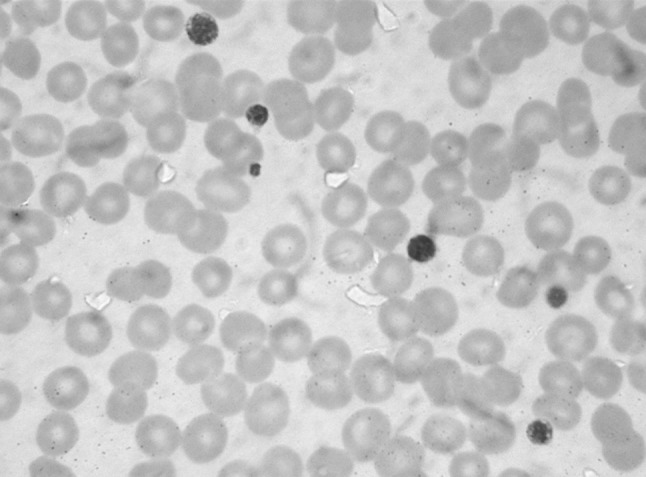
The peripheral blood smear from a patients with macrothrombocytopenia showing large sized platelets. (Leishman ×1000)
Platelet Cytogram Pattern and Large Platelet Flag
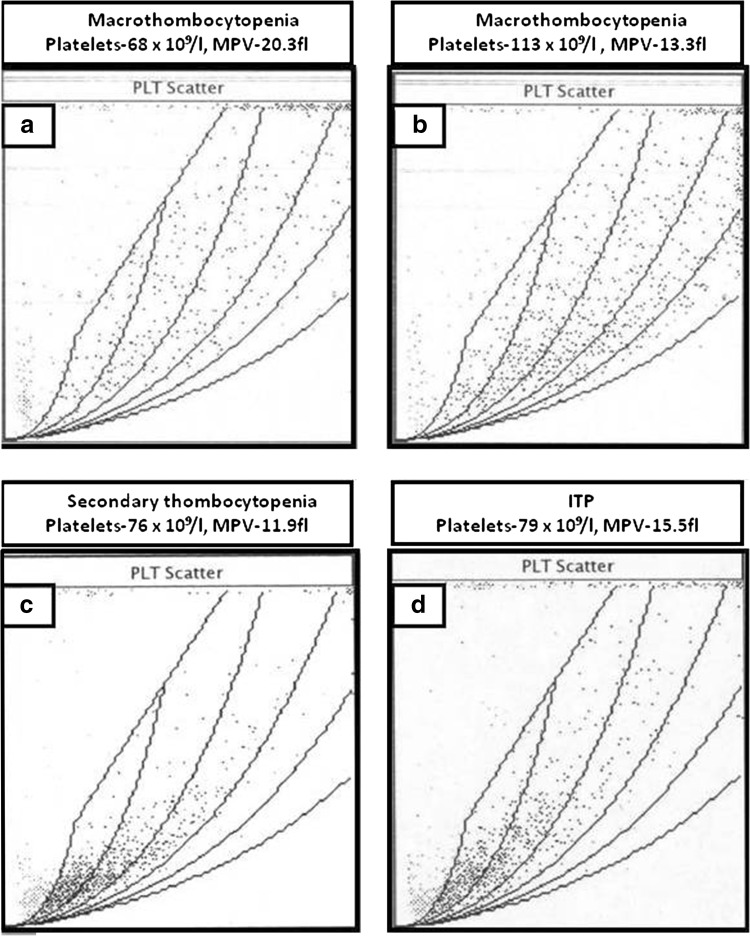
The platelet cytogram in all the patients with macrothrombocytopenia was near identical and showed a dispersed scatter pattern (Fig. 6a, b) with no density pattern as seen in normal individuals, patients with thrombocytosis (Fig. 2) or those with secondary thrombocytopenia (Fig. 6c) or ITP (Fig. 6d). The large platelet flag was consistently seen in all patients. Platelet aggregation studies were not done.
Fig. 6.
The platelet cytogram pattern in two patients with macrothrombocytoenia (a, b) and in patients with secondary thrombocytopenia (c) and ITP (d). Note that a dispersed platelet cytogram pattern in seen in patients with macrothrombocytopenia while a density zone is still preserved in patients with secondary thrombocytopenia and ITP
Macrothrombocytopenia was suggested in all the patients in the absence of (i) history of bleeding, (ii) no evident cause of thrombocytopenia, (iii) LPT on peripheral smear examination (iv) Raised MPV and characteristic dispersed platelet cytogram pattern.
Comparison of MPV with Patients with ITP and Secondary Thrombocytopenia
We also compared the MPVs in patients with macrothrombocytopenia with that seen in patients with ITP and secondary thrombocytopenia (Table 2).
Table 2.
The mean platelet volume in patients with macrothrombocytopenia, secondary thrombocytopenia and immune thrombocytopenic purpura
| Diagnosis | Mean platelet volume (MPV) | |||
|---|---|---|---|---|
| Range | Mean ± SD | 95 % CI | p value | |
| Macrothrombocytopenia (n = 75) | 10.9–23.3 | 15.1 ± 3.1 | 14.4–15.8 | <0.00001 |
| Secondary thrombocytopenia (n = 78) | 6.4–16.1 | 10.9 ± 2.6 | 10.3–11.7 | |
| Immune thrombocytopenic purpura (ITP) (n = 31) | 6.4–18.7 | 10.8 ± 3.5 | 9.5–12.1 | |
Of 78 patients (selected randomly over 1 month from the laboratory’s routine workflow) with thrombocytopenia due to various causes (sepsis, leukemia, drug induced thrombocytopenia, disseminated intravascular thrombocytopenia and megaloblastic anemia) detected on a complete blood count examination, the MPV was 10.9 ± 2.6. In a retrospective analysis of 31 patients with immune thrombocytopenic purpura (ITP) who had increased megakaryocytes in the bone marrow, the mean MPV was 10.8 ± 3.5. The difference in mean MPV between the groups was statistically significant (p < 0.00001).
Exclusion of other causes
Secondary thrombocytopenia was excluded by a detailed clinical examination (absence of Organomegaly) and no other evident cause of the thrombocytopenia: drugs, infection or other causative factors. Autoimmune panel were done to rule out an immunological cause. As most of the patients were adults with no history of previous bleeding since childhood, Glanzmann’s thrombaesthenia and Bernard Soulier Syndrome (BSS) were not considered.
Clinical Management
Since all patients were asymptomatic, they were only reassured. We advised tapering and discontinuation of the medications (corticosteroids) that eight patients were using for low platelets (having been diagnosed as ITP elsewhere). Seven patients in whom elective surgical procedures and one patient in whom emergency appendicectomy was being considered were given clearance for the surgeries and they had uneventful intra operative and post operative outcomes with no increased bleeding. All patients were given patient information pamphlets about this condition.
Discussion
This study has shown presence of macrothrombocytopenia in the North Indian population which to the best of our knowledge has not been reported earlier. It also highlights the utility of automated platelet data in its detection.
Acquired thrombocytopenia can be seen in a variety of clinical disorders including infections, autoimmune diseases, marrow aplasia, ITP and in patients on drugs or radiation therapy. Macrothrombocytes or LPT associated with thrombocytopenia may be seen in acquired or inherited disorders [9]. ITP and myelodysplasia are the common conditions leading to acquired macrothrombocytopenia. Inherited macrothrombocytopenia is a group of disorders characterized by variable degrees of thrombocytopenia and large sized platelets in the peripheral blood. These disorders have a clinical heterogeneity that ranges from benign asymptomatic thrombocytopenia to severe thrombocytopenia causing bleeding [3].
Of the inherited macrothrombocytopenias, BSS and MHY9 (May Hegglin gene) related disease are the most common. Patients with BSS have a prolonged bleeding time and show an impaired platelet aggregation response to ristocetin. May-Hegglin anomaly, Sebastian, Fechtner and Epstein syndromes have an autosomal dominant inheritance and have macrothrombocytopenia. Some of these syndromes are associated with other abnormalities like nephritis, hearing loss and cataracts. Except for Epstein syndrome, the other syndromes also show granulocytic inclusion bodies. The molecular defect in these disorders is located in the MYH9 gene encoding for non-muscle myosin heavy chain IIA [2, 3, 10–13].
Other less commonly seen forms of inherited macrothrombocytopenia include gray platelet syndrome, Mediterranean macrothrombocytopenia, platelet-type von Willebrand disease, Montreal platelet syndrome, macrothrombocytopenia with mitral valve insufficiency and familial macrothrombocytopenia with glycoprotein (GP) IV abnormality. All these disorders have specific clinical features [12].
None of the patients in the present study had leukocyte inclusions, stomatocytic hemolysis, bleeding manifestations or other associations like cataracts, nephritis or deafness seen with other inherited macrothrombocytopenias.
Mediterranean macrothrombocytopenia is characterized by varying degree of thrombocytopenia and the presence of large sized platelets on the blood smear. The exact molecular nature of this defect is still unclear although a few patients have been shown to be heterozygous carriers of the BSS [14]. Clinically, most patients are asymptomatic or have mild bleeding [15, 16].
Previous reports from India have shown macrothrombocytopenia to be prevalent in the population from eastern India and Nepal in a study done in a South Indian hospital with majority of patients from these areas. This entity had been named Harris syndrome and since then there have been no further reports from India of its occurrence [6–8].
Inherited macrothrombocytopenia is often misdiagnosed as ITP, an acquired disease with a much higher incidence. Automated hematology analyzers, especially the three-part instruments that rely only on electrical impedance have a limited ability to detect giant platelets and hence underestimate the platelet count and MPV. Sophisticated instruments like the Advia-120 can give more accurate counts in blood samples that have significant number of LPT.
Falsely low platelet counts obtained by three part hematology analyzers, if not validated by a careful peripheral blood smear examination can lead to unnecessary diagnostic evaluation and inappropriate treatment in patients. There have been reports where patients have received high dose steroids and even splenectomy has been done [4, 5].
In a study in Japan on Advia-120 hematology counter, Gohda et al. evaluated 112 patients who had initially been diagnosed with ITP. They identified 11 patients as having probable macrothrombocytopenia (average MPV of 19.2 ± 3.8 fl). Blood counts done earlier by a conventional automated hematology analyzer had overlooked giant platelets in these patients, and all of them had received high-dose steroid therapy and/or splenectomy before the study [4].
Young et al. reviewed six African children with macrothrombocytopenia with mild bleeding symptoms. All of them had been diagnosed to have ITP. Of these, five children had undergone treatment with steroids, IVIG and one patient had also undergone splenectomy. Their platelet count ranged from 10 to 125 × 109/l and MPV ranged from 8 to 20 fl [5].
The mean MPV in patients with macrothrombocytopenia in our study was 15.1 ± 3.1 fl. Two of the patients in our study had also been initially diagnosed to have ITP and bone marrow was done elsewhere.
Norris et al. studied MPV and mean platelet diameter (MPD) in 35 patients with inherited macrothrombocytopenias and 56 patients with ITP. They reported that platelets were larger in inherited macrothrombocytopenias than in ITP. Combining MPD with MPV increased sensitivity and specificity of macrothrombocytopenia diagnosis to 0.97 and 0.89, respectively [17]. In our study too, the MPV in patients with macrothrombocytopenia was significantly higher (p < 0.00001) than that seen in patients with ITP and secondary thrombocytopenia.
Depending on the hematology analyzers used and the characteristic of the blood sample, some very LPT (>30–40 fl) may be missed. This can be vital in thrombocytopenic patients and can adversely affect the management of bleeding. Some authors even recommend the use of immunologic markers by fluorescent flow cytometers integrated with hematology analysers [18].
Our study has some limitations. The cytogram interpretations presented in this study are specific to Advia-120 and other analyzers using similar technologies. Laboratory haematologists in other centres running samples on different analyzers need to characterize the histogram or cytogram patterns in their set ups. Also, since no molecular characterisation was done, we were unable to subtype the exact cause of the macrothrombocytopenia among the vast array of inherited platelet disorders. Further studies are required for definite syndromal categorization of this under reported condition.
Conclusion
Macrothrombocytopenia is an under diagnosed condition in North India and may be initially suspected on automated blood counts. Along with a blood smear examination, automated data (MPV and platelet cytogram pattern) aids the diagnosis and can avoid unnecessary investigations and interventions for these patients.
Acknowledgments
Conflict of interest
None.
References
- 1.Nayean Y, Lecompte T. Genetic thrombocytopenia with autosomal dominant transmission: a review of 54 cases. Br J Haematol. 1990;74:203–208. doi: 10.1111/j.1365-2141.1990.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 2.Noris P, Spedini P, Belletti S, Magrini U, Balduini CL. Thrombocytopenia, giant platelets, and leukocyte inclusion bodies (May-Hegglin anomaly): clinical and laboratory findings. Am J Med. 1998;104:355–360. doi: 10.1016/S0002-9343(98)00062-X. [DOI] [PubMed] [Google Scholar]
- 3.Kunushima S, Saito H. Congenital macrothrombocytopenias. Blood Rev. 2006;20:111–121. doi: 10.1016/j.blre.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Gohda F, Uchiumi H, Handa H, Matsushima T, Tsukamoto N, Morita K, et al. Identification of inherited macrothrombocytopenias based on mean platelet volume among patients diagnosed with idiopathic thrombocytopenia. Thromb Res. 2007;119:741–746. doi: 10.1016/j.thromres.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Young G, Luban N, White JG. Giant platelet disorders in African-American children misdiagnosed as idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 1999;21:231–236. doi: 10.1097/00043426-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Naina HV, Nair SC, Harris S, Woodfield G, Rees MI. Harris syndrome: a geographic perspective. J Thromb Haemost. 2005;3:2581–2582. doi: 10.1111/j.1538-7836.2005.01601.x. [DOI] [PubMed] [Google Scholar]
- 7.Naina HVK, Harris S. Harris platelet syndrome-underdiagnosed and unrecognized. Arch Pathol Lab Med. 2008;132:1546. doi: 10.5858/2008-132-1546a-HPSUAU. [DOI] [PubMed] [Google Scholar]
- 8.Naina HV, Nair SC, Daniel D, George B, Chandy M. Asymptomatic constitutional macrothrombocytopenia among West Bengal blood donors. Am J Med. 2002;112:742–743. doi: 10.1016/S0002-9343(02)01114-2. [DOI] [PubMed] [Google Scholar]
- 9.Drachman JG. Inherited thrombocytopenia: when a low platelet count does not mean ITP. Blood. 2004;103:390–398. doi: 10.1182/blood-2003-05-1742. [DOI] [PubMed] [Google Scholar]
- 10.The May-Hegglin/Fechtner Syndrome Consortium Mutations in MYH9 result in the May-Hegglin anomaly, and Fechtner and Sebastian syndromes. Nat Genet. 2000;26:103–105. doi: 10.1038/79063. [DOI] [PubMed] [Google Scholar]
- 11.Kunishima S, Saito H. Advances in the understanding of MYH9 disorders. Curr Opin Hematol. 2010;17:405–410. doi: 10.1097/MOH.0b013e32833c069c. [DOI] [PubMed] [Google Scholar]
- 12.Mhawech P, Saleem A. Inherited giant platelet disorders: classification and literature review. Am J Clin Pathol. 2000;113:176–190. doi: 10.1309/FC4H-LM5V-VCW8-DNJU. [DOI] [PubMed] [Google Scholar]
- 13.Lopez JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard-Soulier syndrome. Blood. 1998;91:4397–4418. [PubMed] [Google Scholar]
- 14.Savoia A, Balduini CL, Savino M, Noris P, Del Vecchio M, Perrotta S, et al. Autosomal dominant macrothrombocytopenia in Italy is most frequently a type of heterozygous Bernard-Soulier syndrome. Blood. 2001;97:1330–1335. doi: 10.1182/blood.V97.5.1330. [DOI] [PubMed] [Google Scholar]
- 15.Behrens WE. Mediterranean macrothrombocytopenia. Blood. 1975;46:199–208. [PubMed] [Google Scholar]
- 16.Von Behrens WE. Splenomegaly, macrothrombocytopenia and stomatocytosis in healthy Mediterranean subjects (splenomegaly in Mediterranean macrothrombocytopenia) Scand J Haematol. 1975;14:258–267. doi: 10.1111/j.1600-0609.1975.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 17.Noris P, Klersy C, Zecca M, Arcaini L, Pecci A, Melazzini F, et al. Platelet size distinguishes between inherited macrothrombocytopenias and immune thrombocytopenia. J Thromb Haemost. 2009;7:2131–2136. doi: 10.1111/j.1538-7836.2009.03614.x. [DOI] [PubMed] [Google Scholar]
- 18.Zandecki M, Genevieve F, Gerard J, Godon A. Spurious counts and spurious results on haematology analysers: a review. Part I: platelets. Int J Lab Hematol. 2007;29:4–20. doi: 10.1111/j.1365-2257.2006.00870.x. [DOI] [PubMed] [Google Scholar]