Abstract
There are an estimated 200 million carriers of the β-thalassemia gene worldwide, 20 million being in India. The mean prevalence in India is 3.3 %. Objective To evaluate the clinico-investigational profile and the demographic characteristics of patients with thalassemia major (TM). Methods This was a retrospective analysis of the clinico-demographic profile at presentation of patients of TM diagnosed in the Paediatric Hematology Clinic of our hospital. Results The clinical profile of 964 patients of TM was analyzed. The mean age at presentation of untransfused children was 13.2 ± 9.7 months. Nearly 2/3rd children presented before 1 year of age. Almost 40 % had symptoms for 3 months prior to presentation. The manifestations at presentation included pallor and failure to thrive. About 40 % presented with severe anemia, with a hemoglobin of <5.0 gm/dl. A large number received blood transfusions prior to establishment of the diagnosis. Half of the families had ancestors who hailed originally from Pakistan. Approximately 50 % belonged to the Khatri/Arora castes. The parental literacy rate was about 90 %. Conclusions Thalassemia needs greater public awareness and prevention strategies in our country. Some communities are at high risk as compared to others. Education programs and compulsory antenatal screening appear to be the order of the day.
Keywords: Thalassemia major, Age, Blood transfusion, Caste, Literacy, Education
Introduction
The March of Dimes Global Report on birth defects estimates that 7.9 million infants are born annually with a serious birth defect. Most of these (7.4 million) occur in the middle and low-income countries [1]. The hemoglobin disorders, sickle cell anemias and thalassemias contribute significantly to this global toll. Approximately 7 % of the world’s population is a carrier for hemoglobin disorders with 300,000–500,000 births every year with the severe heterozygous form of disease [2–4]. Beta thalassemia is the commonest inherited hemoglobin disorder in the Indian subcontinent with an uneven distribution among the different endogenous populations. Carrier frequency ranges between 3.7 and 10 % [5–14]. With national programmes the epidemiology of the disease has changed worldwide. However, in India, though immense progress has been made in the management and antenatal diagnosis of the disease, an accurate estimation of the disease burden at the district level is as yet not available [5, 6]. Certain communities have been identified to have a high carrier rate, and could be responsible for 50 % of the disease burden. We need awareness of the burden of the disease to evolve early detection, management and prevention strategies.
Objective
To determine the clinico-investigational profile and the racial demographic pattern of patients with thalassemia major (TM) presenting to our centre.
Setting
Hematology Oncology unit, Advanced Pediatric Centre, PGIMER, Chandigarh.
Study Design
This was a retrospective analysis.
Patients and Methodology
Patients diagnosed to have beta TM between January 1987 and December 2007 were analyzed. Data pertaining to racial demographics, symptomatology and investigations was retrieved from the case files and pooled for statistical analysis. Case files are arranged chronologically and maintained in the central record department of the Advanced Pediatric Centre.
Definition of TM [4, 12, 15]
Children presenting before the age of 3 years with anemia and hepatosplenomegaly needing regular transfusions [4, 12]
Peripheral blood smear showing evidence of hypochromic microcytosis, hemolysis, anisopoikilocytosis, polychromasia, stippled or target cell and presence of nucleated RBC’S.
Hemoglobin electrophoresis/HPLC for presence of high HbF.
Presence of β thalassemia carrier state in both parents by electrophoresis/HPLC.
Exclusion of other haemoglobinopathies.
Methodology
Automated blood cell counts were done using Sysmex SF-3000/Beckman coulter LH 750. Hemoglobin electrophoresis at alkaline pH of 8.6 and HbA2 quantization by micro column chromatography was performed using diethylaminoethyl cellulose (DE-52) and fetal Hb (HbF) quantization by Singer 1 min alkali denaturation test were done till 2004. After 2005 automated HPLC was performed using the BioRad Variant (BioRad, Hercules, CA, USA) for quantization of HbF and HbA2.
Institutional ethical approval was taken to conduct the study.
Statistical Analysis
Descriptive analysis was used for data analysis. For comparison and analysis of categorical variables, Chi square test was used. A p value of <0.05 was categorized as being significant. Fisher’s exact test was used to calculate the p value. Graph Pad Prism version 4.0 for windows was used for analysis. Graph pad software, San Diego, California, USA www.graphpad.com.
Results
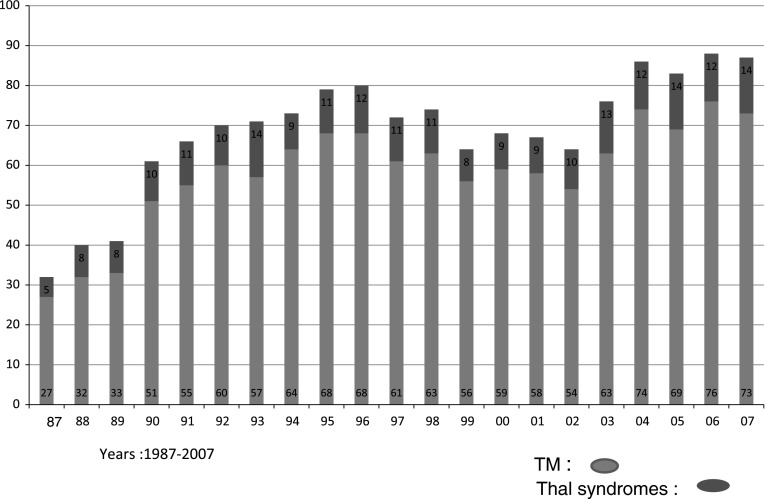
One thousand, two hundred and twenty one children were diagnosed to have TM between 1987 and 2007. The files of 964 children, 688 boys and 276 girls, were retrieved. We see 300–350 new cases with hematological disorders in the pediatric hematology clinic every year. The number of children with thalassemia during these years is given in Fig. 1. Analysis of 964 patients is given.
Fig. 1.
Thalassemia patients in the Pediatric Hematology Clinic
The age at presentation of the whole cohort was 17.2 ± 19.8 months, median being 10 months. Fifty two percent presented before 1 year of age. One third presented between 12 and 24 months of age and 17 % presented after 24 months (Table 1). Sixty percent had received a transfusion before presentation.
Table 1.
Age distribution of patients at presentation
| Age (months) | Whole cohort number (%) | Untransfused patients number (%) |
|---|---|---|
| 0–3 | 25 (2.5) | 19 (4.9) |
| >3–6 | 173 (17.9) | 98 (25.5) |
| >6–12 | 305 (31.6) | 129 (33.6) |
| >12–24 | 295 (30.6) | 110 (28.7) |
| >24 | 164 (17.0) | 26 (6.7) |
| 964 | 382 |
There were 382 children who presented untransfused, the mean age being 13.2 ± 9.7 months. In this group 64 % presented in the first year of life, and 6.7 % presented after 2 years of age (Table 1). The difference in ages between these two groups was not significant. (p > 0.05).
Clinical Features
The presenting features included a history of pallor, jaundice, poor growth and fever (Table 2). Nearly 40 % presented with hemoglobin of <5.0 gm/dl. In addition, the peripheral blood film revealed anisocytosis in 98 % cases, target cells in 96 %, basophilic stippling in 27 % and nucleated red blood cells in 63 % of the patients.
Table 2.
Presenting profile of patients (n = 964)
| Feature | N | % |
|---|---|---|
| Time interval (Symptom to hospital) | ||
| 0–12 weeks | 411 | 42.6 |
| 13–24 weeks | 270 | 28.0 |
| 25–52 weeks | 95 | 9.9 |
| >1 year | 188 | 19.6 |
| Pallor | 952 | 99 |
| Jaundice | 49 | 5 |
| Poor growth | 296 | 30.7 |
| Fever | 155 | 16.1 |
| Weight: normal | 333 | 34.5 |
| Undernutrtion (≤70 % expected) | 257 | 26.7 |
| Hepatomegaly | ||
| Nil | 72 | 7.5 |
| <2 Cm | 215 | 22.3 |
| >2–5 Cm | 529 | 54.9 |
| >5 Cm | 148 | 15.4 |
| Splenomegaly | ||
| Nil | 75 | 7.8 |
| <5 Cm | 599 | 62 |
| >5 Cm | 290 | 30.1 |
| Hemolytic facies | 373 | 38.7 |
| Family history | ||
| Affected sibling | 104 | 10.7 |
| Affected relative | 72 | 7.4 |
| Hb At presentation | ||
| <5 gm/dl | 372 | 38.6 |
| 5.1–7.1 gm/dl | 370 | 38.4 |
| BT before presentation (582) | ||
| ≤6 months | 81 | 13.9 |
| 7–12 months | 176 | 30.2 |
| 13–24 months | 187 | 32.0 |
| >24 months | 138 | 23.7 |
| Hb F value | Percentage received prior BT | |
| <25 | 255 | 89 |
| 25–50 | 205 | 80.4 |
| 50–75 | 227 | 41 |
| >75 | 277 | 33 |
Forty two percent presented within 3 months of symptomatology (Table 2). One hundred eighty eight children came with a history of greater than 1 year.
An initial alternate diagnosis was considered in 128 children (13.2 %). This included: (i) nutritional anemia in 62; (ii) infection: 49; (iii) storage disorder: 10; (iv) malignancy: 2; (v) other hemoglobinopathies. Nutritional anemia was thought of mainly in infancy in patients who had presented with mild-moderate anemia and mild organomegaly. The commonest infection considered was malaria which was thought of in the children who came with pallor, fever and splenomegaly. A storage disorder was deliberated as the initial diagnosis in ten children who had presented with massive splenomegaly and mild to moderate pallor. Malignancy was thought of in two children who had been referred with blasts in the peripheral smear. A review of the film showed these to be nucleated red blood cells and not blasts. Hereditary spherocytosis was considered in those who presented with moderate anemia without a transfusion.
Transfusion
Five hundred and eighty two (60 %) patients had received a transfusion prior to referral (Table 2). Two hundred and fifty seven (44.2 %) had received a transfusion before 12 months of age. The number of transfusions varied. Two third children had received 1–3 transfusions prior to the establishment of diagnosis of TM. Though 582 children had received a blood transfusion prior to an institute referral, only 50 had been investigated for thalassemia by doing a hemoglobin electrophoresis before receiving a transfusion. Sixty nine children had been on a regular transfusion (>12 transfusions) prior to referral. Amongst these 69, only 30 children had been investigated for thalassemia and the rest were on regular transfusions with no definite diagnosis.
The HbF value at presentation varied from <25 to >75 %. This variance was seen because of previous transfusions. Around 85 % children who had an HbF value of less 50 % had received prior transfusions. The HbF level was significantly inversely proportional to receipt of a transfusion (Table 2). Thalassemia carrier status was documented in all parents by an Hb Electrophoresis/HPLC showing an elevated HbA2 ( >3.5 %). Eight mothers had a low HbA2 secondary to iron deficiency. After treatment the value recorded was >3.5 %.
Religion and Caste
More than three fourth, (745) patients were Hindu; 184 (19.1 %): Sikh; 34 (35 %): Muslim and 1 patient was a Christian. The caste, known in 711 (73.7 %) cases, mainly of the Hindu and Sikh religions, is given in Table 3. Khatri’s and Arora’s predominated, followed by the Sharma and the Aggarwal communities. A very small percentage belonged to the marginalized weaker sections of society.
Table 3.
Caste distribution
| Caste | Number | Percent |
|---|---|---|
| Khatri | 176 | 24.7 |
| Arora | 162 | 22.7 |
| Sharma | 72 | 10.1 |
| Baniya/Aggarwal | 44 | 6.1 |
| SC/OBC | 54 | 7.5 |
| Rajput | 31 | 4.3 |
| Lohana | 2 | 0.2 |
| Jat | 61 | 8.5 |
| Suni | 15 | 2.1 |
| Other* | 94 | 13.2 |
| Total | 711 |
* Other castes include Sindhi, Jain, Mujail, Roar, Bhat, Kaeuitz, Sarsi, Thapa and Gadavia
State of Origin
Nearly half of the cohort, 480 (49.8 %) belonged to Punjab followed by 237 (24.6 %) patients from Haryana. There were 88 patients from Himachal Pradesh (9.1 %). A small number of patients were from Rajasthan (47); Uttar Pradesh (45) and Jammu and Kashmir (9). A small number, 58, hailed from Bihar, Orissa and Madhya Pradesh.
Ancestors Belonging to Areas now in Pakistan
The ancestry of 457/872 was traced back to areas in Pakistan. This is essentially people who migrated to India when India and Pakistan achieved independence from the British in 1947. Information was not available in 92 patients. History of consanguinity was seen in 61 cases (6.3 %).
Education of Parents
Seventy nine (8.2 %) fathers and 124 (12.8 %) mothers were illiterate. Two hundred and forty (25 %) fathers and 198 (20 %) mothers had primary school education. About 1/3rd of the parents (37 % fathers and 35 % mothers) had studied up to high school. Two hundred and twenty one (22.9 %) fathers and 233 (24.2 %) mothers had a bachelor’s degree. A small percentage (3.5 %) had university education. The symptom diagnosis interval was compared with the education of the father. This difference was not significant (p = 0.213).
Discussion
The severe and most frequently encountered hemoglobinopathies in India include the thalassemias and sickle cell disease. These hemoglobinopathies are geographically distributed in the country. TM is the commonest hemoglobinopathy seen in the northern part of the country, sickle cell being confined mainly to central India [5, 6]. Our hospital is one of the major referral centers in north India, draining the states of Punjab, Haryana, Himachal Pradesh, eastern Uttar Pradesh, western Rajasthan and Jammu and Kashmir. This retrospective analysis was done to determine the epidemiological spectrum of TM cases at our centre.
The mean age at presentation in our whole cohort was 17.2 ± 19.9 months, with 50 % presenting in the first year of life. Children presenting directly to our centre had a mean age of 13.2 ± 9.7 months with 64 % being diagnosed within the first year of life.
A small percentage, 2.5 %, presented before 3 months of age and 2.7 % were diagnosed after the age of 2 years. Most children with transfusion dependent thalassemia present in the first year of life. Kattamis et al. [16] in 1975 noted age at presentation to be 13.1 (2–36) months. Cao [17] reported the mean age of children who presented with TM to be 8.4 ± 9.1 months. In 1984, Modell and Berdukas [18] reported 60 % of patients to present in the first year of life, mean age being 6 months. Nine percent children in their population presented after 2 years of age. Children who present late often need observation for a while before a definite diagnosis of TM or thalassemia intermedia can be made. These patients probably have a milder form of disease.
We have a male to female ratio of 2.5:1. In literature, only Indian studies have reported a male preponderance of up to 68 % [19] and 69.5 % [13]. Studies from the West, Mediterranean and Middle East [4, 20–23] have shown an equal incidence of disease in both sexes. The male preponderance seen in India has been attributed to a gender bias, rather than an actual preponderance of disease in boys. The existence of female disadvantage in large parts of the country, especially the northern states, has been clearly identified. Studies across India have found that boys are much more likely than girls to be taken to a health facility when sick [24, 25].
Nearly all (99 %) children had pallor as a presenting complaint. A small percentage had jaundice. Significant malnutrition was seen in 27 % of patients (Grade 2 and above, as per Indian Academy of Pediatrics classification [26]). Contributing factors to growth retardation include recurrent infections, nutritional deficiency, chronic hypoxia, iron toxicity from transfusion hemosiderosis, poor transfusional status and inadequate chelation [27, 28]. Hemolytic facies were present in 39 % of patients and 78 % came with moderate to severe anemia. This gives an insight to late presentation in our country as compared to the west where the mean Hb at diagnosis has been reported as 8.2 gm/dl [18].
A mere 50 of the 582 children who received transfusion prior to referral had been investigated for TM. None of the families who had children on a regular transfusion regimen had proper comprehension of the disease. In addition, the symptom diagnosis interval prior to reaching a tertiary care centre was more than 3 months in 58 % of patients. This is a reflection on the inadequacy of the health system and lack of awareness amongst the medical personnel. It is important to note, that though children had received transfusions prior to presentation, there was no significant difference in the clinical features, except hemoglobin, amongst those who came without a transfusion and those who received a transfusion.
Caste was known to us in 711 patients (73.7 %). Khatris and Aroras constituted 47.4 % of the cases, followed by the Brahmin community accounting for 10 % and the Aggarwal community 6 % of cases. The Khatris and Arora communities are concentrated in Punjab (India and Pakistan), Haryana, Delhi and the Sindh province of Pakistan and constitute about 15 % of the population in north India. Studies from these regions have shown 50–65 % cases to belong to the Khatri and Arora communities [9, 10, 23, 29]. The thalassemia carrier frequency in India varies from 3.0 to 15 % and is estimated to be about 3–6.5 % in Punjab [2, 6, 8, 10, 11]. Though carrier frequency has not been described in all states, states with a high carrier frequency include Gujarat, Maharashtra, Tamil Nadu and Punjab [6, 8, 10–12, 14].
Nearly 50 % patients 457 (47.4 %) had ancestors who had migrated from Pakistan. Studies have shown that 65 to 80 % families [10, 11, 13, 29] with thalassemia in Punjab, India are first/second generation migrants from Pakistan, Punjab. The main areas of Pakistan from where our patients have migrated are Rawalpindi, Multan, Lahore, Lyallpur, Faizabad, Bhawalpur, Sargodha, Montgomery, Gujranwala and Punjab.
A family history of transfusion dependent thalassemia was elicited in 176 (18 %) patients. One hundred and four had an affected sibling. The inability to prevent disease recurrence within the family reflects the poor awareness of disease in the general population and health care professionals. In addition there is often denial and resistance to antenatal diagnosis amongst affected families.
Only 8.2 % of fathers and 12.8 % mothers were illiterate. One third of the cohort had parents educated up to high school. 22.9 % fathers and 24.2 % mothers had a graduate degree. The average literacy rate in this area is around 68–77 % as per the 2001 census [30]. This information is significant to enable population based education and screening at high school/college level.
Despite education, improvement in health services, availability of genetic testing and ante natal diagnosis the number of patients coming to the center have remained about the same over the years. This in itself calls for a crying need of the necessity for community awareness and mandatory screening programs in the high risk areas.
Conclusions
Thalassemia is a public health problem which needs to be addressed in our country. There is need for strategies for increasing awareness about this condition and providing high quality management services. From our study it is significant that
-
(i)
Patients often get blood transfusions, (even regular transfusions) prior to the establishment of a diagnosis, and referral is delayed.
-
(ii)
This disease is more prevalent amongst the Khatri/Arora communities in Punjab.
-
(iii)
Significant level of literacy is seen up to high school and graduate level.
-
(iv)
Late presentation is evident by poor hemoglobin and growth failure.
-
(v)
The number of children coming to hospital every year with TM is increasing.
This information can help in formulating public awareness education programs at high school/college levels. This approach has good success stories in various parts of the world. India had genetic heterogeneity and is an endogamic society. This helps and makes it imperative that high risk communities be targeted. As nearly 50 % cases belong to these communities in the north, targeting defined communities would cover a huge number of at risk population. This is best advised during the antenatal period [31, 32].
Community based survey to know the exact burden of disease and carrier status in India is needed. Also, as is clear from our study, girl children are often not brought to hospital.
Acknowledgments
Conflict of interest
There is no conflict of interest.
References
- 1.Management of birth defects and hemoglobin disorders (2006) Report of a joint WHO-March of Dimes meeting. Geneva, Switzerland, 17–19 May
- 2.Higgs DR, Thein SL, Wood WG. The molecular pathology of thalassemias. In: Weatherall DJ, Clegg B, editors. The thalassemia syndromes. 4. Oxford: Blackwell Sciences; 2001. pp. 133–191. [Google Scholar]
- 3.Rund D, Rachmilewitz E. Beta-Thalassemia. N Engl J Med. 2005;353:1135–1146. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 4.Gibbon R, Higgs DR, Olivieri NF, Wood WG. The beta-thalassemias. In: Weatherall DJ, Clegg JB, editors. The thalassemia syndromes. 4. Oxford: Blackwell Science; 2001. pp. 288–289. [Google Scholar]
- 5.Verma IC, Saxena R, Kohli S. Past, present and future scenario of thalassemic care and control in India. Indian J Med Res. 2011;34:507–521. [PMC free article] [PubMed] [Google Scholar]
- 6.Mohanty D, Colah RB, Gorakshakar AC, Patel RZ, Master DC, Mahanta J, Sharma SK, et al. Prevalence of β-thalassemia and other haemoglobinopathies in six cities in India: a multicentre study. J Community Genet. 2013;4(1):33–42. doi: 10.1007/s12687-012-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asha S. Thalassemia syndromes. Ind J Med Sci. 2004;58:449–455. [PubMed] [Google Scholar]
- 8.Marwaha RK, Lal A. Present status of hemoglobinopathies in India. Indian Pediatr. 1994;31:267–271. [PubMed] [Google Scholar]
- 9.Ambedkar SS, Phadke MA, Mokashi GD, Bankar MP, Khedkar VA, Venkat V, et al. Pattern of hemoglobinopathies in western Maharashtra. Indian Pediatr. 2001;38:530–534. [PubMed] [Google Scholar]
- 10.Garewal G, Das R. Spectrum of beta thalassemia mutations in Punjabis. Int J Hum Genet. 2003;3(4):217–219. [Google Scholar]
- 11.Dash S. Beta thalassemia trait in the Punjab (North India) Br J Haematol. 1985;61:185–191. doi: 10.1111/j.1365-2141.1985.tb04075.x. [DOI] [PubMed] [Google Scholar]
- 12.Mohan N, Sarkar R. Hematological status of β-thalassemia in Madras. Indian J Pediatr. 1994;61(3):237–248. doi: 10.1007/BF02752216. [DOI] [PubMed] [Google Scholar]
- 13.Chhotray GP, Dash BP, Ranjit M. Spectrum of hemoglobinopathies in Orissa, India. Hemoglobin. 2004;28(2):117–122. doi: 10.1081/HEM-120034244. [DOI] [PubMed] [Google Scholar]
- 14.Patel Ashwin P, Naik Madhuben R, Shah Nilam M, Sharma Narmadeshwar P, Parmar Prakash H. Prevalence of common hemoglobinopathies in Gujarat: an analysis of a large population screening program. Natl J Community Med. 2012;3:112–117. [Google Scholar]
- 15.Old JM, Olivieri NF, Thein SL. Diagnosis and management of thalassemia. In: Weatherall DJ, Clegg B, editors. The thalassemia syndromes. 4. Oxford: Blackwell Sciences; 2001. pp. 551–686. [Google Scholar]
- 16.Kattamis C, Ladis V, Metaxatou-Mavromati A. Hemoglobins F and A2 in Greek patients with β and β/δβ thalassemia. In: Schmidt RM, editor. Abnormal hemoglobin’s and thalassemia: diagnostic aspects. New York: Academic Press; 2005. p. 209. [Google Scholar]
- 17.Cao A, Galanello R. Effect of consanguinity on screening for thalassemia. N Engl J Med. 2000;347:1200–1202. doi: 10.1056/NEJMe020086. [DOI] [PubMed] [Google Scholar]
- 18.Modell B, Berdoukas V. The clinical approach to thalassemia. New York: Grune & Stratton; 1984. p. 125. [Google Scholar]
- 19.Bandyopaadhyay B, Nandi S, Mitra K, Mandal PK, Mukhopadhayay S, Biswas AB. A comparative study on perceptions and practices among parents of thalassemic children attending two different institutions. Indian J Community Med. 2007;28(3):1–5. [Google Scholar]
- 20.Ganczakowski M, Bowden DK, Maitland K, William TN, Shaughnessy DO, Viji J. Thalassemia in Vanuatu, south-west Pacific: frequency and hematological phenotypes of young children. Br J Haematol. 1995;89:485–495. doi: 10.1111/j.1365-2141.1995.tb08353.x. [DOI] [PubMed] [Google Scholar]
- 21.Pearson HA, Cohen AR, Giadina PJV, Kazazian HH. The changing profile of homozygous beta thalassemia: demography, ethnicity, and age distribution of current North American patients and changes in two decades. Pediatrics. 1996;97:352–356. [PubMed] [Google Scholar]
- 22.Deyde VM, Lo BB, Khalifa DO, Ly B, Ball A, Fattoum S. Epidemiological profile of hemoglobinopathies in the Mauritanian population. Ann Haematol. 2002;81(6):320–321. doi: 10.1007/s00277-002-0471-6. [DOI] [PubMed] [Google Scholar]
- 23.Hafeez M, Aslam M, Ali A, Rashid Y, Jafri M. Regional and ethnic distribution of beta thalassemia mutations and effect of consanguinity in patients referred for prenatal diagnosis. J Coll Physicians Surg Pak. 2007;17(3):144–147. [PubMed] [Google Scholar]
- 24.Murthi N, Guio AC, Dreze J. Mortality, fertility and gender bias in India: a district level analysis. In: Dreze J, Sen AK, editors. Indian development: selected regional perspective. Oxford: Clarendon Press; 1997. p. 357. [Google Scholar]
- 25.Arokiasany P, Pradhan J (2006) Gender bias against female children in India: regional differences and their implications for MDGs. International Institute for population Sciences, Princeton.edu, Mumbai
- 26.Proceedings of the workshop on protein calorie malnutrition ecology and management (1975) Indian Pediatr 12:57–117. Nutrition sub committee of the Indian Academy of Pediatrics Report (1972). Indian Pediatr 9:360–372
- 27.Eshghi P, Alavi S, Ghavami S, Rashidi A. Growth impairment in beta thalassemia major: the role of trace element deficiency and other potential factors. J Pediatr Hematol Oncol. 2007;29(1):5–8. doi: 10.1097/MPH.0b013e31802d74f3. [DOI] [PubMed] [Google Scholar]
- 28.Louis CK. Growth of children with beta thalassemia major. Indian J Pediatr. 2005;72(2):159–164. doi: 10.1007/BF02760702. [DOI] [PubMed] [Google Scholar]
- 29.Grewal G, Fearon CW, Waren TC, Marwaha N, Marwaha RK, Mahadik C, et al. The molecular basis for beta thalassemia in Punjabi and Maharashtra Indians includes a multilocus aetiology involving triplicated alpha-globin loci. Br J Haematol. 1994;86(2):372–376. doi: 10.1111/j.1365-2141.1994.tb04742.x. [DOI] [PubMed] [Google Scholar]
- 30.Census of India (2001) www.censusindia.gov.in
- 31.Tamhankar PM, Agarwal S, Arya V, Kumar R, Gupta UR, Agarwal SS. Prevention of homozygous beta thalassemia by premarital screening and prenatal diagnosis in India. Prenat Diagn. 2009;29(7):732. doi: 10.1002/pd.2318. [DOI] [PubMed] [Google Scholar]
- 32.Gorakshakar AC, Colah R. Cascade screening for β-thalassemia: a practical approach for identifying and counseling carriers in India. Indian J Community Med. 2009;34(4):354–356. doi: 10.4103/0970-0218.58399. [DOI] [PMC free article] [PubMed] [Google Scholar]