Abstract
Acquired haemophilia A is an uncommon, potentially life-threatening disorder caused by onset of auto-antibodies against coagulation factor VIII. The association of acquired haemophilia and multiple myeloma is extremely rare. Prompt diagnosis of this acquired bleeding disorder is essential for management, aimed at haemorrhage control and inhibitor suppression. We describe a case of acquired haemophilia in a patient with multiple myeloma.
Acquired haemophilia A (AHA) is a rare but potentially life-threatening haemorrhagic disorder caused by the onset of auto-antibodies against coagulation factor VIII. Nearly 50 % of instances are associated with other conditions such as the gestational and the postpartum periods, autoimmune disorders and drug allergies [1].
The association of AHA and multiple myeloma (MM) is an extremely exceptional occurrence.
A 64-year-old Indian male with a history of human immunodeficiency virus (HIV) and hepatitis B infection presented with gingival bleeding, epistaxis, and haemoptysis for 5 days. He was on anti-retroviral treatment consisting of zidovudine, lamivudine, and efavirenz for the past 18 months. There were few ecchymoses over bilateral arms, thighs and lower back. Aspiration of stomach contents showed no evidence of bleeding in the gastrointestinal tract.
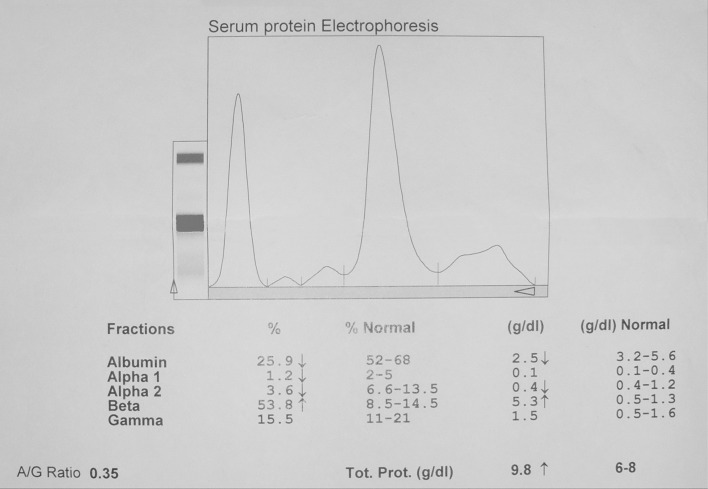
On the day of admission, the haemoglobin concentration was 5.3 g/dL, increased to 10.7 g/dL after transfusion of 6 U of packed red cells. The prothrombin time (PT) was 14.2 s (normal 12–13.4) but APTT was prolonged (69.3 s, normal 30–33). Serial monitoring is shown in Table 1. The correction study profile is detailed in Table 2. Factor VIII C level was decreased at 17.3 %. ELISA for Anti-cardiolipin antibody (ACA) was negative (Table 3). His creatinine level was 1.1 mg/dL. Total serum protein was 9.8 g/dL. Protein fractions and Immunofixation results are populated in Table 4.
Table 1.
Serial monitoring of APTT
| Date | APTT (Control—30″) |
|---|---|
| (At admission) | 69.3 |
| Day 3 | 65.0 |
| Day 5 | 56.2 |
| Day 9 | 50.7 |
| Day 16 (at discharge) | 42.3 |
| Day 23 (on follow up) | 31.6 |
Table 2.
APTT correction study profile
| Lupus anticoagulant work up | Patient | Control |
|---|---|---|
| PT | 14.2″ | 12″ (INR:1.2) |
| APTT | ||
| 4 part patient + l part control | 58″ | 30″ |
| ½ patient + ½ control | 31 .6″ | 30″ |
| ½ patient + ½ adsorbed plasma | 42.9″ | 30″ |
| ½ patient + ½ aged serum | 48.6″ | 30″ |
Table 3.
Anti cardiolipin antibody (ACA) profile
| ACA (ELISA) | Patient | Normal values |
|---|---|---|
| IgG | 2 GPL | <15 GPL |
| IgM | 3 MPL | <15 MPL |
Table 4.
Serum protein electrophoresis and immunofixation profile at diagnosis
| β-2microglobulin (mg/L) | 8.69 (0.7–1.8) |
| Immunofixation (mg/dL) | |
| IgA | 232.0 (90–410) |
| IgG | 1380.0 (600–1560) |
| IgM | 6,350 (30–360) |
| Kappa light chains (mg/L) | 77.0 (3.30–19.40) |
| Lambda light chains (mg/L) | 13.2 (5.71–26.30) |
| Kappa: lambda ratio | 5.83 (0.26–1.65) |
| Protein fractions (g/dL) | |
| Total proteins | 9.8 (6–8) |
| A1bumin | 2.5 (3.2–5.6) |
| Globulins | |
| α1 | 0.1 (0.1–0.4) |
| α2 | 0.4 (0.4–1.2) |
| β | 5.3 (0.5–1.3) |
| γ | 1.5 (0.5–1.6) |
| M-Spike (g/dL) | 3.99 |
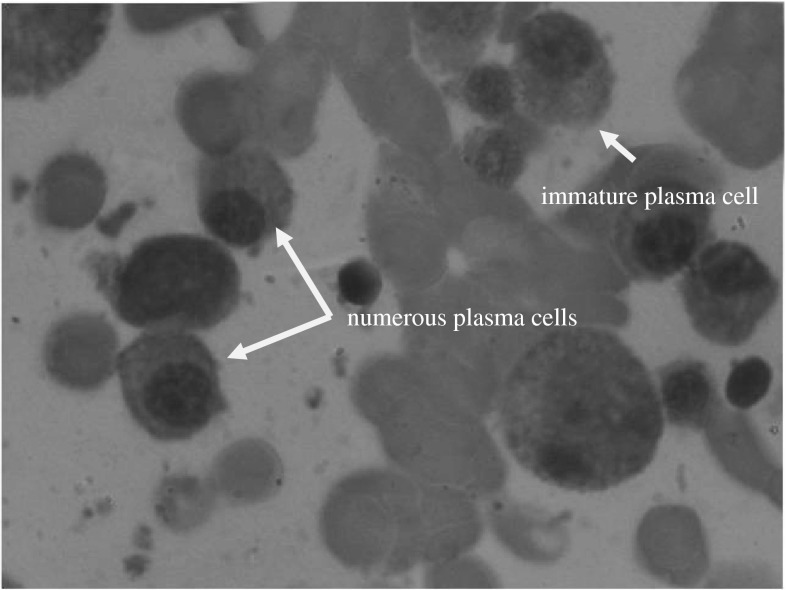
Immunofixation showed IgM kappa monoclonal gammopathy (Fig. 1). The bone marrow aspirate showed features of MM with plasma cell of 18 % (Fig. 2).
Fig. 1.
Serum protein electrophoresis with M-spike in β region suggestive of Multiple Myeloma
Fig. 2.
Bone marrow biopsy showing increased plasma cells
Abdominal ultrasound and CT showed no evidence of intra abdominal bleeding. Transfusions of packed-cells and fresh frozen plasma were initially administered. The prolonged APTT was not correctable with the 50:50 mix (mixing the patient plasma 1:1 with plasma that contains 100 % of the normal factor level), indicating the presence of an inhibitor. The inhibitor assay to FVIII, showed a titre of 5 Bethesda Units (BU). The patient weighed 88 kg, and a 2 mg vial of recombinant activated factor VII (rFVIIa) was administered as a single bolus dose at 20 mcg/kg to cut the cost. This resulted in rapid haemostasis.
High dose dexamethasone 40 mg/day for 4 days, thalidomide 200 mg/day and bortezomib 1.4 mg/m−2 on day 1 and day 4 was also initiated.
MM as a cause of AHA was considered due to anaemia, elevated serum globulin (with IgM kappa monoclonal gammopathy), mildly elevated creatinine, and increased myeloma cells in the bone marrow biopsy.
The diagnosis of AHA is based on the demonstration of an isolated prolongation of the APTT, and formal evidence of a factor VIII inhibitor in a patient with no personal or family history of bleeding [2].
Review of literature provided 30 cases of AHA associated with hematologic malignancies [3]. Sallah et al. [4] (2000) published the largest case series and reported eight cases of anti-FVIII inhibitors associated with haematologic cancers.
Effective haemostasis can be achieved by replacement with FVIII concentrates (or desmopressin if mild) or bypassing agents such as plasma-derived activated prothrombin complex concentrates and rFVII. The recommended dose of rFVIIa is 90mcg/kg repeated every 2–3 h till cessation of bleeding. Whenever possible, cure of the associated disease leads to the disappearance of the inhibitor.
In conclusion, our patient had MM with AHA, treated with activated factor VII (rFVIIa). Though the recommended dose is 90 mcg/kg, we administered only 20mcg/kg resulting in good outcome.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Delgado J, Jimenez YV, Hernandez NF, Villar A. Acquired haemophilia: review and meta-analysis focused on therapy and prognostic factor. Br J Haematol. 2003;121:21–35. doi: 10.1046/j.1365-2141.2003.04162.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen AJ, Kessler CM. Acquired inhibitors. Bailleres Clin Haematol. 1996;9:331–354. doi: 10.1016/S0950-3536(96)80067-9. [DOI] [PubMed] [Google Scholar]
- 3.Franchini M, Targher G, Manzato F, Lippi G. Acquired factor VIII inhibitors in oncohematology: a systematic review. Crit Rev Oncol Hematol. 2008;66(3):194–199. doi: 10.1016/j.critrevonc.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Sallah S, Nguien NP, Abdallah JM, Hanrahan LR. Acquired hemophilia in patients with hematologic malignancies. Arch Pathol Lab Med. 2000;124:730–734. doi: 10.5858/2000-124-0730-AHIPWH. [DOI] [PubMed] [Google Scholar]