Up to 50 million people worldwide are afflicted with the devastating blinding disease age-related macular degeneration (AMD)1–3. The vast majority of patients have the currently untreatable “dry” or atrophic form of AMD, characterized by NLRP3 inflammasome-driven degeneration of the retinal pigment epithelium (RPE) supportive cell layer4,5. Blockade of the NLRP3 inflammasome is a next-generation therapeutic target in dry AMD; however, it was recently reported that inflammasome-mediated production of IL18 potentially safeguards the retina against the other, often more visually devastating form of AMD, for which dry AMD patients are at greatly increased risk of developing, known as choroidal neovascularization (CNV)6. Therefore, it is essential, prior to initiating inflammasome-targeting clinical trials, to directly and rigorously assess whether modulating IL18 or the NLRP3 inflammasome affects CNV and RPE cell health.
Classically, neovascular AMD is characterized by invasion and leakage of immature blood vessels from the underlying choroid into the neural retina2,3 and causes rapid and severe vision loss if not treated promptly. There is a great need to improve available treatment for CNV: Even with the current standard-of-care – blockade of vascular endothelial growth factor-A (VEGFA) –substantial vision loss still occurs in one-third of CNV patients after seven years of therapy, at which time nearly all patients exhibit central retinal atrophy7, a finding consistent with the toxicity observed following Vegfa blockade in multiple cell types in the rodent retina8,9. As such, there is a pressing need to develop improved, alternative anti-angiogenic strategies to treat neovascular AMD.
The NLRP3 inflammasome, an innate immune complex, and the cytokine IL18, which is processed into a mature form by inflammasome activation, have emerged as targets in atrophic AMD: NLRP3 activation or IL18 upregulation have been identified in the RPE of human atrophic AMD donor eyes5,10,11, and inflammasome activation or IL18 exposure induce cell death of the RPE5,11. These studies suggest that inflammasome inhibition could be therapeutic for atrophic AMD5,12. However, it has been recently reported that blocking either NLRP3 or IL18 inhibits a mouse model of CNV6. To resolve the function of this innate immune pathway in CNV and to determine the viability of modulating this pathway in clinical trials of various forms of AMD, we addressed the putative role of IL18 and NLRP3 in CNV using the standard laser injury-induced model in mice13, which is widely used and has been predictive of therapeutic success in humans.
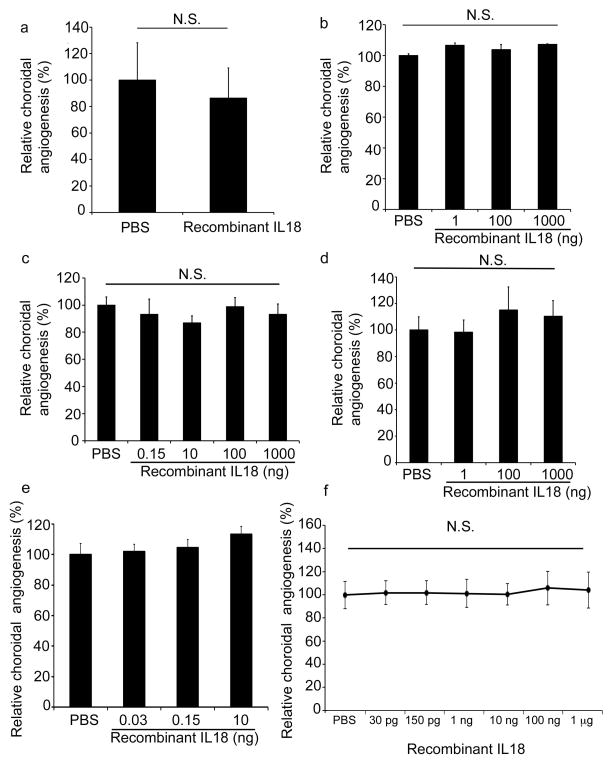
Data from experiments performed independently at five different laboratories (JA, DH, BA, YO, HT) showed that intravitreous administration of recombinant mouse IL18 (MBL International), at doses ranging from 30 pg to 1 μg, did not affect laser CNV in wild-type mice when compared to PBS injection (Fig. 1a–f). Importantly, experiments from two laboratories (JA and HT) found that doses of IL18 comparable to those reported earlier12 did not affect CNV. Meanwhile, all five groups also tested higher levels of IL18 than those tested previously. Yet, despite each of the five laboratories using varied laser injury parameters to explore whether such technical differences could elicit differential sensitivity to IL18 levels, none of the research groups observed any modulatory effect of IL18 on CNV. We also found that IL18 (100 ng) did not modulate CNV volume at either 1 or 2 weeks after laser injury (Supplementary Fig. 1a). To rule out the possibility that the small fraction of additional substances in the recombinant IL18 preparation might be pro-angiogenic, thereby counteracting the purported anti-angiogenic activity of IL18 (which comprises 98.81% of the preparation), we tested whether the concentration of sucrose (1%) in the recombinant IL18 diluent affected CNV; we found that sucrose (1%) did not change CNV compared to PBS (Supplementary Fig. 1b). To further rule out that other substances in the recombinant IL18 preparation were not counteracting the effects of IL-18, we tested the recombinant IL18 preparation in Il18r1−/− mice, which would enable us to unmask the potential pro-angiogenic activity of the non-IL18 components because IL18 would be biologically silent in these mice lacking its cognate receptor. We found that the recombinant IL18 preparation did not increase CNV in Il18r1−/− mice (Supplementary Fig. 1c), which also indicates that the MBL IL18 diluent and excipients are not pro-angiogenic. We also tested another source of recombinant mouse IL18 (US Biological) and found it too did not modulate CNV in doses ranging from 1 ng to 1 ranging from 30 pg tg (Supplementary Fig. 1d). Moreover, in vivo transfection of RPE cells by subretinal injection of expression plasmids encoding inactive precursor (pro-IL-18) or active (mature) mouse IL18 did not reduce CNV in wild-type mice (Supplementary Fig. 2a); expression from plasmids was confirmed using western blotting or immunofluorescence (Supplementary Fig. 2b-c). These data demonstrate that IL18 is not anti-angiogenic in the mouse model of laser-induced CNV.
Figure 1. IL18 administration does not affect choroidal angiogenesis.
a–e. Recombinant IL18 (rIL18) (30 pg–1,000 ng) did not decrease CNV at one week after laser injury in wild-type mice, NS compared to PBS (Independent experiments from different laboratories, a: BA, n = 12; b: DH, n = 8; c: HT, n = 16; d: YO, n = 14; e: JA, n = 17). f. Meta-analysis of datasets presented in Fig. 1a–e, with error bars denoting 95% confidence interval. NS, not significant. All error bars indicate mean ± s.e.m, Mann Whitney U test. Data from laboratories of: BA (Balamurali Ambati), DH (David Hinton), HT (Hiroko Terasaki), YO (Yuichiro Ogura), JA (Jayakrishna Ambati). n = number of eyes per condition.
Recombinant IL18 (Supplementary Fig. 3a), at a dose within the range that did not affect CNV lesion size (Fig. 1), induced RPE degeneration in wild-type mice, as did an expression plasmid for the mature, active form of IL18. Overexpression of pro-IL18, the inactive IL18 precursor did not induce RPE degeneration in wild-type mice, nor did overexpression of mature IL18 in mice lacking its cognate receptor (Il18r1−/−) (Supplementary Fig. 3b). The health of RPE cells, which subserve the neural retina, is essential for vision, and RPE loss is a hallmark of atrophic AMD. Consistent with the observed RPE and, secondarily, photoreceptor degeneration, the electrical response of the eye to light, as measured by full-field electroretinography, of wild-type mice treated with recombinant IL18 was diminished, showing decreased a- and b-wave amplitudes on ERG compared to PBS injection control (Supplementary Fig. 3c). Finally, we found expression of the IL18 receptor (IL18R1) in the RPE of normal human eyes by immunostaining (Supplementary Fig. 3d); it seems reasonable to speculate that increasing IL18 levels in the retina could lead to RPE degeneration and consequently, severe vision loss, in humans. Moreover, the plausibility of this idea is congruent with our findings of increased IL18 expression in human eyes with atrophic AMD5. Altogether, these data do not support the notion that IL18 is anti-angiogenic for CNV, and, importantly, demonstrate that intraocular IL18 administration (40 ng to 100 ng in mice) is toxic to the retina. Furthermore, Doyle et al.12 showed that an even lower dose of IL18 (3 ng) resulted in molecular and visual pathology at just 24 hours after intravitreous administration. Therefore, IL18 therapy could pose a threat to vision and carries the risk of harming the sight of patients.
Although delivery of exogenous IL18 did not reduce CNV, we also tested whether blockade of endogenous IL18 would affect CNV. It was previously reported that intravitreous administration of an anti-IL18 antibody increased CNV lesion size, purportedly via neutralization of endogenous IL18 (ref. 6). However, that experiment compared eyes treated with this antibody to eyes treated with a sham injection, i.e., lack of any injection. We now describe the results of an identical experiment whilst using biologically and chemically robust controls: an intravitreous administration of the isotype control IgG prepared either in PBS or in the identical diluent. The anti-IL18 antibody used in the previous study6 was formulated in a high concentration (50%) of glycerol – a known pro-angiogenic molecule14. We therefore asked whether this antibody increased CNV not due to IL18 targeting, but rather the glycerol content of the diluent.
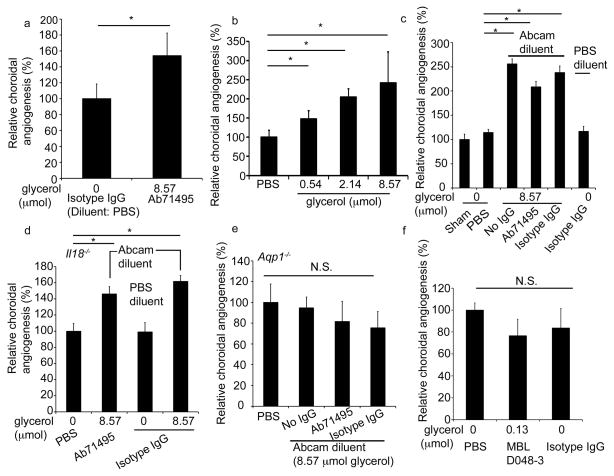
First, two groups (JA and DH) reproduced the finding that the anti-IL18 antibody (Abcam ab71495) used previously6 increased CNV in wild-type mice when compared to sham, i.e., no, injection. We also found that this anti-IL18 antibody increased CNV in wild-type mice when compared to an isotype IgG diluted in PBS (Fig. 2a). Next, we tested the hypothesis that glycerol in ab71495 was responsible for the observed increase in angiogenesis. We found that intravitreous administration of glycerol induced a dose-dependent increase in CNV in wild-type mice (Fig. 2b). The glycerol-containing Abcam diluent alone (Fig. 2c) increased CNV in wild-type mice compared to sham and PBS injection, to a similar extent as ab71495 or isotype IgG constituted in Abcam diluent. In contrast, no increase in CNV was observed for isotype IgG constituted in glycerol-free buffer. In addition, both the anti-IL18 ab71495 and isotype IgG constituted in Abcam diluent similarly increased CNV in Il18−/− mice (Fig. 2d), confirming that their pro-angiogenic effect occurred independent of Il18 blockade.
Figure 2. Glycerol, but not IL18 deficiency, increases choroidal angiogenesis.
a–d. The pro-angiogenic effect of the anti-IL18 antibody ab71495 was due to glycerol in the diluent. a. Ab71495 (1 μg antibody which contains 8.57 μmol glycerol) increased CNV in wild type mice compared to isotype IgG (1 μg antibody; no glycerol). (DH, n = 8). b. Glycerol increased CNV in wild type mice (JA, n = 14–17). c. Ab71495 (1 μg), its diluent (Abcam diluent) alone, and isotype IgG (1 μg) constituted with Abcam diluent increased CNV in wild-type mice compared to sham injection or PBS, whereas isotype IgG (1 μg) in PBS diluent did not increase CNV (n = 11–21). d. Ab71495 (1 μg) or isotype IgG (1 μg) constituted with Abcam diluent, but not an isotype IgG in PBS, increased CNV in Il18−/− mice, compared to PBS injection (n = 6–12). e. Ab71495 (1 μg), its diluent (Abcam diluent) alone, and isotype IgG (1 μg) constituted with Abcam diluent did not increase CNV compared to PBS in mice deficient for aquaporin-1 (Aqp1−/−) (n = 16). f. Neither the neutralizing anti-IL18 antibody MBL D048-3 (30 ng which contains 0.128 μmol glycerol) nor an isotype IgG (30 ng) increased CNV compared to PBS in wild-type mice (n = 18–20). a–f, final glycerol concentration of injections shown in μmol. a–d *, P < 0.05, Mann Whitney U test. e,f NS, not significant. CNV volumes were measured at one week after laser injury. All error bars indicate mean ± s.e.m. DH, data from laboratory of David Hinton; unless specified, data from lab of JA (Jayakrishna Ambati). n = number of eyes per condition.
On the other hand, consistent with the pro-angiogenic property of glycerol, neither ab71495 in diluent nor diluent alone increased CNV in mice deficient for aquaporin-1, a membrane channel protein that is permeable to glycerol15 and promotes angiogenesis16 (Fig. 2e). Next, we dialyzed the Ab71495 antibody to remove substances less than 10,000 Da in molecular weight (including glycerol: 92 Da; and thimerosol: 405 Da), achieving a depletion of 99.95% of glycerol from the antibody preparation. We found that, in contrast to non-dialyzed Ab71495, dialyzed (glycerol-depleted) antibody did not increase CNV in wild-type mice (Supplementary Fig. 4a). After dialysis, the only remaining known constituent in the diluent would be 1% BSA; to rule out any confounding effects of BSA, we found that 1 % BSA did not affect CNV in wild-type mice (Supplementary Fig. 4c).
Further implicating glycerol as the pro-angiogenic component of anti-IL18 ab71495, a different anti-IL18 neutralizing antibody (MBL D048-3) used at a dose that contained 67 times less glycerol than ab71495 (or Abcam diluent) per injection, did not increase CNV in wild-type mice. Three groups (JA, HT, DH) demonstrated that MBL D048-3 antibody against IL18, which prevented RPE degeneration in a mouse model of inflammasome-induced dry AMD at the same dose5, did not change CNV size when compared to an isotype IgG control, or when dialyzed (Fig. 2f and Supplementary Fig. 5a,b). We also injected 1 μg of dialyzed MBL D048-3, and found that it too did not affect CNV (Supplementary Fig. 5b). These data indicate that the pro-angiogenic effect of the anti-pro-IL18 antibody earlier reported6 was due to glycerol and not IL18 neutralization, and that endogenous IL18 does not drive CNV.
Finally, we found that mice genetically deficient for either Il18 or its cognate receptor Il18r1 exhibited reduced CNV size compared to wild-type mice (Supplementary Fig. 5 c,d), which is also contrary to the earlier proposition that IL18 is anti-angiogenic in CNV6. Indeed, these data imply that developmental loss of IL18 function creates a microenvironment that responds with reduced angiogenesis in the face of injury.
In a recent study, Doyle et al.12 reported that intravitreous administration of recombinant IL-18 inhibited laser CNV in wild-type mice. However, their datum consisted only of a single dose (3 μl of 50 ng/ml = 150 pg) of a single IL18 preparation. Apart from the lack of any dose response study (in contrast to our 5-log dose range which encompassed 150 pg), their report also lacked any supporting loss-of-function studies, such as an IL18R1-deficient system, to support the concept that angiosuppression in their model results from a specific biological effect of IL-18. Moreover, the complete composition of their recombinant IL18 preparation (a proprietary GSK formulation) was not disclosed nor were the individual components tested in CNV; as we have demonstrated, the chemical constituents of these biological preparations can profoundly modulate angiogenesis. Another potential source of discrepancy could be the degree of experimental variation: the CNV volumes in vehicle-treated eyes in Doyle et al.12 varied 3-fold between experiments, thereby hindering unambiguous differentiation of drug effects from experimental variability.
They also assessed the effect of systemic administration of IL-18 on laser-induced CNV by administering it subcutaneously in different dosing regimens. Curiously, they reported that this route of IL-18 delivery was equally effective if administered: 1) every day beginning one day prior to laser injury; or 2) in a single dose 14 days prior to laser injury. The half-life of IL18 in the serum of mice is 16 hours17; therefore, at the time of laser injury, the administered IL-18 in mice receiving the second dosing regimen would have undergone 21 half-lives. At the reported effective single dose (1 mg/kg), the amount of recombinant IL18 remaining in the entire mouse (assuming a total body mass of 25 g) at the time of laser injury would be approximately 12 pg (25 μg/221). Assuming uniform distribution throughout the body, the amount of recombinant IL18 in the mouse choroid (~1 mg mass) – the site of CNV – at 14 days after a single systemic injection would be approximately 0.5 fg. Surprisingly, this amount of IL18 was reported to be as effective in inhibiting CNV as a dose containing 2.1 million times as much IL18 administered daily. More confusingly, the same dose administered one day prior to laser injury (but not thereafter) was reported to be completely ineffective in inhibiting CNV. Collectively, the data presented by Doyle et al.12 are both perplexing and difficult to reconcile with a biological model of IL18 signaling. Moreover, systemic IL18 administered to mice at a dose 4–40 times lower than used by Doyle et al.12 has been reported to induce cardiac dysfunction18–20; the effects of such cardiac toxicity on CNV are unknown.
Although inflammasome-targeting drugs have not yet entered clinical trials for AMD, this next-generation treatment strategy is slated to be investigated in the near future. However, inflammasome targeting in pre-clinical investigations has not been limited to IL18 blockade, but extends also to more upstream, common processing events. If a broad approach to inhibiting inflammasome activation were pursued – for example, targeting the inflammasome machinery that processes IL18 (and other cytokines), instead of blocking IL18 directly – it would be important to determine whether such general inflammasome inhibition has any effect on CNV. Therefore, we sought to more generally test whether NLRP3 inflammasome activation influences CNV using genetic ablation and pharmacological inhibition targeting the core inflammasome genes Nlrp3, Pycard, and Casp1.
CNV was unchanged in mice deficient in Casp1, Nlrp3, or Pycard compared to wild-type mice (Supplementary Fig. 6a). Similarly, intravitreous administration of cell-permeant cholesterol-conjugated, non-immunogenic 17+2 nt siRNAs21 targeting Nlrp3 or Pycard in wild-type mice did not change CNV size compared with control siRNA administration (Supplementary Fig. 6b and Supplementary Fig. 7). In addition, intravitreous administration of peptide inhibitor of caspase-1, at a dose that blocked RPE degeneration in vivo5, in wild-type mice did not change CNV compared with a control peptide (Supplementary Fig. 6c). Collectively, these data indicate that NLRP3 inflammasome targeting does not affect CNV.
The development of novel anti-angiogenic strategies to treat neovascular AMD is an important pursuit in providing much-needed improvements to current standard-of-care therapy. IL18 has been reported to promote22,23 and suppress24,25 angiogenesis in various systems, although we found that modulating IL18 levels had no impact on CNV progression. Consistent with the concept that inflammasome inhibition is a safe and viable therapeutic option for AMD, the other major cytokine produced by NLRP3 inflammasome activation, IL-1β, was found to promote CNV, and its blockade reduced CNV without negatively impacting photoreceptor health26. It is not clear why in two of our experiments we did not reproduce the results from previous work6 (Supplementary Fig. 6a; Supplementary Fig. 5), although it is possible that differences in animals (e.g. background mouse strain or age – not published in detail previously6) or technique (e.g. laser CNV parameters27), account for these discrepancies. Differences in gene-environment interactions or microbiomes also might account for the observed differences28,29.
Our work cautions against intraocular administration of IL18 for the treatment of CNV, though it is also important to recognize that laser burn-induced neovascularization model utilized here and by Doyle et al. might differ significantly from CNV in human AMD. In this case, the translation of findings in mice to humans can be hampered by the non-synonymity of immune responses between these species in human inflammatory diseases30.
Our work also highlights the need for guarded interpretation of experiments that are not rigorously controlled. Consistent with precedent literature describing the pro-angiogenic nature of glycerol, we showed that the anti-IL18 antibody reported to induce angiogenesis6 did so only because it is formulated in glycerol and not because it neutralized IL18. Our findings support the idea that inhibition of NLRP3 or IL18, which has been proposed for treatment of dry AMD, Alzheimer’s disease, atherosclerosis, and diabetes, might not exacerbate co-incident CNV.
Supplementary Material
Supplementary Figure 1. IL18 administration does not affect choroidal angiogenesis
a. CNV values were unchanged at one and two weeks after CNV induction by recombinant mouse IL18 (MBL; 100 ng) administration (JA, n = 32). b. Intravitreous injection of sucrose (1%) in wild type mice did not affect CNV. n = 10–15. c. Intravitreous injection of 100 ng recombinant mouse IL18 (MBL) did not affect CNV in mice lacking the cognate receptor (Il18r1−/−). n = 8–14. d. Intravitreous injection of a dose range (1 ng–1 μg) of recombinant mouse IL18 (USBiological Life Sciences) did not affect CNV in wild type mice. n = 5–14 (JA).
Supplementary Figure 2. IL18 administration does not affect choroidal angiogenesis
a. Subretinal injection of expression plasmids encoding pro- or mature-IL18 did not affect CNV (NS) compared to empty vector control plasmid. JA, n = 18.
b. Western blot of IL18 from RPE/choroid lysates of mice treated with plasmid expressing mature IL18 or pNull.
c. Anti-GFP staining of wild type mice injected with a plasmid expressing pro-IL18-GFP, or pNull. Scale bars 50 μm. n = number of eyes per condition.
Supplementary Figure 3. IL18 administration induces RPE degeneration and visual dysfunction. Flat mounts stained for ZO-1 (red) show RPE degeneration in wild-type mice with a. Intravitreous injection of recombinant IL18 (rIL18) (40 ng) compared to PBS-injected eyes (image representative of n = 3–4 per group), and b. Subretinal injection of an expression plasmid encoding mature IL-18 (right panel), but not pro-IL18 or an empty vector control plasmid in wild type-mice, nor mature IL-18 in Il18r1−/− mice (images representative of n =3–4. Scale bars 50 μm. c. Representative amplitude responses (top panel) and wave form (bottom panel) during scotopic flash electroretinography in mice are shown. Intravitreous injection of rIL18 (40 or 100 ng) decreased a- and b- wave responses compared to phosphate-buffered saline (PBS) control (n = 25 per group in 3–4 independent experiments, * P < 0.05, Mann Whitney U test, PBS vs. 100 ng; PBS vs. 40 ng, N.S. all data points). d. Immunolocalization of IL18 receptor (IL18R1; top) in non-diseased human retina, with robust expression in the RPE cell layer (middle panel). Bottom panel: Isotype IgG control. Scale bars 100 μm. n = number of eyes per condition.
Supplementary Figure 4. Dialyzed ab71495 and BSA do not increase CNV. Neither (a) dialyzed anti-IL18 antibody (ab71495) (1 μg, n = 15–21) (JA) nor (b) dialyzed anti-IL18 antibody (MBL D048-3) (1 μg, n = 7–23) (JA) increased CNV in wild type mice. c. 1% BSA did not increase CNV in wild type mice (n = 12–14). n = number of eyes per condition.
Supplementary Figure 5. IL18 deficiency does not increase choroidal angiogenesis. a,b. The anti-IL18 neutralizing antibody MBL D048-3 (30 ng) did not increase CNV compared to isotype IgG (30 ng) in PBS in wild-type mice. a: HT (n = 9); b: YO (n = 12). Mice lacking the (c) IL18 receptor (Il18r1−/−) (n = 16) or (d) IL18 (Il18−/−) (n = 16) exhibit decreased CNV compared to wild-type mice. a,b, final glycerol concentration of injections shown in μmol; NS, not significant. c,d *, P < 0.05, Mann Whitney U test. CNV volumes were measured at one week after laser injury. All error bars indicate mean ± s.e.m. Data from laboratory of: HT (Hiroki Terasaki); YO (Yuichiro Ogura); unless specified data from lab of JA (Jayakrishna Ambati). n = number of eyes per condition.
Supplementary Figure 6. Inflammasome deficiency does not increase choroidal angiogenesis. Mice deficient in a. Nlrp3 (n = 29), Pycard (n = 39) or Caspase1 (n = 16) do not exhibit increased CNV compared to wild-type mice. b. siRNA-mediated knockdown of Pycard or Nlrp3 did not increase CNV compared to control siRNA in wild-type mice (n = 23–29). c. A Caspase-1 inhibitory peptide did not increase CNV compared to a control peptide in wild-type mice (n = 32). NS, not significant. CNV volumes were measured at one week after laser injury. All error bars indicate mean ± s.e.m. n = number of eyes per condition.
Supplementary Figure 7. Confirmation of siRNA-mediated knockdown of Nlrp3 and Pycard in primary mouse RPE cells by real-time quantitative PCR (n = 3). *, P < 0.05, Mann Whitney U test. All error bars indicate mean ± s.e.m.
Acknowledgments
We thank L. Toll, G.R. Pattison R. King, L. Xu, M. McConnell, C. Payne, D. Robertson, G. Botzet, K. Ambati and A. Uittenbogaard for technical assistance. J.A. was supported by NIH grants DP1GM114862, R01EY018350, R01EY018836, R01EY020672, and R01EY022238, and, Doris Duke Distinguished Clinical Scientist Award, Burroughs Wellcome Fund Clinical Scientist Award in Translational Research, Ellison Medical Foundation Senior Scholar in Aging Award, Dr. E. Vernon Smith and Eloise C. Smith Macular Degeneration Endowed Chair, Foundation Fighting Blindness Individual Investigator Research Award, Carl Reeves Foundation, Harrington Discovery Institute Scholar-Innovator Award, and Research to Prevent Blindness departmental unrestricted grant; Y.H. by Alcon Japan Research award; B.J.F. by NIH T32HL091812 and UL1RR033173; A.B.C. by the Programme for Advanced Medical Education (sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde and Fundação para a Ciência e Tecnologia, Portugal) and Bayer Global Ophthalmology Research Award; N.K. by Beckman Initiative for Macular Research; B.D.G. by American Heart Association and International Retinal Research Foundation; B.K.A. by NIH R01EY017182 and R01EY017950, VA Merit Award, and Department of Defense; G.N. by NIH R01AI063331 and R01AR052756; D.R.H. by NIH P30EY003040 and R01EY001545 and Arnold and Mabel Beckman Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions: Y.H., T.Y., T.M., V.T., R.Y., Y.K., A.B.C., N.K., B.D.G., S.H., X.Z., M.N., R.I., and H.K. performed experiments. G.N. and H.N. provided animals and reagents. J.A. and B.J.F. wrote the paper with assistance from A.B.C. The project was jointly directed by J.A., D.R.H., B.K.A., M.N., H.K., Y.O, and H.T. All authors had the opportunity to discuss the results and comment on the manuscript.
References
- 1.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 2.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaneko H, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarallo V, et al. DICER1 Loss and Alu RNA Induce Age-Related Macular Degeneration via the NLRP3 Inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle SL, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-Year Outcomes in Ranibizumab-Treated Patients in ANCHOR, MARINA, and HORIZON: A Multicenter Cohort Study (SEVEN-UP) Ophthalmology. 2013 doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Nishijima K, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda A, et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460:225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan C-C, et al. Inflammasomes in human eyes with AMD and mouse retinas with focal retinal degeneration. Invest Ophthalmol Vis Sci. 2013;54:315. [Google Scholar]
- 11.Tseng WA, et al. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:110–120. doi: 10.1167/iovs.12-10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle SL, et al. IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci Transl Med. 2014;6:230ra244. doi: 10.1126/scitranslmed.3007616. [DOI] [PubMed] [Google Scholar]
- 13.Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010;29:500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobson DE, et al. 1-Butyryl-glycerol: a novel angiogenesis factor secreted by differentiating adipocytes. Cell. 1990;61:223–230. doi: 10.1016/0092-8674(90)90803-m. [DOI] [PubMed] [Google Scholar]
- 15.Abrami L, Tacnet F, Ripoche P. Evidence for a glycerol pathway through aquaporin 1 (CHIP28) channels. Pflugers Arch. 1995;430:447–458. doi: 10.1007/BF00373921. [DOI] [PubMed] [Google Scholar]
- 16.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 17.Hosohara K, et al. Interleukin-18 induces acute biphasic reduction in the levels of circulating leukocytes in mice. Clin Diagn Lab Immunol. 2002;9:777–783. doi: 10.1128/CDLI.9.4.777-783.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platis A, et al. The effect of daily administration of IL-18 on cardiac structure and function. Perfusion. 2008;23:237–242. doi: 10.1177/0267659108101511. [DOI] [PubMed] [Google Scholar]
- 19.Woldbaek PR, et al. Daily administration of interleukin-18 causes myocardial dysfunction in healthy mice. American journal of physiology. Heart and circulatory physiology. 2005;289:H708–714. doi: 10.1152/ajpheart.01179.2004. [DOI] [PubMed] [Google Scholar]
- 20.Yu Q, et al. IL-18 induction of osteopontin mediates cardiac fibrosis and diastolic dysfunction in mice. American journal of physiology Heart and circulatory physiology. 2009;297:H76–85. doi: 10.1152/ajpheart.01285.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinman ME, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park CC, et al. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Cheon S, Cho D. The dual effects of interleukin-18 in tumor progression. Cell Mol Immunol. 2007;4:329–335. [PubMed] [Google Scholar]
- 24.Coughlin CM, et al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101:1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallat Z, et al. Interleukin-18/interleukin-18 binding protein signaling modulates ischemia-induced neovascularization in mice hindlimb. Circ Res. 2002;91:441–448. doi: 10.1161/01.res.0000033592.11674.d8. [DOI] [PubMed] [Google Scholar]
- 26.Lavalette S, et al. Interleukin-1beta inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am J Pathol. 2011;178:2416–2423. doi: 10.1016/j.ajpath.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, et al. Improvement and optimization of standards for a preclinical animal test model of laser induced choroidal neovascularization. PLoS One. 2014;9:e94743. doi: 10.1371/journal.pone.0094743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhardt C, et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature. 2012;483:627–631. doi: 10.1038/nature10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahlsten D, et al. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- 30.Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. IL18 administration does not affect choroidal angiogenesis
a. CNV values were unchanged at one and two weeks after CNV induction by recombinant mouse IL18 (MBL; 100 ng) administration (JA, n = 32). b. Intravitreous injection of sucrose (1%) in wild type mice did not affect CNV. n = 10–15. c. Intravitreous injection of 100 ng recombinant mouse IL18 (MBL) did not affect CNV in mice lacking the cognate receptor (Il18r1−/−). n = 8–14. d. Intravitreous injection of a dose range (1 ng–1 μg) of recombinant mouse IL18 (USBiological Life Sciences) did not affect CNV in wild type mice. n = 5–14 (JA).
Supplementary Figure 2. IL18 administration does not affect choroidal angiogenesis
a. Subretinal injection of expression plasmids encoding pro- or mature-IL18 did not affect CNV (NS) compared to empty vector control plasmid. JA, n = 18.
b. Western blot of IL18 from RPE/choroid lysates of mice treated with plasmid expressing mature IL18 or pNull.
c. Anti-GFP staining of wild type mice injected with a plasmid expressing pro-IL18-GFP, or pNull. Scale bars 50 μm. n = number of eyes per condition.
Supplementary Figure 3. IL18 administration induces RPE degeneration and visual dysfunction. Flat mounts stained for ZO-1 (red) show RPE degeneration in wild-type mice with a. Intravitreous injection of recombinant IL18 (rIL18) (40 ng) compared to PBS-injected eyes (image representative of n = 3–4 per group), and b. Subretinal injection of an expression plasmid encoding mature IL-18 (right panel), but not pro-IL18 or an empty vector control plasmid in wild type-mice, nor mature IL-18 in Il18r1−/− mice (images representative of n =3–4. Scale bars 50 μm. c. Representative amplitude responses (top panel) and wave form (bottom panel) during scotopic flash electroretinography in mice are shown. Intravitreous injection of rIL18 (40 or 100 ng) decreased a- and b- wave responses compared to phosphate-buffered saline (PBS) control (n = 25 per group in 3–4 independent experiments, * P < 0.05, Mann Whitney U test, PBS vs. 100 ng; PBS vs. 40 ng, N.S. all data points). d. Immunolocalization of IL18 receptor (IL18R1; top) in non-diseased human retina, with robust expression in the RPE cell layer (middle panel). Bottom panel: Isotype IgG control. Scale bars 100 μm. n = number of eyes per condition.
Supplementary Figure 4. Dialyzed ab71495 and BSA do not increase CNV. Neither (a) dialyzed anti-IL18 antibody (ab71495) (1 μg, n = 15–21) (JA) nor (b) dialyzed anti-IL18 antibody (MBL D048-3) (1 μg, n = 7–23) (JA) increased CNV in wild type mice. c. 1% BSA did not increase CNV in wild type mice (n = 12–14). n = number of eyes per condition.
Supplementary Figure 5. IL18 deficiency does not increase choroidal angiogenesis. a,b. The anti-IL18 neutralizing antibody MBL D048-3 (30 ng) did not increase CNV compared to isotype IgG (30 ng) in PBS in wild-type mice. a: HT (n = 9); b: YO (n = 12). Mice lacking the (c) IL18 receptor (Il18r1−/−) (n = 16) or (d) IL18 (Il18−/−) (n = 16) exhibit decreased CNV compared to wild-type mice. a,b, final glycerol concentration of injections shown in μmol; NS, not significant. c,d *, P < 0.05, Mann Whitney U test. CNV volumes were measured at one week after laser injury. All error bars indicate mean ± s.e.m. Data from laboratory of: HT (Hiroki Terasaki); YO (Yuichiro Ogura); unless specified data from lab of JA (Jayakrishna Ambati). n = number of eyes per condition.
Supplementary Figure 6. Inflammasome deficiency does not increase choroidal angiogenesis. Mice deficient in a. Nlrp3 (n = 29), Pycard (n = 39) or Caspase1 (n = 16) do not exhibit increased CNV compared to wild-type mice. b. siRNA-mediated knockdown of Pycard or Nlrp3 did not increase CNV compared to control siRNA in wild-type mice (n = 23–29). c. A Caspase-1 inhibitory peptide did not increase CNV compared to a control peptide in wild-type mice (n = 32). NS, not significant. CNV volumes were measured at one week after laser injury. All error bars indicate mean ± s.e.m. n = number of eyes per condition.
Supplementary Figure 7. Confirmation of siRNA-mediated knockdown of Nlrp3 and Pycard in primary mouse RPE cells by real-time quantitative PCR (n = 3). *, P < 0.05, Mann Whitney U test. All error bars indicate mean ± s.e.m.