Abstract
Cdx and Hox proteins are homeodomain transcription factors that regulate hematopoiesis. Transcription of the HOX and CDX genes decreases during normal myelopoiesis, but is aberrantly sustained in leukemias with translocation or partial tandem duplication of the MLL1 gene. Cdx4 activates transcription of the HOXA9 and HOXA10 genes, and HoxA10 activates CDX4 transcription. The events that break this feedback loop, permitting a decreased Cdx4 expression during normal myelopoiesis, were previously undefined. In the current study, we find that HoxA9 represses CDX4 transcription in differentiating myeloid cells, antagonizing activation by HoxA10. We determine that tyrosine phosphorylation of HoxA10 impairs transcriptional activation of CDX4, but tyrosine phosphorylation of HoxA9 facilitates repression of this gene. As HoxA9 and HoxA10 are phosphorylated during myelopoiesis, this provides a mechanism for differentiation stage-specific Cdx4 expression. HoxA9 and HoxA10 are increased in cells expressing Mll-Ell, a leukemia-associated MLL1 fusion protein. We find that Mll-Ell induces a HoxA10-dependent increase in Cdx4 expression in myeloid progenitor cells. However, Cdx4 decreases in a HoxA9-dependent manner on exposure of Mll-Ell-expressing cells to differentiating cytokines. Leukemia-associated, constitutively active mutants of Shp2 block cytokine-induced tyrosine phosphorylation of HoxA9 and HoxA10. In comparison with myeloid progenitor cells that are expressing Mll-Ell alone, we find increased CDX4 transcription and Cdx4 expression in cells co-expressing Mll-Ell plus constitutively active Shp2. Increased Cdx4 expression is sustained on exposure of these cells to differentiating cytokines. Our results identify a mechanism for increased and sustained CDX4 transcription in leukemias co-overexpressing HoxA9 and HoxA10 in combination with constitutive activation of Shp2. This is clinically relevant, because MLL1 translocations and constitutive Shp2 activation co-exist in human myeloid leukemias.
Introduction
HOX genes are found in four groups on four chromosomes and encode a set of highly conserved homeodomain (HD) transcription factors.1 During hematopoiesis, Hox1–4 are maximally expressed in hematopoietic stem cells, whereas Hox7–11 are expressed in committed progenitors.2 HOX transcription decreases during normal myelopoiesis, but a subset of poor prognosis, acute leukemias are characterized by increased and sustained transcription of a group of HOX genes (HoxB3, B4, A7–11).3, 4, 5 This includes leukemias with chromosomal translocations of the MLL1 gene (referred to as 11q23-leukemia).6, 7, 8, 9 The Mixed Lineage Leukemia 1 (Mll1) protein activates HOX transcription in hematopoietic stem and progenitor cells, but ubiquitination-mediated degradation of Mll1 decreases this effect during normal hematopoiesis.6, 7, 8, 9, 10 Fusion proteins generated by MLL1 gene translocations lack ubiquitinated domains, resulting in sustained HOX transcription.10 Engineered loss of Mll1 in mice impairs HOX transcription and results in hematopoietic defects.11 Conversely, mice that are transplanted with bone marrow expressing an Mll-fusion protein, or overexpressing HoxA9 or HoxA10, develop a myeloproliferative neoplasm that progresses to acute myeloid leukemia (AML) over time.12, 13, 14, 15
Cdx proteins are HD transcription factors that also regulate HOX transcription. Loss of Cdx4 in mice decreases the expression of HoxA9 and HoxA10 and impairs hematopoiesis.16, 17 Engineered overexpression of Cdx4 is leukemogenic in mice, and rescues Hox expression in MLL1−/− bone marrow.16, 17 HOXA9 and HOXA10 are Cdx4 target genes and CDX4 is a sHoxA10 target gene.18, 19 This defines a positive feedback mechanism whereby Cdx4 enhances expression of HoxA9 and HoxA10, and HoxA10 enhances Cdx4 expression. A decrease in CDX4 transcription during myelopoiesis implies the existence of a mechanism that disrupts this feedback. In the current study, we hypothesize that HoxA9 represses CDX4 in differentiating cells, antagonizing HoxA10.
HoxA9 and HoxA10 co-regulate CYBB and NCF2; genes that encode components of the phagocyte NADPH-oxidase.20, 21, 22, 23, 24, 25, 26 HoxA10 binds to and represses homologous CYBB and NCF2 cis elements in myeloid progenitor cells. Differentiating cytokines induce the phosphorylation of tyrosine residues in the HoxA10-HD, decreasing the binding affinity for these genes.20, 22, 23, 24 HoxA9 binds the same CYBB and NCF2 cis elements and activates transcription during myelopoiesis in a manner that is facilitated by phosphorylation of conserved HD tyrosine residues in HoxA9.21 In myeloid progenitor cells, HoxA9 and HoxA10 are maintained in a nonphosphorylated state by Shp2.24 However, leukemia-associated, constitutively active mutants of Shp2 dephosphorylate HoxA9 and HoxA10 throughout myelopoiesis.24 Mice transplanted with bone marrow that is co-overexpressing HoxA10+activated Shp2 (E76K) develop AML without the lag time that is required with HoxA10 overexpression alone.12 Sustained CYBB and NCF2 repression contributes to phenotypic differentiation block in these mice.12
In the current study, we find that HoxA9 and HoxA10 co-regulate CDX4 via novel mechanisms, activation by HoxA10 in progenitors and repression by HoxA9 during myelopoiesis. Also, HoxA9 and HoxA10 interact with different CDX4 cis elements, unlike previously described common target genes. Mll-fusion proteins increase the expression of both HoxA9 and HoxA10, and we find that Mll-Ell enhances CDX4 transcription in progenitor cells, but decreases transcription in cells exposed to differentiating cytokines. However, Cdx4 expression is increased and sustained in cytokine-stimulated cells co-expressing Mll-Ell plus E76K-Shp2. This is clinically relevant because Shp2 activation coexists with MLL1 translocations in human AML.27 Our studies describe a mechanism for increased and sustained Cdx4 expression in Hox-overexpressing leukemias.
Results
HoxA9 represses CDX4 transcription
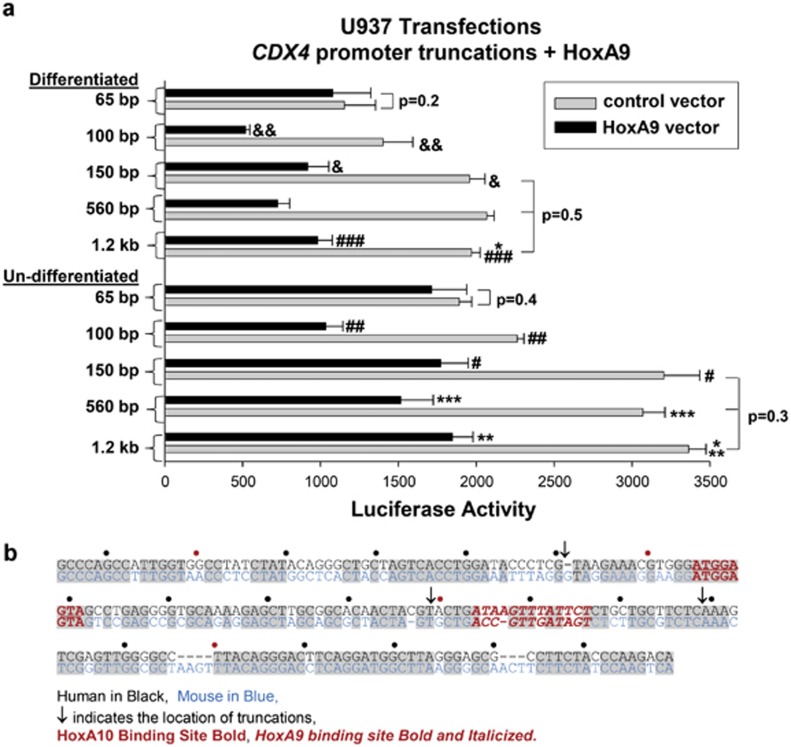
The CDX4 promoter includes other Hox-binding consensus sequences in addition to the previously identified, HoxA10-binding cis element at −139 to −150 bp (relative to the transcription start site).19 To identify HoxA9-regulated cis elements, we employed a series of promoter truncations designed around these consensus sequences.19 CDX4 promoter fragments were subcloned into a reporter vector and co-transfected into U937 myeloid cells with a vector to overexpress HoxA9 or control expression vector. To investigate the dynamics of CDX4 transcription during granulopoiesis, transfectants were analyzed with or without differentiation with retinoic acid/dimethyl formamide (RA/DMF).28, 29 We found that the overexpression of HoxA9 significantly decreased the activity of constructs with 1.2 kb, 560, 150 and 100 bp of CDX4 promoter in transfectants relative to control transfectants (P<0.01, n=6 for HoxA9 versus control expression vector; Figure 1a). This effect of HoxA9 was not observed with the 65 bp CDX4 promoter construct (P>0.5, n=6), suggesting that HoxA9 influences a cis element between −65 and −100 bp rather than the more distal HoxA10-binding cis element (Figure 1b).
Figure 1.
HoxA9 represses CDX4 transcription. (a) HoxA9 decreases CDX4 promoter activity. U937 myeloid cells were transfected with a series of CDX4 promoter–reporter constructs and a vector to overexpress HoxA9 or control expression vector. The reporter activity was determined with or without differentiation with retinoic acid/dimethyl formamide. A statistically significant difference in reporter activity with versus without differentiation is indicated by *. Statistically significant differences in reporter activity with HoxA9 overexpression versus control vector are indicated by **, ***, #, ##, ###, & or &&. Differences with P<0.01 are considered statistically significant. Some nonstatistically significant differences are also indicated. (b) The CDX4 promoter includes multiple Hox DNA-binding consensus sequences. The sequence of the proximal human CDX4 5′ flank (from the transcription start site) is depicted in black and the murine sequence in blue. Conserved sequences are indicated in gray, the distal HoxA10-binding cis element is indicated in red, the proximal HoxA9-binding cis element is indicated in red and italicized and the site of truncations used in reporter assays are indicated by arrows.
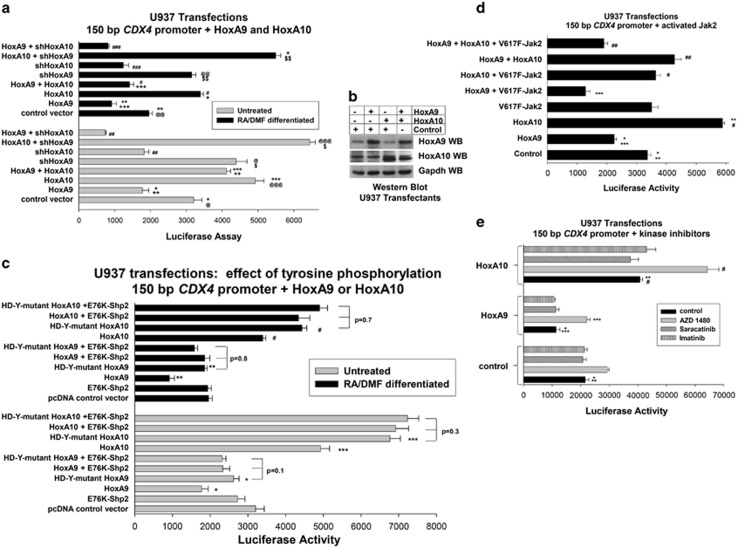
We investigated the combined effects of HoxA9 and HoxA10 by co-transfecting U937 cells with the 150-bp CDX4 reporter construct (the shortest one influenced by both Hox proteins) and vectors to overexpress these proteins (or control vector). The activity of the 150-bp construct in undifferentiated U937 cells co-overexpressing HoxA9+HoxA10 was significantly greater than transfectants with control expression vector (P<0.01, n=6), but significantly less than transfectants overexpressing HoxA10 alone (P<0.02, n=6; Figure 2a). In differentiated transfectants, the activity of the 150-bp construct was significantly less in cells co-overexpressing HoxA9+HoxA10 in comparison with control vector transfectants (P<0.001, n=6), but repression was more efficient with HoxA9 overexpression alone versus co-overexpression of HoxA9+HoxA10 (P<0.01, n=6; Figure 2a). HoxA9 and HoxA10 were equivalently overexpressed in these cells (Figure 2b).
Figure 2.
HoxA9 and HoxA10 are antagonists for CDX4 promoter activity during myelopoiesis. (a) HoxA9 antagonizes the effect of HoxA10 on the CDX4 promoter. U937 cells were transfected with a reporter construct with 150 bp of CDX4 promoter and combinations of vectors to overexpress or knockdown HoxA9 or HoxA10. Reporter activity was determined with or without differentiation. Statistically significant differences in reporter activity are indicated by *, **, ***, #, ##, ### for with versus without overexpressed HoxA9; @, @@, @@@ or + for with versus without HoxA9 shRNA; ++, +++, $ or $$ for with versus without HoxA10 overexpression. (b) HoxA9 and HoxA10 protein are equivalently overexpressed in U937 transfectants. U937 cells were transfected with vectors to overexpress HoxA9, HoxA10, HoxA9+HoxA10 or control expression vector. Western blots were serially probed with antibodies to HoxA9, HoxA10 and Gapdh (as a loading control). (c) Tyrosine phosphorylation decreases the repression of the CDX4 promoter by HoxA9 and increases activation by HoxA10. U937 cells were transfected with a reporter construct with 150 bp of CDX4 promoter and vectors to overexpress tyrosine mutant HoxA9 (HD-Y-mutant HoxA9) or tyrosine mutant HoxA10 (HD-Y-mutant HoxA10) or vectors to overexpress wild-type or HD-Y-mutant HoxA9 or HoxA10 plus constitutively active Shp2 (E76K). Reporter activity was determined with or without differentiation. Statistically significant differences in reporter activity with wild-type versus tyrosine mutant protein are indicated by *, **, *** or #. Some differences that are not statistically significant (P>0.1) are indicated. (d) Constitutively active Jak2 increases repression of the CDX4 promoter by HoxA9 and decreases activation by HoxA10. U937 cells were transfected with a reporter construct with 150 bp of CDX4 promoter and a vector to express constitutively active Jak2 (V617F) with or without vectors to overexpress HoxA9, HoxA10 or both (or control vector). Statistically significant differences in reporter activity with HoxA9 or HoxA10 versus control vector are indicated by * or **. Statistically significant differences with versus without V617F-Jak2 are indicated by ***, # or ##. (e) Inhibition of Jak2 blocks repression of the CDX4 promoter by HoxA9 but increases activation by HoxA10 in differentiating myeloid cells, but inhibition of Src or Abl tyrosine kinase do not have a similar effect. U937 cells were transfected with a reporter construct with 150 bp of CDX4 promoter and vectors to overexpress HoxA9 or HoxA10 (or control vector). Cells were differentiated with retinoic acid/dimethyl formamide (RA/DMF) and some were treated with AZD 1480 (Jak2 inhibitor), Sarcatinib (Src inhibitor) or Imatinib (Ableson kinase inhibitor). Statistically significant differences in reporter activity with HoxA9 or HoxA10 overexpression versus control vector are indicated by * and **. Statistically significant differences with versus without Jak2-inhibition are indicated by *** or #.
To further investigate the relative effects of HoxA9 and HoxA10, we co-transfected U937 cells with the 150-bp CDX4 promoter construct and vectors to express HoxA9- or HoxA10-specific short hairpin RNA (shRNAs; or scrambled control shRNAs) or to overexpress one Hox protein and knockdown the other. Activity of the 150-bp CDX4 promoter construct was significantly greater in transfectants with HoxA9-specific shRNA relative to transfectants with control shRNA and knockdown of HoxA9 also augmented activation by overexpressed HoxA10 (P<0.01, n=6 for both comparisons; Figure 2a). HoxA10 knockdown significantly decreased CDX4 promoter activity relative to transfectants with control shRNA and augmented repression by HoxA9 (P<0.001, n=6 for both comparisons; Figure 2a). The effects of HoxA9 knockdown were significantly greater in differentiated versus undifferentiated transfectants (P=0.003, n=6), and effects of HoxA10 knockdown were significantly greater in undifferentiated transfectants versus differentiated transfectants (P=0.01, n=6).
We investigated the contribution of tyrosine phosphorylation of HoxA9 or HoxA10 to CDX4 regulation by expressing forms of these proteins with conserved HD tyrosine residues mutated to phenylalanine (Y212F/Y225F-HoxA9 or Y326F/Y343F-HoxA10). These mutations influence the transcription of the CYBB and NCF2 genes, but not Hox protein stability.21, 23, 24 We co-transfected U937 cells with the 150-bp CDX4 reporter construct and vectors to overexpress HD-Y-mutant HoxA9 or HD-Y-mutant HoxA10. Repression of CDX4 by HD-Y-mutant HoxA9 was significantly less efficient than wild-type HoxA9 (P<0.0001, n=6; Figure 2c). Conversely, HD-Y-mutant HoxA10 was significantly more efficient in activating the CDX4 promoter than wild-type HoxA10 (P<0.001, n=6; Figure 2c). As an additional method to prevent HoxA9 and HoxA10 phosphorylation, some cells were co-transfected with vectors to express constitutively active Shp2 (E76K-Shp2).12, 24 E76K-Shp2 significantly decreased the efficiency of CDX4 repression by overexpressed HoxA9 (P<0.01, n=4 for comparison with HoxA9 alone), but E76K-Shp2 significantly increased CDX4 activation by overexpressed HoxA10 (P<0.001, n=4 for comparison with HoxA10 alone; Figure 2c).
In these studies, we titrated the expression of E76K-Shp2 so that it had an minimal effect on reporter activity in the absence of overexpressed HoxA9 or HoxA10. As an additional control experiment, we investigated the impact of this level of constitutive Shp2 activity on overexpressed, HD-Y-mutant HoxA9 or HoxA10. We found that the activity of the 150 bp CDX4 promoter construct was not significantly different in transfectants co-overexpressing HD-Y-mutant HoxA9+E76K-Shp2 versus HD-Y-mutant HoxA9 (P⩾0.1, n=4), or in transfectants co-overexpressing HD-Y-mutant HoxA10+E76K-Shp2 versus HD-Y-mutant HoxA10 (P⩾0.3, n=4; Figure 2c).
As we previously determined that various cytokines induce tyrosine phosphorylation of HoxA9 and HoxA10 in a Jak2-dependent manner, we investigated the effect of Jak2 on CDX4 promoter activity.30 For these studies, we co-transfected U937 cells with the 150-bp CDX4 promoter construct, a vector to express constitutively active Jak2 (V617F), and vectors to overexpress HoxA9 or HoxA10 (or relevant control vectors). We found less activation of the CDX4 promoter in transfectants overexpressing HoxA10+V617F-Jak2 in comparison with HoxA10 alone (P<0.001, n=3; Figure 2d). Conversely, we found significantly greater repression of the CDX4 promoter in transfectants overexpressing HoxA9+V617F- Jak2 versus HoxA9 alone (P<0.01, n=3; Figure 2d). Similar to differentiation, the addition of activated Jak2 to transfectants co-overexpressing HoxA9+HoxA10 switched the net effect from activation to repression (Figure 2d).
We also investigated the effect on CDX4 promoter activity of inhibiting Jak2 or other tyrosine kinases involved in myelopoiesis. In these studies, we co-transfected U937 cells with the 150-bp CDX4 promoter construct and a vector to overexpress HoxA9 or HoxA10 (or control vector). Cells were differentiated with retinoic acid/dimethyl formamide and analyzed for reporter activity after treatment with a Jak2 inhibitor (AZD 1480), a Src inhibitor (Saracatinib) or an Abl inhibitor (imatinib).31, 32, 33 We found that treatment with the Jak2 inhibitor blocked HoxA9-induced repression of the 150-bp CDX4 promoter (P<0.001, n=6 with versus without AZD 1480), but increased activation of the CDX4 promoter by HoxA10 (P<0.001, n=6 with versus without AZD 1480; Figure 2e). In contrast, inhibition of Src or Abl did not influence activity of the CDX4 promoter, with or without overexpressed HoxA9 or HoxA10 (Figure 2e).
Activity of the empty reporter vector was not influenced by differentiation or manipulation of HoxA9 or HoxA10 and was subtracted as background. We previously demonstrated that these shRNA vectors are specific for HoxA9 or HoxA10 and decrease the endogenous proteins by ~70%.28, 34
HoxA9 and HoxA10 influence adjacent CDX4 cis elements
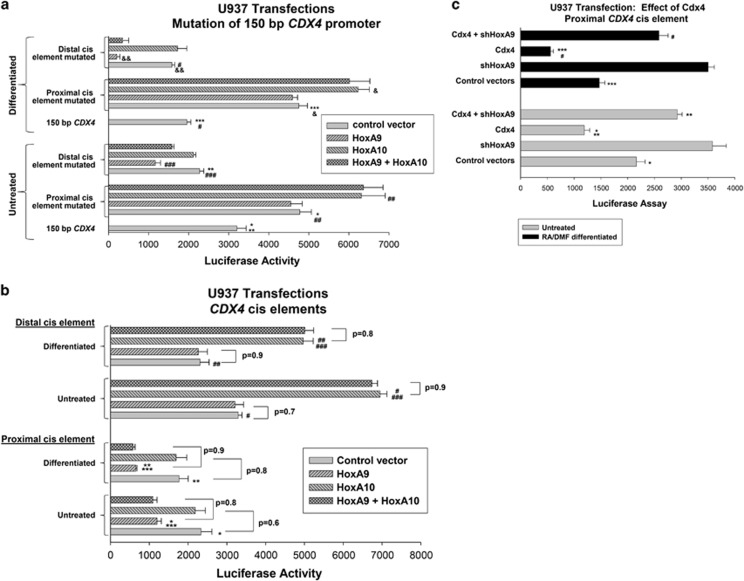
These studies identify a cis element between −65 and −100 bp in the CDX4 promoter that is influenced by HoxA9 (Figure 1b). We introduced mutations into the 150-bp CDX4 promoter construct to disrupt this cis element −86 to −94 bp, or the more distal, HoxA10-binding cis element (−139 to −146 bp). The promoter constructs were co-transfected into U937 cells with vectors to overexpress HoxA9, HoxA10 or both. Mutation of the proximal cis element blocked repression by overexpressed HoxA9 and increased activation by overexpressed HoxA10 relative to the effect of these proteins on the wild-type 150-bp construct (P<0.001, n=6; Figure 3a). Reporter expression from the proximal-cis-element-mutant construct in HoxA10-overexpressing transfectants was not significantly different with versus without HoxA9 overexpression (Figure 3a). HoxA10 did not activate a 150-bp CDX4 construct with mutation of the distal cis element, but HoxA9 was significantly more efficient at repressing this construct versus the wild-type 150-bp CDX4 construct (P<0.002, n=6; Figure 3a). The effect of co-overexpressing HoxA9+HoxA10 on activity of the distal-cis-element-mutant construct was not significantly different than HoxA9 alone (Figure 3a).
Figure 3.
HoxA9 and HoxA10 influence distinct CDX4 promoter cis elements. (a) The HoxA9-induced repression requires the proximal CDX4 promoter cis element and activation by HoxA10 the distal cis element. U937 cells were transfected with a reporter construct with 150 bp of CDX4 promoter with mutation in the Hox-binding consensus between −80 and −93 bp, the Hox-binding consensus between −141 and −148 or neither. Cells were co-transfected with vectors to overexpress HoxA9, HoxA10 or both (or control vector) and reporter assays performed with or without differentiation. Statistically significant differences in reporter activity are indicated by * and *** for mutation of the HoxA10-binding cis element; ** or # for mutation of the HoxA9 influenced cis element; ## or & for the overexpression of HoxA10 versus control expression vector; ### or && for the overexpression of HoxA9 versus control expression vector. Differences of P<0.01 are considered statistically significant. Some nonstatistically significant differences are also indicated. (b) HoxA9 represses the proximal CDX4 cis element; HoxA10 activates the distal CDX4 cis element. U937 cells were transfected with an artificial promoter/reporter construct with three copies of the −65 to −100 bp CDX4 sequence (proximal cis element) or −100 to −150-bp CDX4 sequence (distal cis element) or empty minimal promoter/reporter control vector (subtracted as background). Cells were co-transfected with vectors to overexpress HoxA9, HoxA10 or both (or control expression vector) and analyzed for reporter activity with or without differentiation. Statistically significant differences in reporter activity are indicated by * and ** for HoxA9 overexpression versus control expression vector; *** for differentiated versus nondifferentiated transfectants overexpressing HoxA9; # and ## for HoxA10 overexpression versus control expression vector; and ### for differentiated versus nondifferentiated in transfectants overexpressing HoxA10. Some differences that are not statistically significant are also indicated. (c) Cdx4 influences the proximal CDX4 cis element in a HoxA9-dependent manner. U937 cells were transfected with an artificial promoter/reporter construct with multiple copies of the proximal CDX4 cis element (or control vector) and combinations of vectors to overexpress Cdx4 and knockdown HoxA9 (or control vectors). Reporter assays were performed with or without differentiation. Statistically significant differences in reporter activity are indicated by * and *** for Cdx4 overexpression versus control expression vector and ** and # for HoxA9 knockdown versus control vector in assays with Cdx4 overexpression.
To eliminate cross-influences between CDX4 cis elements, we generated artificial promoter constructs with three copies of either the −100 to −150 or −65 to −100 bp CDX4 sequence linked to a minimal promoter and reporter. These constructs (or minimal promoter/reporter control) were co-transfected into U937 cells with vectors to overexpress HoxA9, HoxA10 or both (or control vector). Overexpressed HoxA9 significantly decreased activity of the −65 to −100 bp CDX4 construct (P<0.0001, n=6 versus control expression vector) and this effect was greater in differentiated versus undifferentiated transfectants (P=0.01, n=6; Figure 3b). HoxA10 overexpression did not alter activity of this construct (P=0.4, n=6 relative to control expression vector) and there was no difference in activity in transfectants with HoxA9 versus HoxA9+HoxA10 (P=0.6, n=6). The −100 to −150 bp CDX4 construct was activated by overexpressed HoxA10 (P<0.001, n=6 versus control expression vector) and this effect was more efficient in undifferentiated transfectants (P<0.001, n=6; Figure 3b). Overexpression of HoxA9 did not influence the −100 to −150 bp construct (P=0.7, n=6) and there was no difference in activity in transfectants with HoxA10+HoxA9 versus HoxA10 alone (P=0.6, n=6).
We hypothesized that the overexpression of Cdx4 would decrease the activity of the proximal CDX4 cis element as HoxA9 represses this cis element and Cdx4 activates HOXA9 transcription. To test this, we co-transfected U937 cells with the −65 to −100 bp CDX4 reporter construct and vectors to overexpress Cdx4 with or without HoxA9 knockdown. Cdx4 overexpression significantly decreased the activity of the proximal CDX4 cis element (P<0.001, n=6 versus control expression vector) and this was reversed by HoxA9 knockdown (Figure 3c).
Activity of the empty minimal promoter/reporter vector was not influenced by overexpression of HoxA9, HoxA10 or Cdx4 or differentiation and was subtracted as background.
HoxA9 interacts with the CDX4 promoter
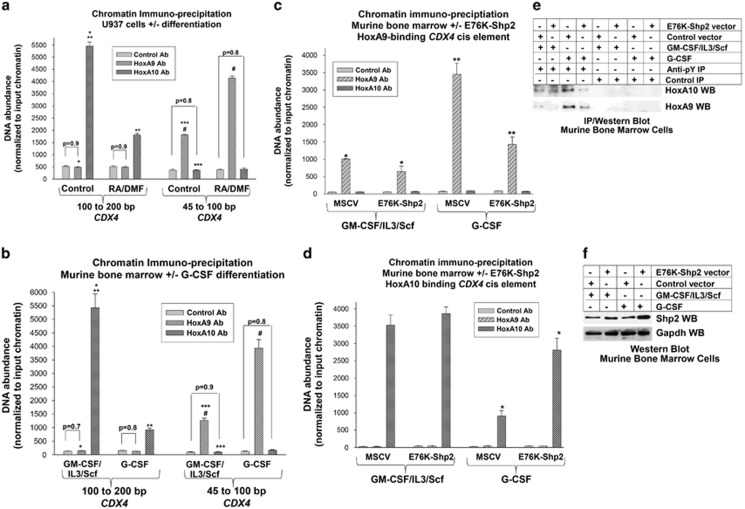
We investigated HoxA9 binding to the CDX4 promoter by chromatin immunoprecipitation. For these studies, chromatin was co-precipitated from U937 cells with an antibody to HoxA9, or HoxA10, or irrelevant antibody. Precipitated chromatin (sheared to <100 bp) was amplified by PCR with primers flanking the proximal (−45 to −100 bp) or distal (−100 to −200 bp) cis element. HoxA9 specifically interacted with the proximal CDX4 cis element with a significant increase in interaction on differentiation (P<0.001, n=3; Figure 4a). HoxA9 did not co-precipitate the distal cis element or two irrelevant CDX4 sequences (from the distal promoter or intron 1; not shown). HoxA10 interacted with the distal cis element with a significant decrease in interaction during differentiation (P<0.01, n=3), but did not interact with the proximal cis element (Figure 4a). Cdx4 did not interact with either CDX4 cis element (not shown).
Figure 4.
HoxA9 interacts with the proximal CDX4 promoter cis element in vivo. (a) In vivo interaction of HoxA9 with the proximal CDX4 cis element increases during differentiation of U937 cells. U937 cells were analyzed by chromatin co-immunoprecipitation with antibody to HoxA9, HoxA10 or irrelevant control antibody. Precipitating chromatin was amplified by real-time PCR with primers flanking regions of the CDX4 gene. Cells were analyzed with or without differentiation. Statistically significant differences in co-precipitation of chromatin sequences are indicated by * and *** for HoxA9 antibody versus HoxA10 antibody; ** and # for differentiated versus undifferentiated cells. Differences of P<0.01 were considered statistically significant. (b) In vivo interaction of HoxA9 with the proximal CDX4 cis element increases during ex vivo differentiation of murine bone marrow cells. Bone marrow mononuclear cells were harvested from wild-type mice and cultured in GM-CSF, interleukin 3 (IL3) and Scf followed by separation of CD34+ cells (myeloid progenitor conditions) or differentiation with G-CSF. Chromatin was co-precipitated and analyzed as above. Statistically significant differences in co-precipitation of chromatin sequences are indicated by * and *** for HoxA9 antibody versus HoxA10 antibody, ** and # for G-CSF-differentiated cells versus cells cultured under myeloid progenitor conditions. (c) Constitutively active Shp2 blocks binding of HoxA9 to the CDX4 promoter during myeloid differentiation. Bone marrow mononuclear cells were harvested from mice, transduced with a retroviral vector to express E76K-Shp2 (or control vector) and cultured in GM-CSF, IL3 and Scf followed by separation of CD34+ cells (myeloid progenitor conditions) or differentiation with G-CSF. Chromatin was co-precipitated and analyzed by real-time PCR with primers flanking the proximal, HoxA9-binding CDX4 cis element. Statistically significant differences with versus without expression of E76K-Shp2 are indicated by * or **. (d) Constitutively active Shp2 increases the binding of HoxA10 to the CDX4 promoter during myeloid differentiation. Bone marrow cells were transduced and cultured as described above and co-precipitating chromatin was analyzed for binding to the distal, HoxA10-binding CDX4 cis element. Statistically significant differences with versus without expression of E76K-Shp2 are indicated by *. (e) Constitutive Shp2 activity decreases tyrosine phosphorylation of HoxA9 and HoxA10 in murine bone marrow myeloid progenitor cells undergoing G-CSF-induced differentiation. Murine bone marrow cells were transduced and treated as described above. Cell lysates were immunoprecipitated with an antibody to phosphotyrosine and immunoprecipitates were analyzed by western blots probed for HoxA9 or HoxA10. Equivalence of protein in the immunoprecipitation samples is addressed in f. (f) Shp2 expression is increased in cells transduced with E76K-Shp2 expression vector. Lysates from the cells described in e above were also analyzed for by western blots probed with antibodies to Shp2 and Gapdh (as a loading control).
We performed similar chromatin immunoprecipitation using primary murine cells. Bone marrow mononuclear cells were cultured 48 h in granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin 3 and Scf, and CD34+ cells were isolated (referred to as myeloid progenitor conditions; >70% of these cells are Sca1−ckit+CD34+CD38−Gr1−). Unlike human bone marrow populations, CD34+ murine bone marrow cells are predominantly committed progenitor populations (and CD38+ cells are differentiating progenitors), not cells with HSC function.35, 36 Some cells were differentiated with G-CSF (>80% of these cells are ckit−CD34−CD38+Gr1+). We found specific binding of endogenous HoxA9 to the proximal, but not the distal, CDX4 cis element that was significantly greater in G-CSF differentiated cells in comparison with myeloid progenitor cells (P<0.0001, n=3; Figure 4b). Conversely, the specific binding of endogenous HoxA10 to the distal CDX4 cis element decreased significantly in response to G-CSF (P<0.0001, n=3; Figure 4b).
To investigate the influence of tyrosine phosphorylation on binding of endogenous HoxA9 and HoxA10 to the CDX4 promoter, bone marrow mononuclear cells were transduced with a retroviral vector to express constitutively active Shp2 (E76K) or control vector (MSCV). Transduced cells were analyzed by chromatin immunoprecipitation, as above. We found significantly less binding of HoxA9 to the CDX4 promoter in G-CSF-differentiated cells that were transduced with E76K-Shp2 vector versus MSCV control vector (P<0.001, n=3; Figure 4c). Conversely, HoxA10 binding to the CDX4 promoter was significantly greater in G-CSF-differentiated, E76K-Shp2 transduced cells in comparison with control cells (P<0.001, n=3; Figure 4d).
To confirm the effect of constitutive Shp2 activity on phosphorylation of HoxA9 or HoxA10 during G-CSF-induced differentiation, lysates from the transduced cells were analyzed by phosphotyrosine immunoprecipitation followed by western blot for HoxA9 or HoxA10. Consistent with our prior studies, we found less tyrosine phosphorylation of HoxA9 and HoxA10 in cells transduced with E76K- Shp2 vector versus control vector during G-CSF-induced differentiation (Figure 4e). In control studies with these lysates, we verified increased Shp2 expression in E76K-Shp2 vector-transduced cells and equivalent input protein in the immunoprecipitation experiments (Figure 4f). In control studies, we confirmed that the expression of E76K-Shp2 does not alter expression of endogenous HoxA9 or HoxA10, as we demonstrated in prior studies.12, 21, 24
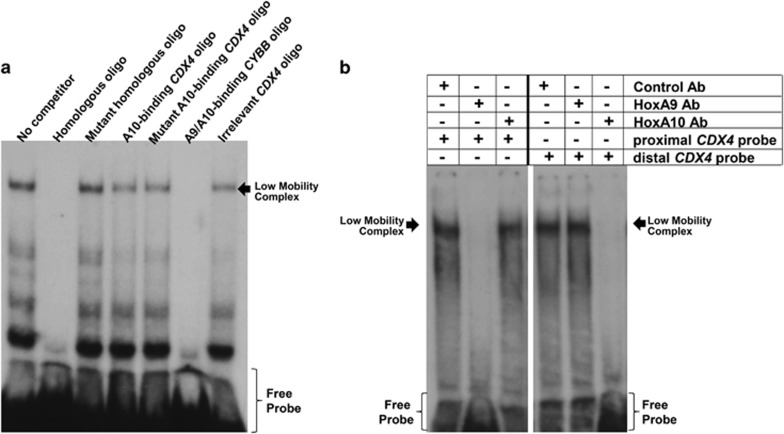
We also investigated HoxA9 interaction with the CDX4 promoter by electrophoretic mobility shift assays using oligonucleotide probes representing the proximal (−65 to −100 bp) or distal (−100 to −150 bp) cis elements and U937 nuclear proteins. Some assays with the proximal cis element probe were incubated with unlabeled, oligonucleotides competitors. We found the proximal cis element bound a low-mobility complex (Figure 5a), similar to the HoxA10-containing complex that binds the distal CDX4 cis element (Figure 5b). Protein binding to the proximal cis element was disrupted by excess homologous oligonucleotide, but not homologous oligonucleotide with mutation of the Hox-consensus, or distal cis element oligonucleotide (Figure 5a). Other assays were incubated with HoxA9, HoxA10 or irrelevant control antibody. Antibody to HoxA9, but not HoxA10, disrupted the proximal cis element complex, and antibody to HoxA10, but not HoxA9, disrupted the distal cis element complex (Figure 5b).
Figure 5.
HoxA9 interacts with the proximal CDX4 cis element in vitro. (a) The proximal CDX4 cis element binds a protein complex in vitro that is not cross-reactive with the distal cis element. Electrophoretic mobility shift assay (EMSA) was performed with nuclear proteins from U937 cells and a radiolabeled, double-stranded oligonucleotide probe representing the −68 to −104 bp sequence from the CDX4 promoter with or without unlabeled double-stranded oligonucleotide competitors including homologous CDX4 sequence (± mutation in the Hox-binding consensus), the −138 to −150-bp HoxA10-binding CDX4 sequence (± mutation in the Hox-binding consensus), the HoxA9/HoxA10-binding cis element from the CYBB promoter or an irrelevant oligonucleotide, as indicated. (b) HoxA9 binds to the proximal, but not distal, CDX4 cis element. EMSA was performed with U937 nuclear proteins and radiolabeled, double-stranded oligonucleotide probe representing −68 to −104 or −138 to −150-bp CDX4 promoter sequence with or without an antibody to HoxA9 or HoxA10 or an irrelevant control antibody.
HoxA9 decreases expression of Cdx4 in myeloid cells
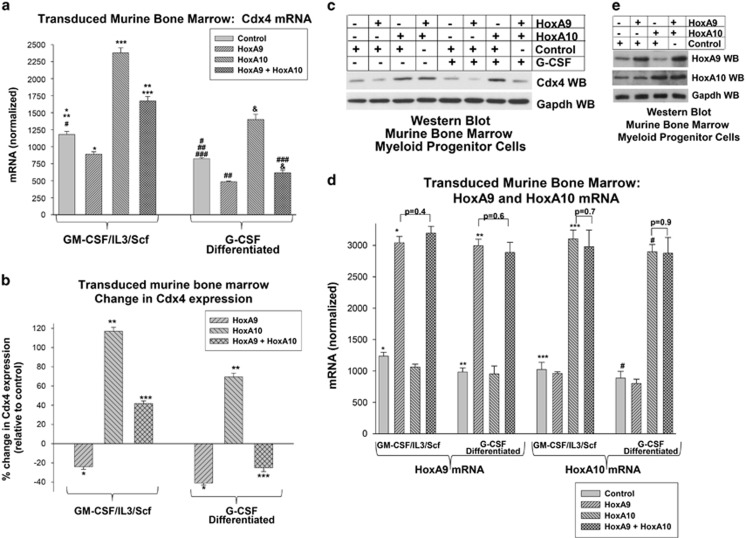
We investigated the impact of HoxA9 on endogenous Cdx4 expression using murine bone marrow cells that were transduced with vectors to express HoxA9, HoxA10 or HoxA9+HoxA10 (or empty vector). Cells were cultured under myeloid progenitor conditions or differentiated with G-CSF (see above) and analyzed for Cdx4 mRNA. We found significantly less Cdx4 mRNA in HoxA9-overexpressing cells versus cells transduced with control vector (P<0.01 for three independent experiments, analyzed in duplicate; Figure 6a). The HoxA9-induced difference in Cdx4 expression was significantly greater in G-CSF-treated cells versus myeloid progenitors (P<0.001, n=3; Figure 6b). Conversely, the effect of HoxA10 overexpression on Cdx4 mRNA expression was significantly greater in myeloid progenitor cells versus G-CSF-treated cells (P<0.001, n=3; Figure 6b). Cdx4 mRNA was significantly greater in HoxA9+HoxA10-overexpressing myeloid progenitors versus control vector-transduced cells (P<0.01, n=3; Figure 6a). However, Cdx4 mRNA was significantly less in HoxA9+HoxA10-overexpressing, G-CSF treated cells in comparison with similarly treated control vector-transduced cells (P<0.01, n=3; Figure 6a). Therefore, the effect of co-overexpressing HoxA9+HoxA10 switched from increasing Cdx4 mRNA in comparison with control vector in myeloid progenitor cells to decreased Cdx4 mRNA in comparison with control in G-CSF-differentiated cells (P<0.001, n=3; Figure 6b).
Figure 6.
Overexpression of HoxA9 decreases Cdx4 murine myeloid progenitor cells. (a) Overexpression of HoxA9 in murine myeloid progenitor cells decreases Cdx4 mRNA and antagonizes HoxA10-induced Cdx4 expression. Murine bone marrow cells were transduced with vectors to overexpress HoxA9, HoxA10, both or control expression vector. Cells were cultured in GM-CSF, interleukin 3 (IL3) and Scf followed by CD34+ cells separation (myeloid progenitor conditions) or differentiation with G-CSF. Cells were analyzed for Cdx4 mRNA by real-time PCR. Statistically significant differences in Cdx4 mRNA abundance are indicated by * and ## for HoxA9 overexpression versus control expression vector; *** and & for HoxA9 overexpression versus control expression vector in cells co-overexpressing HoxA10; ** or ### for HoxA9+HoxA10 overexpression versus control expression vector; and # for GMP versus G-CSF-differentiation. Differences of P<0.01 are considered statistically significant. (b) Differentiation with G-CSF switches the effect of co-overexpressing HoxA9+HoxA10 from increasing to impairing Cdx4 expression. Data from the experiments above were analyzed as % change in Cdx4 expression in HoxA9, HoxA10 or HoxA9+HoA10 overexpressing cells versus control vector-transduced cells. Statistically significant differences in Cdx4 expression in myeloid progenitor cells versus G-CSF differentiated cells are indicated by *, ** or ***. (c) HoxA9 overexpression decreases Cdx4 protein in primary murine bone marrow cells. Lysate proteins from the transduced murine bone marrow cells, described above, were analyzed for protein expression. Western blots were serially probed with antibodies to Cdx4 or Gapdh (loading control). (d) HoxA9 and HoxA10 are equivalently overexpressed in transduced primary murine bone marrow cells. The transduced bone marrow cells, describe above, were analyzed for HoxA9 and HoxA10 mRNA by real-time PCR. Statistically significant differences in mRNA are indicated by * and ** for HoxA9 overexpression versus control expression vector; *** and # for HoxA10 overexpression versus control expression vector. Some nonstatistically significant differences are also indicated. (e) Expression of HoxA9 and HoxA10 protein correlates with mRNA expression in the transduced cells. Western blots of cell lysates from these transduced murine bone marrow cells were analyzed for expression of HoxA9 and HoxA10 protein. Western blots were serially probed with antibodies to HoxA9, HoxA10 and Gapdh (loading control).
In these studies, Cdx4 mRNA expression correlated with Cdx4 protein expression (Figure 6c). HoxA9 and HoxA10 mRNA (Figure 6d) and protein (Figure 6e) were equivalently overexpressed.
Mll-Ell influences CDX4 transcription in a HoxA9/HoxA10-dependent manner
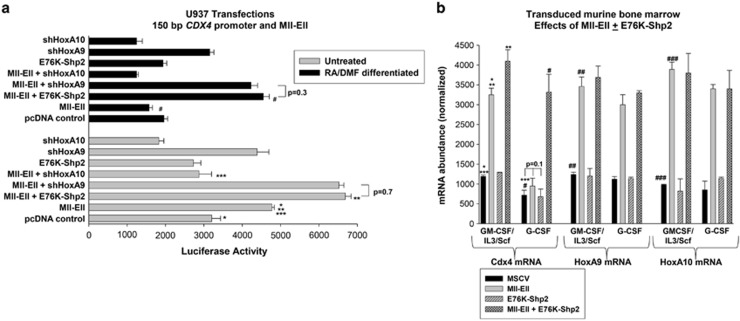
As Mll-fusion proteins increase the expressions of both HoxA9 and HoxA10, the influence of such proteins on CDX4 transcription might be similar to co-overexpressing HoxA9+HoxA10. To investigate this, we co- transfected U937 cells with the 150-bp CDX4 reporter construct and a vector to express Mll-Ell or empty expression vector, with or without vectors to knockdown HoxA9 or HoxA10. We found that Mll-Ell significantly increased the activity of the CDX4 promoter in untreated transfectants (P<0.001, n=3 versus control expression vector), but slightly repressed promoter activity in transfectants undergoing differentiation (Figure 7a). HoxA10 knockdown significantly decreased CDX4 promoter activation by Mll-Ell in undifferentiated transfectants (P<0.001, n=3 for Mll-Ell versus Mll-Ell+HoxA10 shRNA) and HoxA9 knockdown significantly increased activation by Mll-Ell with or without differentiation (P<0.0001, n=3 for Mll-Ell versus Mll-Ell+HoxA9 shRNA; Figure 7a). As inhibiting phosphorylation of HoxA9 and HoxA10 increases CDX4 promoter activity, we co-transfected U937 cells with the 150-bp CDX4 promoter construct and vectors to express Mll-Ell plus E76K-Shp2. We found that E76K-Shp2 significantly increased Mll-Ell-induced CDX4 promoter activation in undifferentiated and differentiating transfectants (P<0.001, n=3 for Mll-Ell versus Mll-Ell+E76K-Shp2; Figure 7a).
Figure 7.
Mll-Ell influences Cdx4 expression in a HoxA9/HoxA10-dependent manner. (a) Mll-Ell increases CDX4 promoter activity in myeloid progenitors, but not differentiating myeloid cells; the effect of differentiation is blocked by HoxA9-knockdown or constitutive Shp2 activation. U937 cells were co-transfected with a reporter construct with 150 bp of CDX4 promoter and vectors to express Mll- Ell with or without vectors to express specific shRNAs for HoxA9 or HoxA10 or E76K-Shp2. Reporter gene assays were performed with or without differentiation. Statistically significant differences in reporter activity are indicated by * for Mll-Ell versus control expression vector; ** and # for expression of E76K-Shp2 versus control expression vector in Ml-Ell expressing transfectants; and *** for expression of HoxA10-specific shRNA versus control shRNA in Mll-Ell expressing transfectants. (b) Mll-Ell increases Cdx4 expression in murine myeloid progenitor cells, but not G-CSF-treated cells, and this effect of G-CSF is blocked by constitutive Shp2 activation. Primary murine bone marrow cells were transduced with a vector to express Mll-Ell, E76K-Shp2, Mll-Ell+E76K-Shp2 or control expression vector. Cells were cultured in GM-CSF, interleukin 3 (IL3) and Scf followed by either separation of CD34+ cells (myeloid progenitor conditions) or differentiation with G-CSF, and analyzed for Cdx4, HoxA9, and HoxA10 mRNA by real-time PCR. Statistically significant differences in expression of these genes are indicated by *, ## and ### for with versus without Mll-Ell; ** or # for with versus without E76K-Shp2 in Mll-Ell expressing cells; and *** for myeloid progenitor versus G-CSF-differentiated cells.
We also investigated the effect of Mll-Ell on the expression of endogenous Cdx4 in murine bone marrow. Bone marrow mononuclear cells were transduced with retroviral vectors to express Mll-Ell, E76K-Shp2, both or control vector and cultured under myeloid progenitor or G-CSF-differentiation conditions (defined above). We found significantly more Cdx4 mRNA in Mll-Ell-transduced versus control vector-transduced myeloid progenitor cells (P<0.001, n=3; Figure 7b). This effect of Mll-Ell on Cdx4 mRNA was abolished by G-CSF differentiation (Figure 7b). In cells co-expressing Mll-Ell+E76K-Shp2, Cdx4 mRNA was significantly greater than in control vector-transduced cells under myeloid progenitor and G-CSF differentiation conditions (P<0.01, n=3; Figure 7b). HoxA9 and HoxA10 mRNA were both increased by Mll-Ell expression (P<0.0001, n=3 versus control expression vector; Figure 7b). Mll-Ell expression was verified by real-time PCR with primers spanning the fusion (not shown).
Shp2 inhibition decreases Cdx4 expression in Hox-overexpressing AML
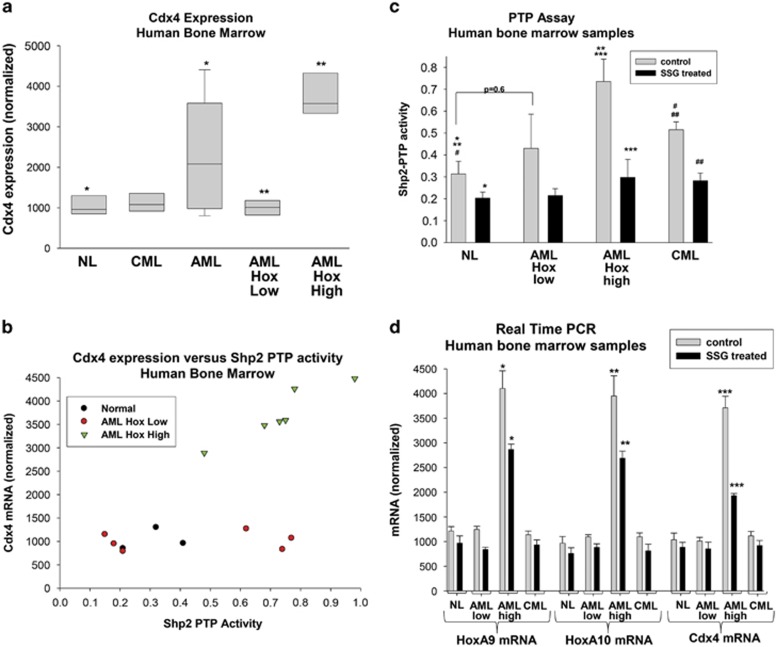
We also investigated the impact of HoxA9 and HoxA10 expression and Shp2 activity on Cdx4 expression in human AML. We analyzed CD34+ bone marrow cells from subjects with AML (16 total), chronic myeloid leukemia (5 total) or normal control individuals (4 total). Cells were assayed for HoxA9, HoxA10 and Cdx4 mRNA. AML samples were grouped for analysis as high Hox-expressing (⩾2 s.d. above the control mean; six samples) or low Hox-expressing (<2 s.d. above control; six samples). None of these samples were known to harbor translocations or duplications involving the MLL1 gene, but the Hox-high group included three samples that evolved from a myelodysplasia and two with relapsed/refractory disease. The Hox-low samples were all de novo AML. We found that Cdx4 mRNA expression was significantly greater in AML bone marrow cells relative to bone marrow from either chronic myeloid leukemia or control subjects (P<0.01 by three-way analysis of variance; Figure 8a). Cdx4 mRNA was significantly greater in the Hox-high AML group versus Hox-low AML (P<0.0001, n=3; Figure 8a).
Figure 8.
Shp2 activity influences Cdx4 expression in Hox-overexpressing human AML. (a) Increased Cdx4 expression correlates with increased expression of HoxA9 and HoxA10 in AML. Human bone marrow CD34+ cells from subjects with chronic myeloid leukemia (CML), AML or non-leukemic control subjects (NL) were analyzed by real-time PCR for expression of HoxA9, HoxA10 or Cdx4 mRNA. AML samples were separated into groups with increased HoxA9/HoxA10 expression (AML Hox high) or without increased Hox expression (AML Hox low). Statistically significant differences are indicated by * or **. (b) Cdx4 expression correlates with Shp2 activity in AML. Cells in the AML Hox-high and AML Hox-low groups (and control cells) above were analyzed for Shp2 activity by functional protein tyrosine phosphatase assay. Results were graphed as Shp2 activity versus Cdx4 mRNA. (c) Treatment with SSG decreases Shp2 activity in human bone marrow cells. The cells described above were also assayed for Shp2 protein tyrosine phosphatase activity with or without treatment with SSG. Statistically significant differences with versus without SSG are indicated by *, *** and ##. Statistically significant difference between control and leukemia samples are indicated by ** and #. (d) Treatment with SSG decreases the expressions of Cdx4, HoxA9 and HoxA10 in AML cells. The cells described above were also analyzed by real-time PCR for the expressions of Cdx4, HoxA9 and HoxA10 mRNAs. Statistically significant differences with versus without SSG in Hox-high AML are indicated by *, ** or ***.
We also analyzed the Shp2 phosphatase activity in some samples. Linear regression analysis identified a positive correlation between Shp2-protein tyrosine phosphatase activity and Cdx4 mRNA in the bone marrow of Hox-high AML (P=0.01, n=6), but not Hox-Low AML (P>0.6, n=6) or control bone marrow (P>0.4, n=4; Figure 8b). We treated a subset of samples with sodium stibogluconate (SSG), a specific inhibitor of Shp1/Shp2.36 Although these samples were not characterized for Shp2 mutations, we previously determined that SSG inhibits both wild-type and constitutively active Shp2 (not shown). We found that SSG treatment significantly decreased Shp2-PTP activity in all of the samples (P<0.01; Figure 8c). Cdx4 expression decreased significantly in the high-Hox-expressing group on treatment with SSG (P<0.0001, n=4), as did the expressions of HoxA9 and HoxA10 (P<0.01, n=4; Figure 8d). Effects of Shp2 inhibition on expression of HoxA9, HoxA10 and Cdx4 mRNA in the other groups of samples did not reach statistical significance.
Discussion
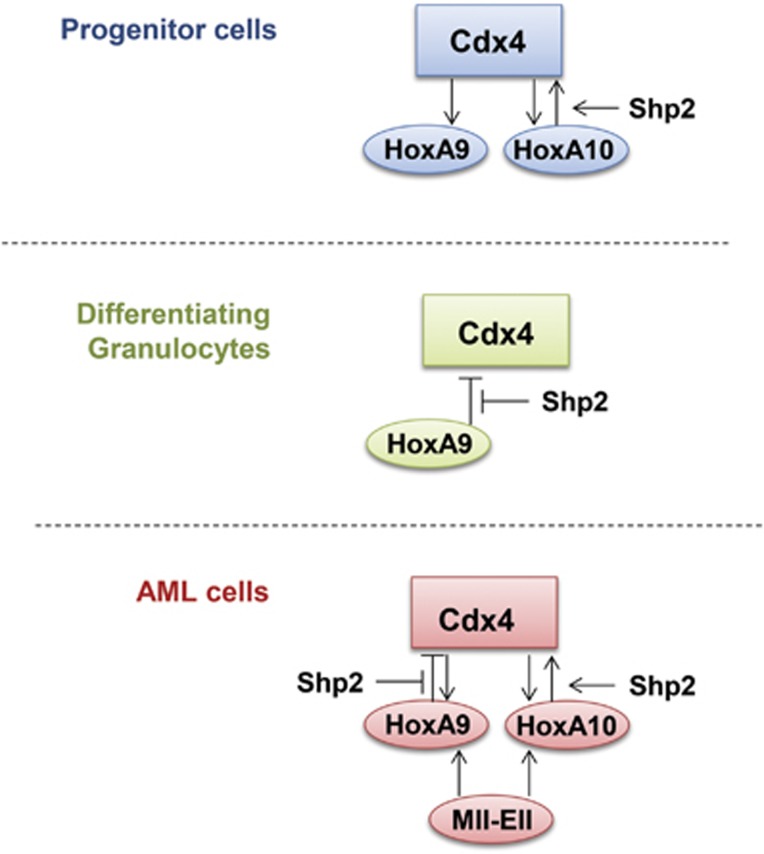
In these studies, we identify a mechanism that decreases CDX4 transcription during myelopoiesis. As Cdx4 activates transcription of genes involved in expanding hematopoietic stem and progenitor cells, decreased expression of Cdx4 contributes to proliferation arrest as myelopoiesis proceeds. We find that HoxA9 breaks the positive feedback loop between Cdx4 and HoxA10 in a differentiation stage-specific manner. Therefore, HoxA10 and Cdx4 activate each other's promoters in myeloid progenitor cells, leading to cytokine hypersensitivity and progenitor expansion via HoxA10 target genes such as FGF2, TGFB2 and ITGB3.19, 34, 37 In contrast, HoxA9 represses the CDX4 promoter in differentiating myeloid cells; inhibiting Cdx4-dependent events. Our current studies also determine that this mechanism is circumvented by constitutive activation of Shp2 in Hox-overexpressing forms of leukemia (Figure 9). This results in the sustained activation of CDX4 transcription by nonphosphorylated HoxA10 and impaired CDX4 repression by nonphosphorylated HoxA9 in cells with dysregulated Shp2 activity.
Figure 9.
Graphical representation of the influence of HoxA9 and HoxA10 on Cdx4 expression. Influences in myeloid progenitor cells are compared with differentiating granulocytes and AML cells.
A subset of poor prognosis AML is characterized by aberrant expression of a group of HD proteins, including HoxB3, B4, A7–11, Cdx2, Cdx4 and Meis1.3, 4, 5, 38 This includes AML with MLL1 translocation or partial tandem duplication, but it also a poor prognosis subset with normal cytogenetics.3, 4, 38 We found that co-overexpression of HoxA9+HoxA10, or expression of a leukemia-associated Mll-fusion protein (Mll-Ell), facilitates HoxA10-dependent CDX4 transcription in myeloid progenitor cells. However, Cdx4 expression decreases in cells exposed to differentiating cytokines. We found that this balance was shifted and Cdx4 expression was sustained by maneuvers that impair cytokine-induced phosphorylation of HoxA9 and HoxA10, including constitutive activation of Shp2. Therefore, sustained, aberrant CDX4 transcription in 11q23-AML requires a secondary, Shp2-activating mutation. Consistent with this, we found that inhibition of Shp2 in human AML samples with increased HoxA9, HoxA10 and Cdx4 decreased the expression of these HD proteins.
In previous studies, we found that HoxA10 represses CYBB and NCF2 transcription in myeloid progenitor cells by interacting with tandem cis elements in these genes, but HoxA9 activates the same cis elements during phagocyte differentiation.20, 21, 22, 23, 24 We also identified FGF2 as a target gene for HoxA9 and HoxA10, but FGF2 is activated by cooperative interaction of the two Hox proteins with a common cis element throughout myelopoiesis.28 Mll-Ell increases FGF2 transcription and autocrine production of Fgf2 in myeloid cells, and tyrosine phosphorylation of HoxA9 and HoxA10 does not influence this activity.25 Fgf2 expands bone marrow progenitor cells and participates in phagocyte effector functions.25, 34, 39 In contrast, CDX4 represents the first target gene regulated by the interaction of HoxA9 and HoxA10 with different cis elements. The mode of CDX4 regulation by HoxA9 and HoxA10 is also previously undescribed; transcription is activated by HoxA10 in myeloid progenitors and repressed by HoxA9 in differentiating cells. In all three genes with antagonistic regulation by HoxA9 and HoxA10 (CYBB, NCF2, CDX4), phosphorylation of conserved tyrosine residues in the DNA-binding HD decreases the binding of HoxA10, but increases HoxA9 binding.20, 21, 22, 23, 24
Our studies identify three classes of HoxA9/HoxA10 target genes. The first are the genes involved in phagocyte effector functions that are repressed by HoxA10 in progenitors, but activated by HoxA9 during myelopoiesis. The second are the genes involved in myeloid progenitor expansion that are activated by HoxA10 in progenitors, but repressed by HoxA9 during myelopoiesis. The third are the genes involved in both phagocyte function and progenitor expansion that are activated by cooperation between HoxA9 and HoxA10 throughout myelopoiesis. Ongoing studies in our laboratory will determine if these hypothetical categories are substantiated.
The mechanism for aberrant CDX4 transcription described in these studies is clinically relevant as activating mutations of the Shp2 gene are found 11q23-AML.27 Shp2 is also activated by leukemia-associated, activating Flt3 mutations.40 Such mutations occur frequently in AML and are enriched in the Hox-overexpressing subset.27, 41 Consistent with this, gene expression profiling studies correlate increased Cdx4 expression with MLL1 gene translocations, Flt3 mutations and progression from MDS to AML, but not with progression from chronic phase to blast crisis in chronic myeloid leukemia.42, 43, 44 Although our sample size is small, we also found correlation between Hox expression, Cdx4 expression and Shp2-PTP activity in a subset of AML. Determining the overall incidence of Hox overexpression and altered expression of target genes, such as Cdx4, will require larger studies. The incidence of Hox overexpression in our small cohort may be slightly skewed by the overrepresentation of patients with advanced and refractory disease in our referral center cohort.
Understanding the cooperation between these leukemia-associated mutations will be of interest for identifying potential, specific therapeutic targets for this treatment refractory form of AML. Our studies suggest Shp2 would be one targetable candidate.
Materials and methods
Plasmids
HoxA10 cDNA was obtained from C Largman (University of California, San Francisco).45 Mll-Ell cDNA was obtained from DE Zhang (University of California, San Diego). Cdx4 and HoxA9 cDNAs were obtained by PCR from U937 cells. HoxA9, HoxA10, Cdx4 or Mll-Ell cDNAs were subcloned into pcDNAamp (for transfections) and MSCV (for retrovirus production; Stratagene, La Jolla, CA, USA).46 Tyrosine mutant HoxA9 (Y212F/Y225F-HoxA9) or HoxA10 (Y326F/Y343F-HoxA10) was generated by site-directed mutagenesis, as described.21, 23 Shp2 and E76K-Shp2 plasmids have been previously described.21, 23 HoxA9- or HoxA10-specific shRNAs were designed with the Promega website (Madison, WI, USA). Double-stranded oligonucleotides with complementary sequences separated by a hairpin were subcloned into pLKO.1puro vector (from K Rundell, Northwestern University, Chicago). Several sequences were tested and the most efficient combined. Matched shRNAs with scrambled sequences were controls.
The CDX4 5′ flank was amplified by PCR from U937 chromatin, sequenced to ensure identity with the ENSEMBL data base and subcloned into pGL3-E vector (Promega).19 Constructs with three copies of −139 to −150 or −68 to −104 bp of the CDX4 promoter were generated in the pGL3- promoter vector.
Oligonucleotides
Oligonucleotides were synthesized by MWG Biotech (Piedmont, NC, USA). For electrophoretic mobility shift assay :-139 to −150 bp of CDX4 (5′-GTGGGATGATGTAGCCTGAGGG-3′, mutant 5′-GTGGGATGGCTTAGCCTGAGGG-3′) or −68 to −104 bp of CDX4 (5′-ACAACTACGTACTGATAAGTTTATTCTCTGCTGCTT-3′, mutant (5′-ACAACTACGTACTGATAAGCACATTCTCTGCTGCTT-3′). For PCR for chromatin immunoprecipitation (5′–3′): CDX4 (HoxA10-binding site) (F-TATGTAAAAGCCTGAAGCCCCTT, R-AAGCTCTTTTGCACCCCTC), CDX4 (HoxA9-binding site) (F-TGCAAAAGAGCTTGCGGCACAACTAC, R-TAAGCCATCCTGAAGTCCCTGTAA). Real-time PCR primers for mRNA expression were previously described.19, 28, 34
Myeloid cell line transfections and assays
The human myeloid cell line U93729 was obtained from Andrew Kraft (Hollings Cancer Center, University of South Carolina, Charleston). Cells were maintained as described (as per manufacturer's instructions: Stratagene). U937 cells were co-transfected with a luciferase reporter vector (pGL3-E) containing 1.4 kb, 450, 150, 100 or 65 bp of CDX4 5′ flank sequences, or pGL3-E control (30 pg) and a HoxA9 expression vector or control (50 pg). The 150-bp construct was co-transfected with combinations of vectors (50 pg) to overexpress or knockdown HoxA9 and HoxA10; vectors to overexpress HoxA9 or HoxA10 with HD-Y residues changed to F (HD-Y-mutant); vectors to co-overexpress combinations of Wt or HD-Y mutant HoxA9 or HoxA10+constitutively active Shp2 (E76K); or vectors to express Mll-Ell+E76K-Shp2. Other cells were co-transfected with a minimal promoter-luciferase reporter vector with three copies of the distal (−139 to −146 bp) or proximal (−84 to −96 bp) CDX4 cis element and combinations of vectors to overexpress HoxA9, HoxA10 or Cdx4 (or control vector)+vectors to express specific shRNAs for HoxA9 or HoxA10 (or scrambled control; 50 pg). Cells were transfected with a E-galactosidase reporter to control for transfection efficiency.
Murine bone marrow studies
Animal studies were performed according to the ACUC-approved protocols at Northwestern University and Jesse Brown VA.
Bone marrow mononuclear cells were obtained from femurs of C57/BL6 mice and cultured for 24 h in DME with 10% fetal calf serum, 1% penicillin–streptomycin, 20 ng/ml murine GM-CSF (R&D Systems Inc., Minneapolis, MN, USA), 20 ng/ml murine recombinant IL3 (R&D Systems) and 100 ng/ml murine recombinant stem cell factor (Scf; R&D Systems; 2 × 105 cells/ml). Cells were maintained 48 h in GM-CSF+IL3+Scf followed by isolation of CD34+ cells (using the Miltenyi magnetic bead system, Miltenyi Biotechnology, Auburn, CA, USA) or differentiation with 20 ng/ml of G-CSF.12
Retrovirus (~107 PFU/ml) was generated with HoxA10/MSCV, HoxA9/MSCV, E76K-Shp2/MSCV or control MSCV using the Phoenix packaging line according to manufacturer's instructions (Stratagene). Bone marrow mononuclear cells were cultured 24 h in 20 ng/ml IL3, 20 ng/ml GM-CSF and 100 ng/ml SCF, incubated with retroviral supernatant supplemented with polybrene (6 pg/ml) and cultured 48 h in GM-CSF, IL3 and Scf±G-CSF.12 Transduced cells were selected in the appropriate antibiotic (G418 or puromycin with the various vectors) post transduction. We find that 70–75% if the cells are consistently transduced (preselection) with this technique.
Human leukemia cells
Human studies were performed with the approval of the Northwestern University institutional review board. Bone marrow was obtained from leukemia subjects at the time of diagnostic evaluation or from individuals without leukemia. CD34+ cells were isolated (using the Miltenyi magnetic bead system) and cultured for 24 h in human GM-CSF, IL3 and Scf (at concentrations indicated above).
Quantitative real-time PCR
RNA was isolated using Trizol (Gibco-BRL, Gaithersburg MD, USA) and tested for integrity by denaturing electrophoresis. Primers were designed with Applied Biosystems software and real-time PCR performed using the SYBR green ‘standard curve' method. Results were normalized to 18S and actin (mRNA) or input chromatin (co-precipitated chromatin).
Chromatin immunoprecipitation
U937 cells were briefly treated with formaldehyde to generate DNA–protein cross links. Cell lysates were sonicated to generate chromatin fragments of <100 bp and immunoprecipitated with antibody to HoxA9, HoxA10 or Cdx4 or control antibody (Abcam, Cambridge, MA, USA).28, 34, 47 Precipitated chromatin was analyzed by PCR.
Western blots and phosphatase assays
Cells were lysed by boiling in 2 × SDS sample buffer, proteins (50 pg), separated by SDS–polyacrylamide gel electrophoresis, transferred to nitrocellulose and western blots serially probed with antibodies to HoxA9, HoxA10, Cdx4 and GAPDH (loading control). Each experiment was repeated three times with different lysates and a representative blot is shown. Immunoprecipitation studies were performed with anti-phosphotyrosine antibody (clone 4G10, Millipore, Billerica, MA, USA) under nondenaturing conditions.12, 23, 24 Immunoprecipitates were separated by SDS–polyacrylamide gel electrophoresis and western blots probed with antibodies to HoxA9 or HoxA10. Nonimmunoprecipitated lysates were used for control western blots that were probed with Shp2 and GAPDH antibodies. For phosphatase assays, cells were lysed in RIPA buffer and immunoprecipitated with Shp2 or irrelevant control antibody and assayed for functional PTP activity as previously described.12, 23, 24
Electrophoretic mobility shift assays
Nuclear proteins were extracted by Dignam's method.48, 49 Oligonucleotides probes were prepared and electrophoretic mobility shift assay was performed as described.23, 47, 48 HoxA9, HoxA10 or irrelevant control antibody was added to some assays. Competition studies were performed with double-stranded oligonucleotides at 200-fold molar excess.49, 50 At least three batches of nuclear proteins were tested in two independent experiments. Protein integrity and loading was determined in electrophoretic mobility shift assay with a CCAAT box probe.
Genomic sequence analysis
Conserved sequences and Hox-consensus sequences were identified using VISTA (Genomics Division of the Lawrence Berkley National Laboratory, Berkley, CA, USA51, 52, 53, 54).
Statistical analysis
Statistical significance was determined by Student's t-test and analysis of variance using SigmaPlot software (SigmaStat, Chicago, IL, USA). Error bars represent s.e.
Acknowledgments
This work was supported by a Translational Research Award from the Leukemia and Lymphoma Society, a VA Merit Review, NIH HL87717, NIH CA174205 (to EAE) and NIH CA77816 and NIH CA155566 (to LCP).
The authors declare no conflict of interest.
References
- Acampora D, D'Esposito M, Faiella A, Pannese M, Migliaccio E, Morelli F, et al. The human HOX, gene family. Nucleic Acids Res. 1989;17:10385–10400. doi: 10.1093/nar/17.24.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau G, Lansdorp PM, Eaves CJ, Hogge DE, Dragowska WH, Reid DS, et al. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc Natl Acad Sci USA. 1994;91:12223–12227. doi: 10.1073/pnas.91.25.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe H, Humphries RK, Blair A, Sutherland HJ, Hogge DE. Expression of HOX genes, HOX cofactors, and MLL in phenotypically and functionally defined subpopulations of leukemic and normal human hematopoietic cells. Leukemia. 1999;13:687–698. doi: 10.1038/sj.leu.2401410. [DOI] [PubMed] [Google Scholar]
- Armstrong SA, Staunton JE, Silverman LB, Pieters R, denBoer ML, Minden MD, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- Camos M, Esteve J, Jares P, Colomer D, Rozman M, Villamor N, et al. Gene expression profiling of acute myeloid leukemia with translocation t(8;16)(p11;p13) and MYST3-CREBBP rearrangement reveals a distinctive signature with a specific pattern of HOX gene expression. Cancer Res. 2006;66:6947–6954. doi: 10.1158/0008-5472.CAN-05-4601. [DOI] [PubMed] [Google Scholar]
- Guenther MG, Jenner RG, Chevalier B, Nakamura T, Croce CM, Canaani E, et al. Global and hox- specific roles for the MLL1 methyltransferase. Proc Natl Acad Sci USA. 1995;102:8603–8608. doi: 10.1073/pnas.0503072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10:1107–1117. doi: 10.1016/s1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- Ernst P, Mabon M, Davidson AJ, Zon LI, Korsmeyer SJ. An Mll-dependent Hox program drives hematopoietic progenitor expansion. Curr Biol. 2004;14:2063–2069. doi: 10.1016/j.cub.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Horton SJ, Grier DG, McGonigle GJ, Thompson A, Morrow M, De Silva I, et al. Continuous MLL- ENL expression is necessary to establish a "Hox Code" and maintain immortalization of hematopoietic progenitor cells. Cancer Res. 2005;65:9245–9252. doi: 10.1158/0008-5472.CAN-05-1691. [DOI] [PubMed] [Google Scholar]
- Wang J, Muntean AG, Wu L, Hess JL. A subset of mixed lineage leukemia proteins has plant homeodomain (PHD)-mediated E3 ligase activity. J Biol Chem. 2012;287:43410–43416. doi: 10.1074/jbc.M112.423855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BD, Hanson RD, Hess JL, Horning SE, Korsmeyer SJ. MLL, a mammalian trithorax-group gene, functions as a transcriptional maintenance factor in morphogenesis. Proc Natl Acad Sci USA. 1998;95:10632–10636. doi: 10.1073/pnas.95.18.10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lindsey S, Konieczna I, Bei L, Huang W, Eklund EA. Constitutively active SHP2 cooperates with HoxA10-overexpression to induce acute myeloid leukemia. J Biol Chem. 2009;284:2549–2567. doi: 10.1074/jbc.M804704200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsdottir U, Sauvageau G, Hough MR, Dragowska W, Lansdorp PM, Lawrence HJ, et al. Overexpression of HoxA10 in murine hematopoietic cells perturbs both myeloid and lymphoid differentiation and leads to acute myeloid leukemia. Mol Cell Biol. 1995;17:495–505. doi: 10.1128/mcb.17.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Krosl J, Thorsteinsdottir U, Baban S, Buchberg AM, Sauvageau G. HoxA9 transforms primary bone marrow cells through collaboration with Meis1a but not Pbx1b. EMBO J. 1998;17:3714–3725. doi: 10.1093/emboj/17.13.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Luo RT, Mi S, Sun M, Chen P, Bao J, et al. Consistent deregulation of gene expression between human and murine MLL rearrangement leukemias. Cancer Res. 2009;69:1109–1116. doi: 10.1158/0008-5472.CAN-08-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Ernst P, Wang Y, Dekens MPS, Kingsley PD, Pallis J, et al. Cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature. 2003;425:300–306. doi: 10.1038/nature01973. [DOI] [PubMed] [Google Scholar]
- Bansal D, Scholl C, Frohling S, McDowell E, Lee BH, Dohner K, et al. Cdx4 dysregulates Hox gene expression and generates acute myeloid leukemia alone and in cooperation with Meis1a in a murine model. Proc Natl Acad Sci USA. 2006;103:16924–16929. doi: 10.1073/pnas.0604579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Chen YX, Desmond A, Silva A, Yang Y, Wang H, et al. Cdx4 and menin co-regulate Hoxa9 expression in hematopoietic cells. PLoS ONE. 2006;1:e47. doi: 10.1371/journal.pone.0000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei L, Huang W, Wang H, Shah C, Horvath E, Eklund E. HoxA10 activates CDX4 transcription and Cdx4 activates HOXA10 transcription in myeloid cells. J Biol Chem. 2011;286:19047–19064. doi: 10.1074/jbc.M110.213983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund EA, Jalava A, Kakar R. Tyrosine phosphorylation of HoxA10 decreases DNA-binding and transcULSWLRQDO UHSUHVVLRQ GXULQJ,)1û induced differentiation of myeloid leukemia cell lines. J Biol Chem. 2000;275:20117–20126. doi: 10.1074/jbc.M907915199. [DOI] [PubMed] [Google Scholar]
- Bei L, Lu YF, Eklund EA. HoxA9 activates transcription of the gene encoding gp91PHOX during myeloid differentiation. J Biol Chem. 2005;280:12359–12370. doi: 10.1074/jbc.M408138200. [DOI] [PubMed] [Google Scholar]
- Lindsey S, Zhu C, Lu YF, Eklund EA. HoxA10 represses transcription of the gene encoding p67PHOX in phagocytic cells. J Immunol. 2005;175:5269–5279. doi: 10.4049/jimmunol.175.8.5269. [DOI] [PubMed] [Google Scholar]
- Eklund EA, Goldenberg I, Lu Y, Andrejic J, Kakar R. SHP1 protein tyrosine phosphatase regulates HoxA10 DNA-binding and transcriptional repression activity in undifferentiated myeloid cells. J Biol Chem. 2002;39:36878–36888. doi: 10.1074/jbc.M203917200. [DOI] [PubMed] [Google Scholar]
- Lindsay S, Huang W, Wang H, Zhu C, Eklund EA. Activation of SHP2 protein-tyrosine phosphatase increases HoxA10-induced repression of the genes encoding gp91PHOX and p67PHOX. J Biol Chem. 2007;282:2237–2249. doi: 10.1074/jbc.M608642200. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B, Kunkel LM, Monaco AP, Goff SC, Newburger PE, Baehner RL, et al. Cloning the gene for an inherited human disorder-chronic granulomatous disease-on the basis of its chromosomal location. Nature. 1986;322:32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- Francke U, Hsieh CL, Foellmer BE, Lomax KJ, Malech HL, Leto TL. Genes for two autosomal recessive forms of chronic granulomatous disease assigned to 1q25 (NCF2 and 7q11.23 (NCF1. Am J Hum Genet. 1990;47:483–492. [PMC free article] [PubMed] [Google Scholar]
- Balgobind BV, Hollink IH, Arentsen-Peters ST, Zimmermann M, Harbott J, Beverloo HB, et al. Integrative analysis of type-I and type-II aberrations underscores the genetic heterogeneity of pediatric acute myeloid leukemia. Haematologica. 2011;96:1478–1487. doi: 10.3324/haematol.2010.038976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah CA, Bei L, Wang H, Platanias LC, Eklund EA. The leukemia-associated Mll-Ell oncoprotein induces Fibroblast growth factor 2 (Fgf2)-dependent cytokine hypersensitivity in myeloid progenitor cells. J Biol Chem. 2013;288:32490–32505. doi: 10.1074/jbc.M113.496109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick JW, Fischer DG, Anderson SJ, Koren HA. Characterization of a human macrophage-like cell line stimulated in vitro: a model of macrophage functions. J Immunol. 1980;125:6–12. [PubMed] [Google Scholar]
- Kakar R, Kautz B, Eklund EA. JAK2 is necessary and sufficient for interferon gamma-induced transcription of the gene encoding gp91PHOX. J Leukocyte Biol. 2005;77:120–127. doi: 10.1189/jlb.0704429. [DOI] [PubMed] [Google Scholar]
- Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, et al. N-(5-chloro-1,3- benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin- 4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem. 2006;49:6465–6488. doi: 10.1021/jm060434q. [DOI] [PubMed] [Google Scholar]
- Buchdunger E, Zimmermann J, Mett H, Meyer T, Müller M, Druker BJ, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–104. [PubMed] [Google Scholar]
- Shah CA, Bei L, Wang H, Platanias LC, Eklund EA. HoxA10 regulates transcription of the gene encoding Fibroblast Growth Factor 2 (FGF2 in myeloid cells. J Biol Chem. 2012;287:18230–18248. doi: 10.1074/jbc.M111.328401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Ito T, Fackler MJ, Smith OM, Collector MI, Sharkis SJ, et al. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994;84:691–701. [PubMed] [Google Scholar]
- Matsuoka S, Ebihara Y, Xu M, Ishii T, Sugiyama D, Yoshino H, et al. CD34 expression on long-term repopulating hematopoietic stem cells changes during developmental stages. Blood. 2001;97:419–425. doi: 10.1182/blood.v97.2.419. [DOI] [PubMed] [Google Scholar]
- Pathak MK, Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol. 2001;167:3391–3397. doi: 10.4049/jimmunol.167.6.3391. [DOI] [PubMed] [Google Scholar]
- Shah CA, Wang H, Bei L, Platanias LC, Eklund EA. HoxA10 regulates transcription of the gene encoding transforming growth factor beta 2 in myeloid cells. J Biol. Chem. 2011;286:3161–3176. doi: 10.1074/jbc.M110.183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsdottir U, Kroon E, Jerome L, Blasi F, Sauvageau G. Defining roles for HOX and MEIS1 genes in induction of acute myeloid leukemia. Mol Cell Biol. 2001;21:224–234. doi: 10.1128/MCB.21.1.224-234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Takahashi K, Katsura Y, Matsuoka T, Ohsaka A. Basic fibroblast growth factor modulates the surface expression of effector cell molecules and primes respiratory burst activity in human neutrophils. Acta Haematol. 2000;103:78–83. doi: 10.1159/000041024. [DOI] [PubMed] [Google Scholar]
- Nabinger SC, Li XJ, Ramdas B, He Y, Zhang X, Zeng L, et al. The protein tyrosine phosphatase, Shp2, positively contributes to FLT3-ITD-induced hematopoietic progenitor hyperproliferation and malignant disease in vivo. Leukemia. 2013;27:398–408. doi: 10.1038/leu.2012.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. New Engl J Med. 2004;350:1617–1628. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- Wouters BJ1, Löwenberg B, Erpelinck-Verschueren CA, van Putten WL, Valk PJ, Delwel R. Double CEBPA mutations, but not single CEBPA mutations, define a subgroup of acute myeloid leukemia with a distinctive gene expression profile that is uniquely associated with a favorable outcome. Blood. 2009;113:3088–3091. doi: 10.1182/blood-2008-09-179895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez NC1, López-Pérez R, Hernández JM, Isidro I, González B, Delgado M, et al. Gene expression profile reveals deregulation of genes with relevant functions in the different subclasses of acute myeloid leukemia. Leukemia. 2005;19:402–409. doi: 10.1038/sj.leu.2403625. [DOI] [PubMed] [Google Scholar]
- Radich JP, Dai H, Mao M, Oehler V, Schelter J, Druker B, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci USA. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowney P, Corral J, Detmer K, Le Beau MM, Deaven L, Lawrence HJ, et al. A human Hox 1 homeobox gene exhibits myeloid-specific expression of alternative transcripts in human hematopoietic cells. Nucleic Acids Res. 1991;19:3443–3449. doi: 10.1093/nar/19.12.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund EA, Jalava A, Kakar R. PU.1, interferon regulatory factor 1, and interferon consensus sequence binding protein cooperate to increase gp91phox expression. J Biol Chem. 1998;273:13957–13965. doi: 10.1074/jbc.273.22.13957. [DOI] [PubMed] [Google Scholar]
- Oberley MJ, Farnham PJ. Probing chromatin immunoprecipitates with CpG-island micro-arrays to identify genomic sites occupied by DNA-binding proteins. Methods Enzymol. 2003;371:577–596. doi: 10.1016/S0076-6879(03)71043-X. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1479. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei L, Lu YF, Bellis SL, Zhou W, Horvath E, Eklund EA. Identification of a HoxA10 activation domain necessary for transcription of the gene encoding Beta 3 integrin during myeloid differentiation. J Biol Chem. 2007;282:16846–16859. doi: 10.1074/jbc.M609744200. [DOI] [PubMed] [Google Scholar]
- Wang H, Lu YF, Huang W, Papoutsakis ET, Fuhrken P, Eklund EA. HoxA10 activates transcription of the gene encoding Mkp2 in myeloid cells. J Biol Chem. 2007;282:16164–16176. doi: 10.1074/jbc.M610556200. [DOI] [PubMed] [Google Scholar]
- Dubchak I, Brudno M, Loots GG, Mayor C, Pachter L, Rubin EM, et al. Active conservation of noncoding sequences revealed by three-way species comparisons. Genome Res. 2000;10:1304–1310. doi: 10.1101/gr.142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor C, Brudno M, Schwartz JR, Poliakov A, Rubin EM, Frazer KA, et al. VISTA: visualizing global DNA sequence alignments of arbitrary length. Bioinformatics. 2000;16:1046–1053. doi: 10.1093/bioinformatics/16.11.1046. [DOI] [PubMed] [Google Scholar]
- Loots G, Ovcharenko I, Pachter L, Dubchak I, Rubin EM. rVista for comparative sequence-based discovery of functional transcription factor binding sites. Genome Res. 2002;12:832–839. doi: 10.1101/gr.225502. [DOI] [PMC free article] [PubMed] [Google Scholar]