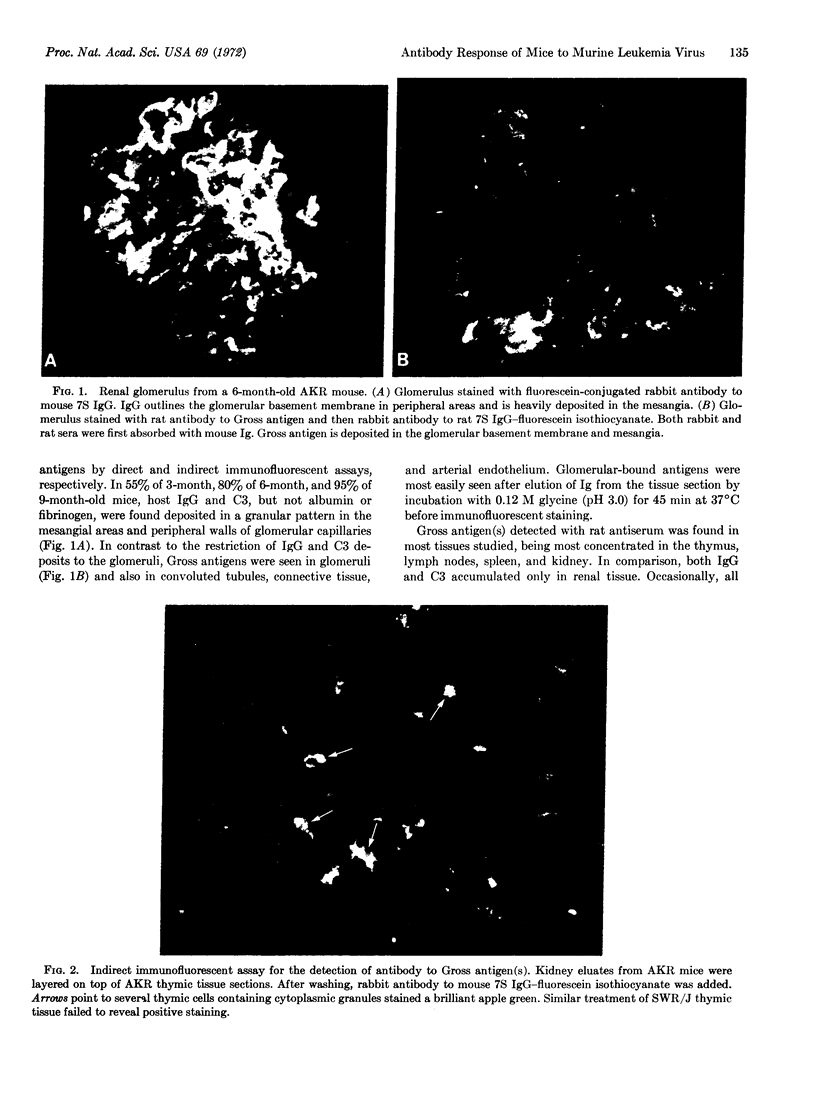
Abstract
Gross murine leukemia virus is the etiologic agent of spontaneous leukemias of AKR mice. Despite the persistence of the Gross virus throughout their life, these mice are not immunologically tolerant to the virus. Specific antibodies to Gross antigens can be detected in the kidney where they have been deposited in the glomeruli, apparently in the form of Gross antigen-antibody complexes.
Keywords: AKR mice, complement-fixing antibodies, lymphocytic choriomeningitis virus, immunofluorescence, glomerular deposits of antigen-antibody complexes
Full text
PDF
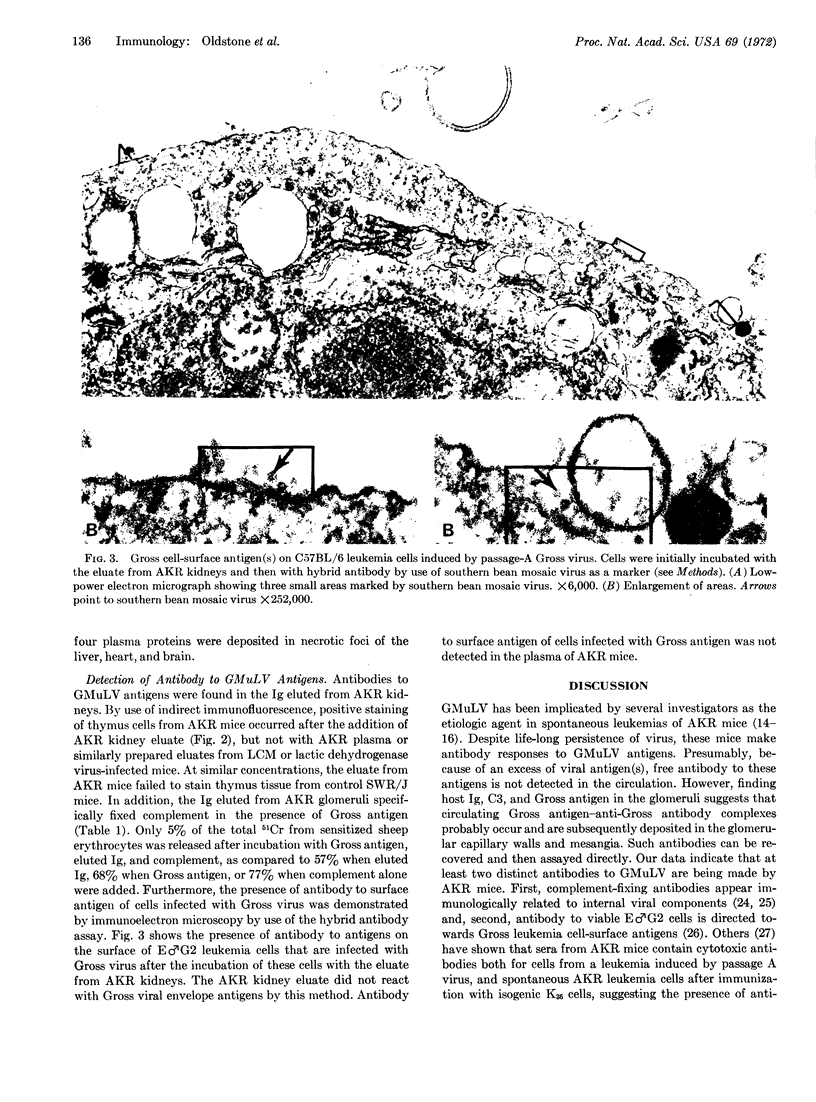


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALFORD C. A., Jr, NEVA F. A., WELLER T. H. VIROLOGIC AND SEROLOGIC STUDIES ON HUMAN PRODUCTS OF CONCEPTION AFTER MATERNAL RUBELLA. N Engl J Med. 1964 Dec 17;271:1275–1281. doi: 10.1056/NEJM196412172712501. [DOI] [PubMed] [Google Scholar]
- AXELRAD A. A. CHANGES IN RESISTANCE TO THE PROLIFERATION OF ISOTRANSPLANTED GROSSVIRUS-INDUCED LYMPHOMA CELLS, AS MEASURED WITH A SPLEEN COLONY ASSAY. Nature. 1963 Jul 6;199:80–83. doi: 10.1038/199080a0. [DOI] [PubMed] [Google Scholar]
- Aoki T., Boyse E. A., Old L. J., De Harven E., Hämmerling U., Wood H. A. G (Gross) and H-2 cell-surface antigens: location on Gross leukemia cells by electron microscopy with visually labeled antibody. Proc Natl Acad Sci U S A. 1970 Mar;65(3):569–576. doi: 10.1073/pnas.65.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Boyse E. A., Old L. J. Occurrence of natural antibody to the G (gross) leukemia antigen in mice. Cancer Res. 1966 Jul;26(7):1415–1419. [PubMed] [Google Scholar]
- Aoki T., Boyse E. A., Old L. J. Wild-type Gross leukemia virus. I. Soluble antigen (GSA) in the plasma and tissues of infected mice. J Natl Cancer Inst. 1968 Jul;41(1):89–96. [PubMed] [Google Scholar]
- Aoki T., Old L. J., Boyse E. A. Serological analysis of leukemia antigens of the mouse. Natl Cancer Inst Monogr. 1966 Sep;22:449–457. [PubMed] [Google Scholar]
- Geering G., Old L. J., Boyse E. A. Antigens of leukemias induced by naturally occurring murine leukemia virus: their relation to the antigens of gross virus and other murine leukemia viruses. J Exp Med. 1966 Oct 1;124(4):753–772. doi: 10.1084/jem.124.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriades A., Old L. J. Isolation and some characteristics of a group-specific antigen of the murine leukemia viruses. Virology. 1969 Feb;37(2):189–202. doi: 10.1016/0042-6822(69)90198-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Complement fixation and tissue culture assays for mouse leukemia viruses. Proc Natl Acad Sci U S A. 1965 May;53(5):931–938. doi: 10.1073/pnas.53.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Allison A. C., Harvey J. J. Immune complexes in mice infected neonatally with Moloney leukaemogenic and murine sarcoma viruses. Nature. 1969 Aug 16;223(5207):739–740. doi: 10.1038/223739a0. [DOI] [PubMed] [Google Scholar]
- Huebner R. J. The murine leukemia-sarcoma virus complex. Proc Natl Acad Sci U S A. 1967 Sep;58(3):835–842. doi: 10.1073/pnas.58.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Aoki T., Wood H. A., Old L. J., Boyse E. A., de Harvin E. New visual markers of antibody for electron microscopy. Nature. 1969 Sep 13;223(5211):1158–1159. doi: 10.1038/2231158a0. [DOI] [PubMed] [Google Scholar]
- Hämmerling U., Aoki T., de Harven E., Boyse E. A., Old L. J. Use of hybrid antibody with anti-gamma-G and anti-ferritin specificities in locating cell surface antigens by electron microscopy. J Exp Med. 1968 Dec 1;128(6):1461–1473. doi: 10.1084/jem.128.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Klein G. Immunological tolerance of neonatally infected mice to the Moloney leukaemia virus. Nature. 1966 Jan 8;209(5019):163–165. doi: 10.1038/209163a0. [DOI] [PubMed] [Google Scholar]
- Lundstedt C. Interaction between antigenically different cells. Virus-induced cytotoxicity by immune lymphoid cells in vitro. Acta Pathol Microbiol Scand. 1969;75(1):139–152. [PubMed] [Google Scholar]
- Notkins A. L., Mage M., Ashe W. K., Mahar S. Neutralization of sensitized lactic dehydrogenase virus by anti-gammglobulin. J Immunol. 1968 Feb;100(2):314–320. [PubMed] [Google Scholar]
- Notkins A. L., Mahar S., Scheele C., Goffman J. Infectious virus-antibody complex in the blood of chronically infected mice. J Exp Med. 1966 Jul 1;124(1):81–97. doi: 10.1084/jem.124.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Howard R. J. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. 1970;24:525–538. doi: 10.1146/annurev.mi.24.100170.002521. [DOI] [PubMed] [Google Scholar]
- Old L. J., Boyse E. A., Stockert E. The G (Gross) leukemia antigen. Cancer Res. 1965 Jul;25(6):813–819. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Lactic dehydrogenase virus-induced immune complex type of glomerulonephritis. J Immunol. 1971 May;106(5):1260–1263. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Lymphocytic choriomeningitis: production of antibody by "tolerant" infected mice. Science. 1967 Dec 1;158(3805):1193–1195. doi: 10.1126/science.158.3805.1193. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Persistent lymphocytic choriomeningitis viral infection. 3. Virus-anti-viral antibody complexes and associated chronic disease following transplacental infection. J Immunol. 1970 Oct;105(4):829–837. [PubMed] [Google Scholar]
- PHILLIPS C. A., MELNICK J. L., YOW M. D., BAYATPOUR M., BURKHARDT M. PERSISTENCE OF VIRUS IN INFANTS WITH CONGENITAL RUBELLA AND IN NORMAL INFANTS WITH A HISTORY OF MATERNAL RUBELLA. JAMA. 1965 Sep 20;193:1027–1029. doi: 10.1001/jama.1965.03090120035008. [DOI] [PubMed] [Google Scholar]
- PLOTKIN S. A., DUDGEON J. A., RAMSAY A. M. LABORATORY STUDIES ON RUBELLA AND THE RUBELLA SYNDROME. Br Med J. 1963 Nov 23;2(5368):1296–1299. doi: 10.1136/bmj.2.5368.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Porter H. G. Deposition of immune complexes in the kidneys of mice infected with lactic dehydrogenase virus. J Immunol. 1971 May;106(5):1264–1266. [PubMed] [Google Scholar]
- RUBIN H. Conditions for establishing immuno logical tolerance to a tumor virus. Nature. 1962 Jul 28;195:342–345. doi: 10.1038/195342a0. [DOI] [PubMed] [Google Scholar]
- Traub E. A FILTERABLE VIRUS RECOVERED FROM WHITE MICE. Science. 1935 Mar 22;81(2099):298–299. doi: 10.1126/science.81.2099.298. [DOI] [PubMed] [Google Scholar]
- WAHREN B. COMPARISON OF TUMOUR ANTIGENS IN AKR AND GROSS VIRUS (PASSAGE A)-INDUCED LEUKAEMIAS. Nature. 1965 Jan 23;205:409–410. doi: 10.1038/205409a0. [DOI] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- WELLER T. H., ALFORD C. A., Jr, NEVA F. A. RETROSPECTIVE DIAGNOSIS BY SEROLOGIC MEANS OF CONGENITALLY ACQUIRED RUBELLA INFECTIONS. N Engl J Med. 1964 May 14;270:1039–1041. doi: 10.1056/NEJM196405142702004. [DOI] [PubMed] [Google Scholar]
- Wahren B., Metcalf D. Cytotoxicity in vitro of preleukaemic lymphoid cells on syngeneic monolayers of embryo or thymus cells. Clin Exp Immunol. 1970 Sep;7(3):373–386. [PMC free article] [PubMed] [Google Scholar]