Abstract
Context:
Establishing and developing minimum data set (MDS), controlled vocabularies, taxonomies and classification systems are requirements of health information system in every society.
Aims:
The aim of this study was to propose an integrated multiple sclerosis (MS) data set by comparing European database for multiple sclerosis (EDMUS Coordinating Center Lyon, France) and iMed© software's (iMed, Merck Serono SA - Geneva). EDMUS is being developed at the EDMUS coordinating centers in Lyon, France and iMed© is owned and distributed by Merck Serono in Geneva, Switzerland.
Settings and Designs:
Retrieval of data of MDS performed through scholars responsible in related agencies and clinics.
Materials and Methods:
This research was an applied. The study was comparative-exploratory. In this study, data elements in iMed© and EDMUS software's were compared. Data collecting tool was data raw form.
Statistical Analysis Used:
Results analyzing was carried out in a descriptive-comparative method. MS data elements were proposed in three general categories: administrative; clinical; and socio-economic. In this study, a MS data set was suggested by studying data elements of EDMUS and iMed© softwares.
Results:
The MS data set includes administrative, clinical and socio-economic data elements that collect information of MS patients during the treatment course. iMed©, EDMUS and other available databases are suitable patterns for determining and recognizing MS key data elements.
Conclusion:
Developing MS data set in this study and studying other available MS information systems result in establishing standardized MS data set. By establishing this data set, it will be possible to present MS MDS internationally. MS MDS is the main base of establishing MS information systems at different levels.
Keywords: Data element, data set, database, information system, minimum data set, multiple sclerosis
INTRODUCTION
Multiple Sclerosis (MS) is a chronic and progressive disease of central nervous system (CNS) with an unknown origin.[1,2] Today, more than 2 million of the world population have the MS.[3,4,5] It is one of the most disabling nervous diseases among young people.[1] The MS causes physical and cognitive disabilities. The disease course is long and varied. There is Still no specific treatment for it.[3,6] The MS has heavy social and economic consequences. This disease also has a significant influence on patients’ quality of life and society.[7]
One of the essential factors in preventing and controlling a disease is the presence of sufficient and accurate data and information about the disease and its patients.[8,9] Recording signs and symptoms, filing and retrieving data are base of medical activities.[10,11,12] Health data are being continuously produced and collected, but collecting data with disorganized content can’t increase knowledge.[13] Standardizing medical records and using a common language has a special importance.[10,14] Therefore, establishing and developing minimum data set (MDS), controlled vocabularies, taxonomies and classification systems are requirements of health information system in every society.[13,15,16]
Information management collects and exchange information among people and organizations by using standard tools and with a common language.[17,18] Standardizing data collection and making a common language may cause some limitations on collecting data process. Although it causes losing details, it has prominent advantages.[10,15,16] Some of them are:
Monitoring health care situation[7]
Assessing health care instructions and standard performance[7]
Comparing collected medical information on different countries[10]
Reducing health care methods differences[7]
Facilitating communications among organizations and care providers[17]
Improving care quality[17]
Improving patients’ quality of life[7]
Supporting care providers and insurance organizations communications[17]
Setting policy and procedure related to prevention of diseases.[19]
Today, advantage of establishing a MS database in improving disease clinical management and facilitating research process is obvious. Presence of databases is effective in optimizing patient care, as a valuable source of information.[20,21] Using a common language is one of the basic requirement in establishing databases.[22] Developing a common language and instituting international databases will facilitate exchange, compatibility and comparability of collecting data from different databases.[7,21] Instituting a MS comprehensive database makes it possible to provide valuable epidemiological information on MS consequences.[2,23]
In Devonshire article, it is discussed on reviewing the advantages of using some MS databases for patients, specialists and researchers. He said that differences among different databases are driven by data collecting source, purpose, duration and methods of retrieving and recording.[21]
The pilot phase of the MS registry in Europe has shown that it is feasible to collect standardized data from different countries in Europe.[7]
Some of valuable MS databases in the world are multiple sclerosis database (MSBase) and European database for multiple sclerosis (EDMUS Coordinating Center Lyon, France) project:
MSBase registry is a unique and international database. This registry is instituted by a collaboration of neurologists at the world-wide. MSBase registry is specified for exchanging, following and assessing data related to MS consequences. In MSBase registry, one of data collecting and recording ways is using iMed© software (iMed, Merck Serono SA - Geneva)[24]
EDMUS project is developed form of France Lyon database. This project, by using software with the same name and with a collaboration of neurologists, is collecting data related to MS patients in the world. This project has had many successes during instituting a comprehensive database.[22]
The aim of this study was to propose an integrated MS data set by comparing EDMUS and iMed© software's.
MATERIALS AND METHODS
This research was an applied and comparative-exploratory study. In this study, data elements in iMed© (5.4.5 version) and EDMUS (5.0 version) software's were compared. EDMUS is being developed at the EDMUS coordinating centers in Lyon, France and iMed© is owned and distributed by Merck Serono in Geneva, Switzerland. Some of the reasons for selecting these two softwares were as follows:
Internationality
Accountability for many clinical and research needs of specialists, researchers and patients
Using by many neurologists and MS healthcare centers, all over the world
Accessibility freely through internet.
Data collecting was done by studying scientific articles, reviewing related websites, studying EDMUS and iMed© software's, corresponding and counseling with specialists in health information management, medical informatics, neurology fields and experienced people in designing and using these two software's in different countries. Data collecting tool was data raw form. Providing data raw form was carried out according to Multiple Sclerosis Common Data Elements (MS CDE) that is presented by the National Institute of Neurological Disorders and Stroke. Data collecting tool validity was proved by specialists’ viewpoint in Neurology and Health Information Management fields. The results were described and compared. Finally MS data elements were proposed in three categories: administrative; clinical; and socio-economic.
RESULTS AND DISCUSSION
Now-a-days, the Internet has provided an excellent opportunity for collaborative and international studies on MS consequences. One of Internet databases is MSBase which is designed for collecting data about MS patients.[23] MSBase registry has provided the possibility of collecting MS data for neurologists all over the world freely. MSBase registry with the collaboration of neurologist is collecting, following and assessing MS patients’ data and other demyelinating diseases in the CNS, on an international level. MSBase registry has facilitated multinational and multicentral epidemiological research, by instituting an Internet website and making it free to access to comparable information resources. This registry promotes MS patients’ care quality, by providing a bed for supporting health care providers.[24,25]
MS patients’ data collecting in MSBase registry is possible in two ways:
MSBase data entering form
Software's such as iMed©, which are compatible with data collecting process in MSBase.[26]
As, it has shown in Figure 1, most of the neurologists, study groups and treatment centers collect MS patients’ data by using iMed© software and then send patients’ nameless data to MSBase registry.[23] The iMed© software is able to institute and maintain comprehensive and available databases of MS patients’ treatment history and medical information. Therefore, iMed© is one of the powerful documentation tools for specialists in MS treatment.[27,28]
Figure 1.
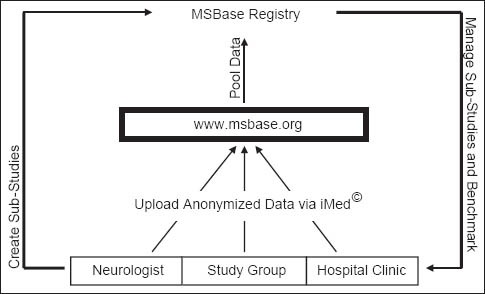
The role of iMed software in MSBase registry
In addition to MSBase and iMed© software's, EDMUS project and its software are available on the Internet. This project is developing to reach research aims, record clinical and paraclincal data of MS patients. By the help of EDMUS, MS MDS are able to be recorded and collected.[21,22] At first, the only aim of instituting EDMUS was promoting and facilitating MS research projects and it was not used as patients’ medical records; unlike databases such as Multiple Sclerosis Computer Storage Ambulatory Record in Canada that is known British Columbia Multiple Sclerosis these days. But today, EDMUS is developed and it can be used as MS patients’ medical records. Now, this database usage range is treatment and research and it improves treatment care and facilitates and promotes research by making a standard and common language for information on MS.[22,29]
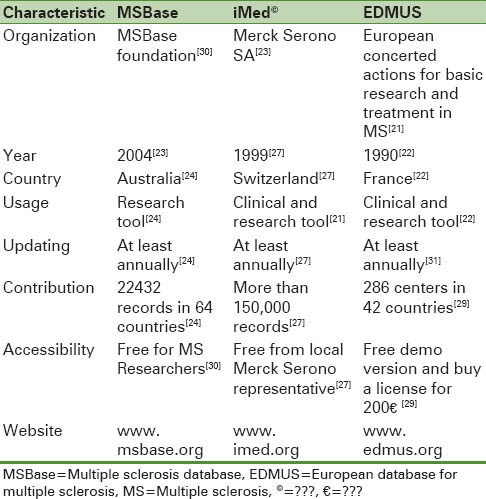
As you can be seen in Table 1, some general characteristics of MS databases discussed in this article are shown.
Table 1.
A brief introduction to MSBase, iMed© and EDMUS
In iMed© software, there are five main categories for patients’ record. Whereas, in EDMUS software, 12 main categories are defined for patients’ record.[32,33] As shown in Figure 2, categories and subcategories of EDMUS and iMed© are shown.
Figure 2.
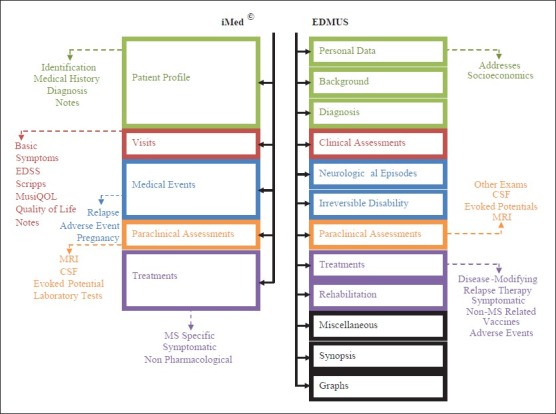
Ahmadi: Categories and subcategories in iMed© and European database for multiple sclerosis
Since a separated part is considered for presenting graphs in iMed©, synopsis and graphs are not introduced in categories. In every category in this software, there is a subcategory named “notes” which plays the role of “comments” or “miscellaneous” in EDMUS. Therefore, although miscellaneous, synopsis and graphs are not introduced in the main categories of iMed©, they are considered as the abilities of the software.
In this study, in order to separate iMed© and EDMUS data elements, standard domains of MS CDE project are used.[34] By studying iMed© and EDMUS software's, software's data elements are recognized.[32,33] After comparing collected data, MS data set was proposed in three groups: administrative, clinical and socio-economic.
In recognizing MS data elements, it should be considered that information system in MS clinics has some significant characteristics as follows:
Collecting data from separating resources
Communicating between patient and treatment team
Improving treatment and care process through monitoring and reporting weaknesses
Patient oriented for data gathering
Using information technology.[35]
Although a long time has passed from instituting MS databases, utilizing them has been limited during the time. Many of centers prefer to focus on some special aspects of MS disease management with their own databases.[36]
It's not easy to link separated databases.[37] Different administrative, clinical and socio-economic data elements are being collected in different information systems.[38] Dispersal of information has undesired effects on MS patients’ disease management and so burdens more expenses on the health system.[39,40] On the other hand, lack of enough accuracy in collecting data reduces the reliability of treatment and consequences effects assessment results.[41]
In combining different information resources and instituting a comprehensive database, there are some limitations as follows:
Incompatibility of data, coding and information receiving time
The impossibility of comparing information at the individual level, due to the lack of a unit identification number of patients
Limitation in following and monitoring the patient's situation, due to deficiencies in collecting data from different information resources.[42]
Establishing and implementing MS comprehensive database is possible by recognizing and determining standard data elements. One of the challenges in integrating health information systems has been the lack of standardizing essential data elements for collecting.[43] Determining standard data elements integrates data collected from different information systems.[44] By determining data elements and establishing MS data set, it's possible to make a framework for collecting data.[45]
The MS data set includes administrative, clinical and socio-economic data elements which collect information on MS patients during the treatment period. Furthermore, it's used as a tool for evaluating health situation and provided healthcare procedures consequences.[46] In Figure 3, a general diagram of data collecting process in MS clinics is provided. Clinical data are written in Italic Font Style.
Figure 3.
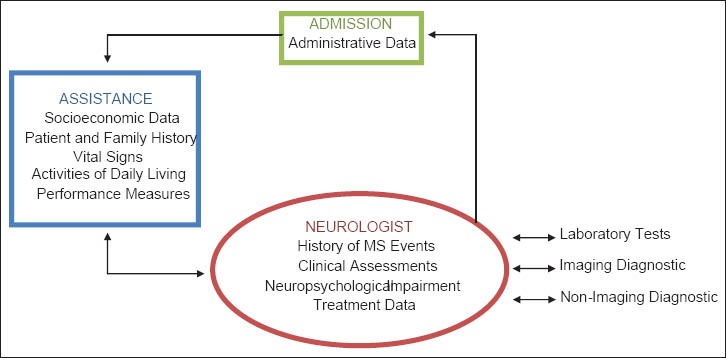
Ahmadi: Overview of data collecting in multiple sclerosis clinics
Proposed MS data set-administrative data elements
They refer to identification and financial data. These data are often collected before admission and during admission for every patient.[47] Identical information is the first data which are collected. These data are useful for facilitating integrating patients’ data at the individual level, improving communication with patients and/or following reimbursements.[48] Financial data originate from the care process.[49]
Suggested administrative data elements in MS data set included:

Determining Identification Code is obligatory. Linking different data elements in information and data sets received from other health databases and reimbursement at an individual level is possible with Unique Identification Code.
In every these software's, a unique identification number is allocated to every record. Types of patients’ record unique identification numbers in EDMUS are:
Local identifier: A unique identification number that is allocated to patients by the treatment center
EDMUS Identifier: An identification number that is allocated to every patient in the database automatically. This number begins with organizing special identical code. Every organization receives a unique code after registering specifications in the EDMUS main database
Unique International Identifier (U2I): An identification number that is made based on patient's first name, last name, gender and birth date and is allocated to the patient's record automatically. This identifier provides the ability to recognize repetitive patents in EDMUS databases.[32]
However, in iMed© they are:
iMed© patient ID: It is patient special number of iMed© network
Patient Code: A unique identification number that is allocated to patients by the treatment center.[33]
Using U2I in EDMUS to recognize patients is a really appropriate solution in preventing instituting a repetitive record for one patient. Determining this identifier for all of the MS patients and defining it as one of basic data elements in the MS data set ensures unique patient's record.
Proposed MS data set-socioeconomic data elements
They refer to data related to patient's economic and social situation.[47] Treatment methods and disease process depend on patient's economic and social situation. Hence collecting these data will effect on choosing treatment ways.[49]
Suggested socio-economic data elements in MS data set included:

Considering MS patient's life quality is one part of disease management. Unfortunately, patients and neurologists still disagree with each other that which aspect of MS has more influence on patient's quality-of-life. Although studying Activities of Daily Living and Performance Measures in clinical examinations provides important information on the person's disabilities, they don’t include many of the important factors related to the patient's quality-of-life. Therefore, life quality measuring tools, generally and especially for MS, is designed and developed. For studying information details related to MS, using this disease special tool is preferred.[50]
In iMed© and EDMUS, Environmental Status Scale (ESS), short form (SF)-36/Physical Component, SF-36/Mental Component, Visual Analogue Scale Quality of Life, Multiple Sclerosis Quality of Life-54 and Multiple Sclerosis International Quality of Life (MusiQOL) are mentioned for measuring MS patients’ life quality. Furthermore, in EDMUS, some tools are introduced to recognize cognitive and depression impairment.[32,33]
Studying patients’ quality of life needs spending time and expense. Furthermore, the presence of trained personnel for guiding in complementing questionnaire is essential. Most of questionnaires are designed for using short-time courses. Selecting suitable tool and time for measuring quality is an essential job.[50]
Proposed MS data set-clinical data elements
These data refer to health care and health situation.[47,51] They are obtained during treatment and diagnosis process. They are efficient in research, policy making, planning and reimbursement process too.[49]
Suggested clinical data elements in the MS data set can be divided into five categories as follows:
Patient and family history
History of MS events
-
Assessments and examinations
- Vital signs
- Clinical assessments
- Activities of daily living/performance
- Neuropsychological impairment
- Performance measures
-
Laboratory tests and diagnostic procedures
- Laboratory tests and bio specimens/biomarkers
- Imaging diagnostics
- Non-Imaging diagnostics
-
Treatment and intervention data
- Disease modifying therapy
- Relapse therapy
- Symptomatic MS treatment
- Non-MS related treatments.
Every named category and subcategory includes MS clinical data elements. These data elements are as follows:

CONCLUSION
In this article, by studying iMed© and EDMUS software's data elements, a MS data set is proposed. The data set is based of instituting information systems such as registries, databases and structured medical records. Recognizing and determining essential data elements make it easy to compare and share data among different information systems. Administrative, clinical and socio-economic data elements play a vital role in standardizing collecting data. Organized collecting and integrating data, will improve disease management, facilitate comparing and sharing information and improve the research process.
MS data set leads to collecting comprehensive data and accounting information needs at international and national levels. Updating data set is one of the important responsibilities in the health system. Editing and expanding iMed© and EDMUS and other databases is possible. According to updating data sets, they can be edited.
Patients referring to MS clinics are three groups:
The first visit
The second referral based on routine follow-up
Referral due to MS relapses.
At first, it may seem time-consuming to collect all of the data elements, but collecting all of the data elements doesn’t happen for all patients. For example, administrative and socioeconomic data are usually completed at the first visit and if it's necessary, they will be updated during routine follow-up referral. It has to be considered that administrative and socioeconomic data elements are essential in analyzing clinical data.
iMed©, EDMUS and other available databases are suitable patterns for determining and recognizing MS key data elements. Using these databases globally has provided a worthwhile experience to determine the importance of collecting different data on MS in information systems.
Developing data elements named in this article and studying other available MS information systems result in instituting the MS standard data set. By instituting this data set, providing the MS MDS internationally is also possible. MS MDS is the main base of instituting MS information systems at different levels.
ACKNOWLEDGMENT
The authors would like to thank Dr. Bernard Frangoulis, Computer Scientist at the EDMUS Coordinating Center for the valuable guidance about EDMUS data elements. We thank especially Dr. Amer Amous, Medical Manager at the Merck Serono (Middle and Near East) for his helpful comments about iMed© data elements.
This article extracted from a part of the Medical Record Education thesis in faculty of Medical Management and Information Sciences of Isfahan University of Medical Sciences with this title: Establishing Multiple Sclerosis MDS in Iran.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Young JJ. Tehran: Andisheye Rafi; 2004. Handbook for Brunner and Suddarth's Textbook of Medical-Surgical Nursing. [Google Scholar]
- 2.Etemadifar M, Janghorbani M, Shaygannejad V, Ashtari F. Prevalence of multiple sclerosis in Isfahan, Iran. Neuroepidemiology. 2006;27:39–44. doi: 10.1159/000094235. [DOI] [PubMed] [Google Scholar]
- 3.About MS. Multiple sclerosis international federation site. [Last Accessed on Aug 23, 2011]. Available from: http://www.msif.org/en/about_ms/index.html .
- 4.Etemadifar M, Maghzi AH. Sharp increase in the incidence and prevalence of multiple sclerosis in Isfahan, Iran. Mult Scler. 2011;17:1022–7. doi: 10.1177/1352458511401460. [DOI] [PubMed] [Google Scholar]
- 5.Etemadifar M, Abtahi SH. Int J Prev Med. 5. Vol. 3. Iran: Past, present and future; 2012. Multiple sclerosis in Isfahan; pp. 301–2. [PMC free article] [PubMed] [Google Scholar]
- 6.Saadatnia M, Etemadifar M, Maghzi AH. Multiple sclerosis in Isfahan, Iran. Int Rev Neurobiol. 2007;79:357–75. doi: 10.1016/S0074-7742(07)79016-5. ISSN: 0074-7742, ISBN: 978012373766. [DOI] [PubMed] [Google Scholar]
- 7.Flachenecker P, Khil L, Bergmann S, Kowalewski M, Pascu I, Pérez-Miralles F, et al. Development and pilot phase of a European MS register. J Neurol. 2010;257:1620–7. doi: 10.1007/s00415-010-5578-4. [DOI] [PubMed] [Google Scholar]
- 8.Jahanbakhsh M. Tehran: Shahid Beheshti University of Medical Sciences and Health Services; 2005. A Comparative Study for Hospital-based Diabetes Registry in the Selected Countries and Designing a Model for Iran [Thesis in M.Sc.] [Google Scholar]
- 9.Ajami S. A Comparative study on Earthquake Information Management Systems (EIMS) in India, Afghanistan and Iran. Journal of Education and Health Promotion. 2012;1:27–34. doi: 10.4103/2277-9531.99963. DOI: 10.4103/2277-9531.99963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Confavreux C, Paty DW. Current status of computerization of multiple sclerosis clinical data for research in Europe and North America: The EDMUS/MS-COSTAR connection. European Database for Multiple Sclerosis. Multiple Sclerosis-Computed Stored Ambulatory Record. Neurology. 1995;45(3 Pt 1):573–6. doi: 10.1212/wnl.45.3.573. [DOI] [PubMed] [Google Scholar]
- 11.Ajami S, Ketabi S. Performance evaluation of medical records departments by analytical hierarchy process (AHP) approach in the selected hospitals in Isfahan: Medical records dep. and AHP. J Med Syst. 2012;36:1165–71. doi: 10.1007/s10916-010-9578-9. [DOI] [PubMed] [Google Scholar]
- 12.Ajami S, Ketabi S, Isfahani SS, Heidari A. Readiness assessment of electronic health records implementation. Acta Inform Med. 2011;19:224–7. doi: 10.5455/aim.2011.19.224-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajab AA. University of South Florida in USA; 2005. [Last Accessed on 2013 Jul 10]. A methodology for developing a nursing education minimum dataset [Thesis] Available from: http://scholarcommons.usf.edu/cgi/viewcontent.cgi?article=1825&context=etd . [Google Scholar]
- 14.Ajami S, Bagheri-Tadi T. Barriers for adopting electronic health records (EHRs) by physicians. Acta Inform Med. 2013;21:129–34. doi: 10.5455/aim.2013.21.129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karimi S, Saghaeian-Nejad-Isfahani S, Farzandipour M, Esmaeli-Ghayoumabadi M. Comparative study of minimum data sets of health information management of organ transplantation in selected countries and presenting appropriate solution for Iran. Health Information Management. 2011;7(S):497–505. [Google Scholar]
- 16.Keyvanara M, Sadeghi M, Saghaeiannejad-Isfahani S, Tadayyon H. A comparative review of national registry systems of acute coronary syndrome in selective countries. Health Inf Manage. 2012;9:172–9. [Google Scholar]
- 17.Carter J, Evans J, Tuttle M, Weida T, White T, Harvell J, et al. Washington, D.C: ASPE/DALTCP and Aplelon, Inc; 2006. Making the Minimum Data Set Compliant with Health Information Technology Standards. [Google Scholar]
- 18.Fard-Azar FE, Tofighi S, Bashardost N, Ajami S. A comparative survey on mortality information management systems in England, United States of America and New Zealand and proposing a suitable MIMS model for Iran. J Qazvin Univ Med Sci. 2004;8:81–8. [Google Scholar]
- 19.Tadayon H. Isfahan: Isfahan University of Medical Sciences and Health Services; 2010. Comparative Study of National Registry of Acute Coronary Syndrome in Selected Countries and Presenting Appropriate Guidelines for Iran. [Google Scholar]
- 20.Trojano M. Can data basing optimize patient care? J Neurol. 2004;251(S5):79–82. doi: 10.1007/s00415-004-1513-x. [DOI] [PubMed] [Google Scholar]
- 21.Devonshire V. Clinical databases in MS: Patient management and research. Int MS J. 2001;8:57–66. [Google Scholar]
- 22.Confavreux C, Compston DA, Hommes OR, McDonald WI, Thompson AJ. EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry. 1992;55:671–6. doi: 10.1136/jnnp.55.8.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butzkueven H, Chapman J, Cristiano E, Grand’Maison F, Hoffmann M, Izquierdo G, et al. MSBase: An international, online registry and platform for collaborative outcomes research in multiple sclerosis. Mult Scler. 2006;12:769–74. doi: 10.1177/1352458506070775. [DOI] [PubMed] [Google Scholar]
- 24.What is MSBase. MSBase registry website. [Last cited on 2013 Jul 23]. Available from: https://www.msbase.org .
- 25.Flachenecker P, Stuke K. National MS registries. J Neurol. 2008;255(S6):102–8. doi: 10.1007/s00415-008-6019-5. [DOI] [PubMed] [Google Scholar]
- 26.Trojano M, Paolicelli D, Lepore V, Fuiani A, Di Monte E, Pellegrini F, et al. Italian Multiple Sclerosis Database Network. Neurol Sci. 2006;27(S5):358–61. doi: 10.1007/s10072-006-0694-8. [DOI] [PubMed] [Google Scholar]
- 27.Merck Serono, Geneva: [Last Accessed on July 24, 2013]. About iMed. iMed Site. Available from: http://www.imed.org/en/index.html . [Google Scholar]
- 28.Merck Serono SA. User Manual. Version 6. Geneva, Switzerland: Merck Serono Company; 2012. iMed: Electronic Multiple Sclerosis Patient Clinical Database. [Google Scholar]
- 29.The EDMUS Project. [Last Accessed on Aug 12, 2013]. Available from: http://www.edmus.org/en/proj/index.html .
- 30.The MSBase Foundation Ltd. MSBase: Free International Online Registry for MS Researchers (MSBase Brochure) [Last Accessed on Aug 12, 2013]. Available from: https://www.msbase.org/msbase/cms: contentasbinary/documents/MSBase_Brochure.pdf .
- 31.Hurwitz BJ. Registry studies of long-term multiple sclerosis outcomes: Description of key registries. Neurology. 2011;76(S3):6. doi: 10.1212/WNL.0b013e3182050225. [DOI] [PubMed] [Google Scholar]
- 32.Free Trial Version of EDMUS 5.0 Software. [Last Accessed on 2013 Sep 23]. Available from: http://www.edmus.org/en/soft/edmus_get.html .
- 33.iMed Version 5.4.5 Software. Iranian Merk Serono Representative. Tehran. 2012. [Last Accessed on 2013 Aug 12]. Available from: http://www.merckserono.com/en/index.html .
- 34.NINDS CDE Team Project: Multiple sclerosis. NINDS common data elements site. [Last Accessed on July 10, 2013]. Available from: http://www.commondataelements.ninds.nih.gov/MS.aspx#tab=Data_Standards .
- 35.Unertl KM, Weinger MB, Johnson KB. Applying direct observation to model workflow and assess adoption. AMIA Annu Symp Proc 2006. 2006:794–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Palace J, Boggild M. The UK multiple sclerosis database. Mult Scler. 1999;5:297–8. doi: 10.1177/135245859900500419. [DOI] [PubMed] [Google Scholar]
- 37.Callaly T, Faulkner P, Hollis G, McIlroy D, Hantz P. The development of a mental health service patient information management system. Aust Health Rev. 1998;21:182–93. doi: 10.1071/ah980182. [DOI] [PubMed] [Google Scholar]
- 38.Treviño FM. Uniform minimum data sets: In search of demographic comparability. Am J Public Health. 1988;78:126–7. doi: 10.2105/ajph.78.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Safdari R, Akbari M, Tofighi S, Moeinolghorabaii M, Karami G. Comparative study of clinical information systems of mental illness caused by the war in America, England and Australia and Offer Appropriate Solutions for Iran. Res J Med Veteran. 2009;3:44–9. [Google Scholar]
- 40.Mehrdad R. Health system in Iran. JMAJ. 2009;52:69–73. [Google Scholar]
- 41.Weinshenker BG. Databases in MS research: Pitfalls and promises. Mult Scler. 1999;5:206–11. doi: 10.1177/135245859900500402. [DOI] [PubMed] [Google Scholar]
- 42.Mittman R. Using Clinical Information Technology in Chronic Disease Care: Exper Workshop Summary: California HealthCare Foundation. 2004 [Google Scholar]
- 43.National Institute of Biomedical Imaging and Bioengineering/National Heart, Lung, and Blood Institute/National Science Foundation Workshop Faculty, Price CP, Kricka LJ. Improving healthcare accessibility through point-of-care technologies. Clin Chem. 2007;53:1665–75. doi: 10.1373/clinchem.2006.084707. [DOI] [PubMed] [Google Scholar]
- 44.Sahay S, Monteiro E, Aanestad M, editors. The 9th International Conference on Social Implications of Computers in Developing Countries. São Paulo, Brazil: 2007. Towards a Political Perspective of Integrative Research: The Case of Health Information Systems in India. [Google Scholar]
- 45.Laing K. Use of the SGNA minimum data set in the clinical area. Gastroenterol Nurs. 2005;28:59–60. doi: 10.1097/00001610-200501000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Buchanan RJ, Wang S, Huang C, Graber D. Profiles of nursing home residents with multiple sclerosis using the minimum data set. Mult Scler. 2001;7:189–200. doi: 10.1177/135245850100700310. [DOI] [PubMed] [Google Scholar]
- 47.Torabi M, Safdari R, Shahmoradi L. Tehran: Jafari; 2010. Health Information Technology Management. [Google Scholar]
- 48.Davis N, Lacour M. 1st ed. USA: W.B. Saunders Company; 2002. Introduction to Health Information Technology. [Google Scholar]
- 49.Hosseini A, Moghaddasi H, Jahanbakhsh M. Designing minimum data sets of diabetes mellitus: Basis of effectiveness indicators of diabetes management. Health Information Management. 2010;7:330–40. [Google Scholar]
- 50.Bandari DS, Vollmer TL, Khatri BO, Tyry T. Assessing quality of life in patients with multiple sclerosis. Int J MS Care. 2010;12:34–41. [Google Scholar]
- 51.Johns M. Chicago: American Health Information Management (AHIMA); 2002. Health Information Management Technology: An Applied Approach. [Google Scholar]


