Abstract
BACKGROUND
Susceptibility weighted imaging is a relatively new MRI sequence that can identify lesions of multiple sclerosis in adults. This study was designed to determine if susceptibility weighted imaging is a useful discriminator between children who develop multiple sclerosis and children with monophasic ADEM.
METHODS
Eighteen children who presented with acute central nervous system demyelination and who had a brain magnetic resonance imaging study including susceptibility weighted imaging within six months of the first clinical attack were studied. Final diagnosis was based on international consensus definitions. Brain lesions detected on the fluid attenuated inversion recovery sequence were assessed for abnormal signal on susceptibility weighted imaging. The burden of susceptibility abnormalities was then analyzed for differences between the multiple sclerosis and ADEM groups.
RESULTS
Eight patients had a final diagnosis of ADEM and ten had multiple sclerosis. Twenty-two percent of fluid attenuated inversion recovery lesions were identified on susceptibility weighted imaging. The percentage of fluid attenuated inversion recovery lesions identified on susceptibility weighted imaging differed between the multiple sclerosis and ADEM groups (p=.04). The median percentage (min, max) of lesions identified on susceptibility weighted imaging in the multiple sclerosis group was 0.22 (0, 0.68) and in the ADEM group was 0.0 (0, 0.17).
CONCLUSION
Susceptibility weighted imaging may be useful as a supporting tool in differentiating ADEM from multiple sclerosis at initial presentation.
Keywords: Multiple sclerosis, acute disseminated encephalomyelitis, ADEM, susceptibility weighted imaging, pediatric demyelination
INTRODUCTION
Pediatric demyelinating diseases are rare, with an overall incidence of 1.66 per 100,000 person-years 1. A single acute central nervous system inflammatory demyelinating episode may represent acute disseminated encephalomyelitis (ADEM), clinically isolated syndrome, or neuromyelitis optica depending on the clinical presentation. Patients may be diagnosed later with multiple sclerosis if they have relapses and fulfill International Pediatric Multiple Sclerosis Study Group criteria (IPMSSG) 2. The risk of conversion to multiple sclerosis from ADEM is low (0–17%), but from clinically isolated syndrome is high (46–72%) 3–5. While multiple sclerosis and ADEM each have a classic clinical presentation, there is substantial overlap. No clinical features, magnetic resonance imaging (MRI), cerebrospinal fluid or serum biomarkers can absolutely distinguish ADEM from multiple sclerosis. However, it is desirable to predict the conversion to multiple sclerosis at the time of the first demyelinating episode in order to assess the risk of relapse and disability, initiate the appropriate immunomodulatory therapy, and potentially spare anxiety.
Much work has been done examining the MRI features of multiple sclerosis and ADEM using standard clinical sequences. MRI criteria have been proposed to diagnose multiple sclerosis in the pediatric population and also to separate children with a first episode of demyelination into those with ADEM and those who have likely experienced a first episode of multiple sclerosis. These criteria are based on T2/fluid attenuated inversion recovery (FLAIR) and T1 characteristics, and they include the total number of T2/FLAIR hyperintense white matter lesions, the location of these lesions (i.e. periventricular, brainstem), whether there is a diffuse and bilateral distribution of the lesions, orientation of the lesions (i.e. perpendicular to the long axis of the corpus callosum), whether the lesions are well-defined, and whether the lesions are hypointense on T1-weighted imaging (“black holes”). MRI parameters used by a Canadian cohort study and the Callen pediatric multiple sclerosis-ADEM criteria have demonstrated the best predictability of multiple sclerosis, with sensitivity of 81–84% and specificity of 93–95% 6,7.
SWI is a relatively new, gradient-echo sequence that provides information about local tissue susceptibility 8. Recently, researchers have investigated the role of SWI as it relates to multiple sclerosis in the adult population. SWI has been shown to identify the demyelinating lesions of multiple sclerosis as having hypointense signal in a number of different patterns, and it has been proposed that the SWI images provide a measure of iron deposition within the lesions 8,9. The goal of this study was to investigate whether SWI can help discriminate children with a first episode of multiple sclerosis from those with ADEM.
METHODS
Patients
Approval for the study was obtained from the Institutional Review Board. The subjects in this study were identified from a dataset of 109 patients prospectively enrolled in an ongoing examination of MRI findings in pediatric demyelinating disease. Entry criteria included a final diagnosis of ADEM or multiple sclerosis, an MRI including SWI obtained within 6 months of diagnosis, and clinical follow-up of 12 months or greater. Detailed serial histories and neurological exams were performed by a pediatric neurologist (SM). The final diagnosis was based on the IPMSSG criteria 2 upon review of the complete medical records. Eighteen total patients met these criteria, including 8 patients with ADEM and 10 patients with multiple sclerosis. The mean length of follow-up for the children in this study was 27.6 months (range 17 – 40 months) for ADEM and 31.0 (range 10 – 53 months) for multiple sclerosis.
MRI protocol and analysis
Patients underwent brain MRI on a 3T Siemens TIM Trio during the acute period (within six months of clinical episode). This consisted of a standard clinical protocol, including T1 weighted imaging pre and post gadolinium contrast, FLAIR, T2, diffusion and SWI. For each MRI, the FLAIR and T1 post-contrast sequences were assessed for the presence, size, location, and enhancement of FLAIR hyperintense lesions. Using a semi-automated technique with standard clinical viewing software, FLAIR images were co-registered with the SWI images and each lesion was assessed for its SWI characteristics.
Two separate criteria (the “Callen” criteria and the “Verhey” criteria) for separating ADEM patients from multiple sclerosis patients using conventional MR were applied to the patients in this study 6,7. In the Callen study, the following criteria were used to distinguish multiple sclerosis from ADEM: any two of 1) absence of a diffuse bilateral lesion pattern, 2) presence of black holes, and 3) presence of two or more periventricular lesions. In the Verhey study, the criteria for multiple sclerosis included either one or more T1-weighted hypointense lesions or one or more periventricular lesions. The Callen and Verhey criteria were applied separately by a board-certified neuroradiologist (JK) and another qualified radiologist (DR). Discrepancies in the application of these criteria were settled by a senior faculty board-certified neuroradiologist (TB).
Data analysis
An exact nonparametric Wilcoxon-rank test was used to assess the effect of the predictor variables (age, months of follow-up, and percentage of lesions visible on SWI) on the final diagnosis. A Fisher’s exact test was used to evaluate the effect of the categorical predictors (the Callen and Verhey criteria) on the final diagnosis.
A logistic regression model was built using the continuous and categorical predictors, with diagnosis (ADEM or multiple sclerosis) as the dependent variable. A univariate analysis was performed using all of the possible predictors. A multivariate model was then built based on the univariate analysis. Since the sample size was small, and since there was no overlap in age between the two groups, the analysis was performed using the “Firth” option in the logistic regression. We also built a model without age as a predictor, using the “exact” option in the logistic regression. For each model, the estimate of coefficient, standard error, and the p-values were calculated for each predictor in the model.
An optimal cut point for percentage of lesions visible on SWI was sought to discriminate ADEM from multiple sclerosis patients. This was done by assigning each possible cut point two scores: one score was derived from an odds ratio estimate using logistic regression, and another score derived from the Fisher exact test p value. The two scores were added and the combined score was ranked to obtain the optimal cut point.
To evaluate the agreement between raters (JK and DR) for the Callen and Verhey criteria, we calculated Cohen’s kappa coefficient, a statistical measure of inter-rater agreement for categorical items.
RESULTS
The results are displayed in table 1 and figures 1 – 3. Ten patients had a final diagnosis of multiple sclerosis and eight patients had a final diagnosis of ADEM. Mean age at initial presentation of the multiple sclerosis cohort was 15.4 (range 13–18) years and of the ADEM cohort was 5.6 (range 0–10) years. Mean follow-up in the multiple sclerosis group was 31 (range 10–53) months and in the ADEM group was 27.6 (range 17–40) months.
Table 1. Individual subject characteristics and results.
Individual patient data for age, time between presentation and MRI, follow-up length, lesion load, and total number and fraction of lesions visible on SWI sequences.
| Patient | Final Diagnosis | Age at first clinical presentation | MRI time from presentation | Months follow-up | Total lesions | SWI (+) lesions | Fraction SWI (+) |
|---|---|---|---|---|---|---|---|
| 1 | ADEM | 10 | 1 day | 40 | 6 | 1 | 0.17 |
| 2 | ADEM | 9 | 1 day | 32 | 4 | 0 | 0 |
| 3 | ADEM | 6 | 5 days | 25 | 32 | 0 | 0 |
| 4 | ADEM | 5 | 0 days | 20 | 19 | 2 | 0.11 |
| 5 | ADEM | 4 | 0 days | 40 | 62 | 10 | 0.16 |
| 6 | ADEM | 0 | 1 day | 30 | 4 | 0 | 0 |
| 7 | ADEM | 8 | 1 day | 17 | 34 | 0 | 0 |
| 8 | ADEM | 3 | 37 days | 17 | 10 | 0 | 0 |
| 9 | MS | 13 | 0 days | 29 | 24 | 6 | 0.26 |
| 10 | MS | 15 | 6 months | 41 | 90 | 22 | 0.24 |
| 11 | MS | 15 | 1 month | 34 | 5 | 1 | 0.2 |
| 12 | MS | 16 | 0 days | 30 | 15 | 6 | 0.4 |
| 13 | MS | 15 | 6 months | 28 | 9 | 0 | 0 |
| 14 | MS | 15 | 5 days | 53 | 20 | 0 | 0 |
| 15 | MS | 17 | 1 day | 46 | 100 | 68 | 0.68 |
| 16 | MS | 15 | 20 days | 26 | 160 | 16 | 0.1 |
| 17 | MS | 18 | 0 days | 13 | 25 | 8 | 0.32 |
| 18 | MS | 15 | 0 days | 10 | 26 | 2 | 0.08 |
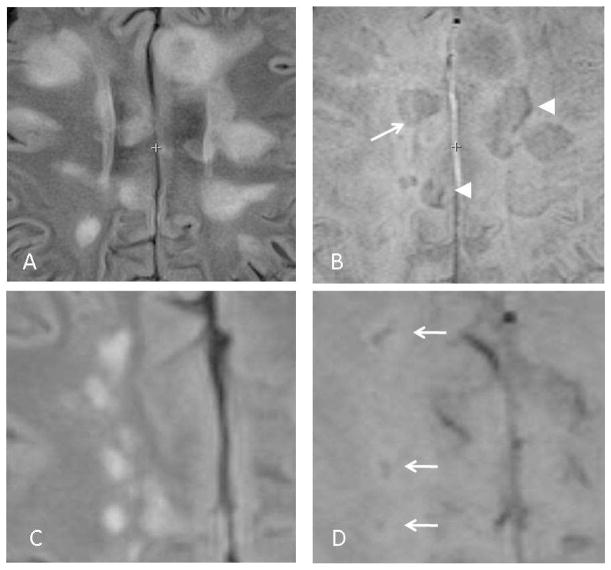
Fig 1.
SWI appearances of demyelinating lesions. Axial FLAIR (A) and SWI (B) images from a patient with multiple sclerosis. Some lesions appear as a vague hypointensity (arrow), while others are seen as a vague lesion accompanied by a a peripheral, linear hypointensity (arrowheads). Axial FLAIR (C) and SWI (D) images from a different multiple sclerosis patient show SWI lesions with a central hypointensity (arrows).
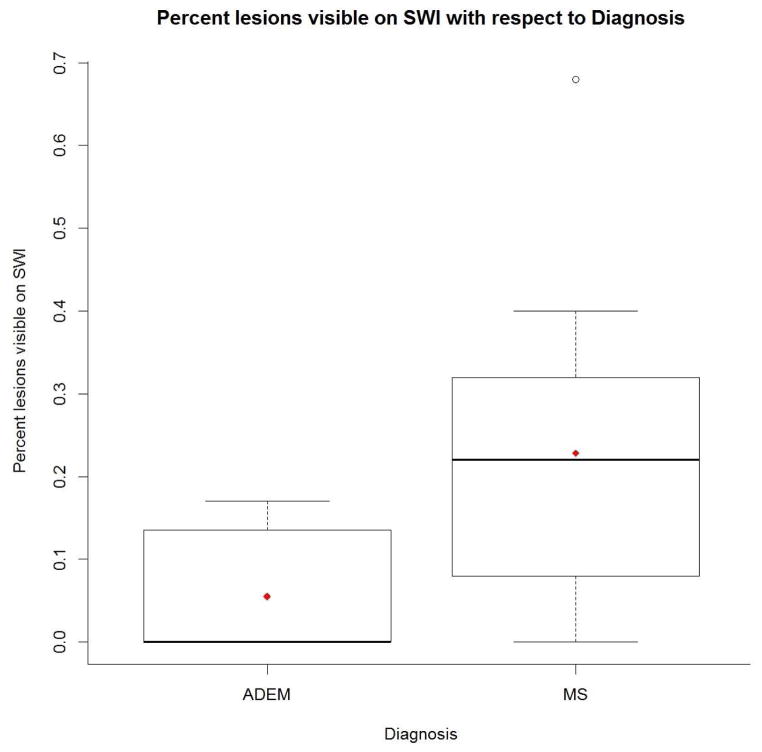
Fig 3.
Boxplot showing differences in percentage of FLAIR lesions visible on SWI between ADEM and multiple sclerosis patients. In each group, the top bar represents the maximum value, the top edge of the box the 75th percentile, the diamond the mean, and the bolded line the median. In the ADEM group, the median, minimum, and 25th percentile are all zero. In the multiple sclerosis group, the bottom edge of the box is the 25th percentile, the bottom bar is the minimum, and the circle is an outlier.
Patients who were ultimately diagnosed with multiple sclerosis had, on average, a larger number of FLAIR hyperintense lesions (mean 47.4, range 5 – 160) compared with the ADEM group (mean 21.4, range 4 – 62), but this was not statistically significant (p=.07). In the multiple sclerosis group, there was a significantly higher percentage of FLAIR lesions which also demonstrated SWI abnormalities. The median percentage (min, max) of lesions identified on SWI for those with a multiple sclerosis diagnosis was 0.23 (0, 0.68) and for those with an ADEM diagnosis was 0 (0, 0.17) (p=.04).
Both the Callen and Verhey criteria were able to identify those patients with multiple sclerosis better than chance according to the Fisher exact test (p=.04 for the Callen criteria and p=.002 for the Verhey criteria). When the Callen criteria were applied to the subjects in this study, they yielded a sensitivity of 90% and specificity of 62.5% for the diagnosis of multiple sclerosis. When the Verhey criteria were used, the sensitivity was 100% and the specificity improved to 75%. For the Callen criteria, the Cohen kappa coefficient was 0.39, and for the Verhey criteria, it was 0.48.
In a model that used all of the predictors, age, percentage of lesions visible on SWI, and the Verhey criteria were all strongly correlated (Kendal’s tau with p < 0.01). The best model included age only. Indeed, all models that included age were superior to those which did not (the next best model included age, percentage of lesions visible on SWI, and the Verhey criteria). This is because, in our sample, age perfectly predicted diagnosis (children 13 years or older had multiple sclerosis while those 10 years or younger had ADEM).
In models that excluded age as a predictor, the Callen and Verhey criteria were highly correlated, but the Verhey criteria performed slightly better. Models using only the Verhey criteria performed better than models using only the percentage of lesions visible on SWI. Models that employed both of these predictors demonstrated intermediate performance.
An optimal cut point of 0.2 was identified to separate the two groups based on the SWI findings (those with a percentage of lesions visible on SWI less than 0.2 were likely to have ADEM, and more than 0.2 were likely to have multiple sclerosis).
FLAIR hyperintense lesions which were visible on SWI had one of three typical appearances (Figures 1 and 2). Some lesions were seen as vague, amorphous, hypointensities on SWI. Others demonstrated a discrete, linear, hypointense signal. Finally, some lesions demonstrated both findings simultaneously.
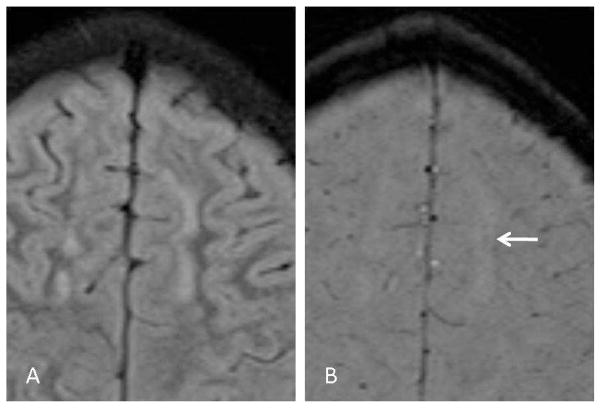
Fig 2.
SWI appearances of demyelinating lesions. Axial FLAIR (A) and SWI (B) images from a patient with ADEM. The large lesion in the left frontal lobe white matter demonstrates a vague, central hypointensity on the SWI image (arrow). Many of the SWI findings in ADEM patients were more subtle than in multiple sclerosis patients.
DISCUSSION
Many MRI criteria have been proposed to diagnose multiple sclerosis in the pediatric population and also to separate children with a first episode of demyelination into those with ADEM and those who have likely experienced a first episode of multiple sclerosis 2,6,7,10. These criteria are based on conventional sequences, specifically T2/FLAIR and T1 sequences. Some of these criteria have high published sensitivity (81–84%) and specificity (93–95%) 6,7. When applied to the patients in our study, sensitivity of these criteria was high (90–100%) but specificity was lower (62.5–75%). However, comparison of sensitivity and specificity between this study and previous studies is limited by poor inter-rater reliability in the current study.
Two radiologists independently applied both the Verhey and Callen criteria to each subject. Some of the disagreement between the two scorers resulted from slightly different interpretations of the criteria. For example, we discovered that the scorers had different thresholds for the degree of T1-hypointensity a lesion must display before it qualifies as a “black hole.” There was also some disagreement in whether certain subjects displayed a diffuse, bilateral pattern of disease. These disputes were settled by consensus, with input from a senior faculty neuroradiologist. This poor inter-rater reliability is a limitation of the study, but it also points to potential difficulties when applying these criteria in the clinical setting. Despite the poor inter-rater reliability, the criteria performed well at predicting disease category based on a subject’s MRI. In fact, they performed better than the percentage of lesions visible on SWI.
The findings in this study are encouraging because they show that incorporating findings on SWI may help separate multiple sclerosis from ADEM patients. There is a significant difference in the prevalence of SWI findings between the pediatric multiple sclerosis and ADEM populations. Those who were ultimately diagnosed with multiple sclerosis had a significantly higher percentage of lesions which were visible on SWI (p = 0.04). The cut-off point of 0.2 (fraction of lesions identified on SWI) provided the best separation between groups. No patient in the ADEM group had a value greater than 0.2. This may provide a quick and useful criterion to apply in difficult cases. In the research setting, combining SWI data with findings on conventional sequences may improve diagnostic accuracy even more. Other advanced sequences may also prove to be useful; work is currently underway using other advanced techniques such as diffusion tensor imaging to improve diagnostic accuracy 11.
The pathophysiology leading to the abnormal findings on SWI in demyelinating lesions remains unclear. SWI sequences are heavily T2*-weighted sequences which are sensitive to paramagnetic substances, such as iron and venous blood 12. In rat models, it has been proposed that abnormal iron accumulation is involved in the pathogenesis of demyelinating lesions 13. Work in the adult multiple sclerosis literature has suggested that iron deposition in multiple sclerosis lesions is accountable for their detection on SWI and can even serve as a biomarker for different lesion characteristics 8. In our study, ADEM patients had fewer abnormalities on SWI, suggesting that perhaps there is less iron deposition in the demyelinating lesions of ADEM.
It is interesting that lesions demonstrated variable appearance on SWI. Some lesions were seen as a vague hypointensity, others demonstrated a linear hypointensity, and others had a combination of both findings (Figure 1). One hypothesis is that the linear hypointensities reflect iron deposition within dilated venous channels, since it is well known that perivascular inflammation is a hallmark of multiple sclerosis pathology 14.
The study is limited by small sample size, but provides an impetus to conduct a larger study to explore the interesting findings. Most patients had their initial MRI close to the time of their initial presentation; two multiple sclerosis patients had their MRIs six months after presentation, which could influence the results (although one of these multiple sclerosis patients had no findings on SWI). Another limitation is the complete separation of the two groups based on age. However, in the clinical setting, caretakers are aware of a patient’s age but still regularly rely on MRI to help arrive at a diagnosis. The study also has a longer follow-up period than most studies on MRI in pediatric demyelinating disease. This provides more confidence in the final groupings of the patients. For example, one patient had been diagnosed with recurrent ADEM for more than three years until ultimately diagnosed with multiple sclerosis, since her relapses initially had encephalopathy. Interestingly, she was the patient with the most profound SWI findings. In the future, a patient with similar findings on SWI will raise our suspicion of multiple sclerosis.
CONCLUSION
In the pediatric population, these preliminary findings suggest that SWI findings may increase the ability of MRI to separate children with an initial episode of multiple sclerosis from those with ADEM. Additional studies will be necessary to confirm these results.
Acknowledgments
This work was supported by the National Multiple Sclerosis Society, PP1361 and RG4190A2/1. We wish to thank the generous contributions of our patients and their families for participating in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer-Gould A, Zhang JL, Chung J, Yeung Y, Waubant E, Yao J. Incidence of acquired CNS demyelinating syndromes in a multiethnic cohort of children. Neurology. 2011;77:1143–8. doi: 10.1212/WNL.0b013e31822facdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krupp LB, Banwell B, Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68:S7–12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 3.Dale RC, Brilot F, Banwell B. Pediatric central nervous system inflammatory demyelination: acute disseminated encephalomyelitis, clinically isolated syndromes, neuromyelitis optica, and multiple sclerosis. Current opinion in neurology. 2009;22:233–40. doi: 10.1097/wco.0b013e32832b4c47. [DOI] [PubMed] [Google Scholar]
- 4.Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain: a journal of neurology. 2000;123(Pt 12):2407–22. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- 5.Tenembaum S, Chitnis T, Ness J, Hahn JS. Acute disseminated encephalomyelitis. Neurology. 2007;68:S23–36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 6.Callen DJ, Shroff MM, Branson HM, et al. Role of MRI in the differentiation of ADEM from MS in children. Neurology. 2009;72:968–73. doi: 10.1212/01.wnl.0000338630.20412.45. [DOI] [PubMed] [Google Scholar]
- 7.Verhey LH, Branson HM, Shroff MM, et al. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: a prospective national cohort study. Lancet neurology. 2011;10:1065–73. doi: 10.1016/S1474-4422(11)70250-2. [DOI] [PubMed] [Google Scholar]
- 8.Haacke EM, Makki M, Ge Y, et al. Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J Magn Reson Imaging. 2009;29:537–44. doi: 10.1002/jmri.21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haacke EM, Garbern J, Miao Y, Habib C, Liu M. Iron stores and cerebral veins in MS studied by susceptibility weighted imaging. International angiology: a journal of the International Union of Angiology. 2010;29:149–57. [PubMed] [Google Scholar]
- 10.Ketelslegers IA, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ. A comparison of MRI criteria for diagnosing pediatric ADEM and MS. Neurology. 2010;74:1412–5. doi: 10.1212/WNL.0b013e3181dc138b. [DOI] [PubMed] [Google Scholar]
- 11.Tillema J, Leach J, Pirko I. Non-lesional white matter changes in pediatric multiple sclerosis and monophasic demyelinating disorders. Mult Scler. 2012;18:1754–9. doi: 10.1177/1352458512447527. [DOI] [PubMed] [Google Scholar]
- 12.Lummel N, Boeckh-Behrens T, Schoepf V, Burke M, Bruckmann H, Linn J. Presence of a central vein within white matter lesions on susceptibility weighted imaging: a specific finding for multiple sclerosis? Neuroradiology. doi: 10.1007/s00234-010-0736-z. [DOI] [PubMed] [Google Scholar]
- 13.Izawa T, Yamate J, Franklin RJ, Kuwamura M. Abnormal iron accumulation is involved in the pathogenesis of the demyelinating dmy rat but not in the hypomyelinating mv rat. Brain research. 2010;1349:105–14. doi: 10.1016/j.brainres.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Adams CW. Perivascular iron deposition and other vascular damage in multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry. 1988;51:260–5. doi: 10.1136/jnnp.51.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]