Abstract
Secreted membrane-enclosed vesicles, collectively called extracellular vesicles (EVs), which include exosomes, ectosomes, microvesicles, microparticles, apoptotic bodies and other EV subsets, encompass a very rapidly growing scientific field in biology and medicine. Importantly, it is currently technically challenging to obtain a totally pure EV fraction free from non-vesicular components for functional studies, and therefore there is a need to establish guidelines for analyses of these vesicles and reporting of scientific studies on EV biology. Here, the International Society for Extracellular Vesicles (ISEV) provides researchers with a minimal set of biochemical, biophysical and functional standards that should be used to attribute any specific biological cargo or functions to EVs.
Keywords: extracellular vesicles, microvesicles, microparticles, exosomes, ectosomes, extracellular RNA
Over the past decade, there has been a rapid growth in studies of secreted membrane vesicles, collectively called extracellular vesicles (EVs). Publications in high-impact journals have proposed exciting functional roles of EVs. In particular, the knowledge that EVs can shuttle functional nucleic acids between cells (mRNA, miRNA or other RNA species) has fundamentally changed the thinking about gene regulation, as the EVs can regulate the recipient cell at a post-transcriptional level (1–3).
However, the extracellular milieu is more complex as several body fluids (especially serum/plasma) harbour extracellular RNA (exRNA) in other non-EV carriers, including protein complexes (AGO2) (4) and lipoproteins [HDL and LDL (5)]. Separation of these non-vesicular entities from EV is not fully achieved by common EV isolation protocols, including centrifugation protocols or commercial kits that claim EV or “exosome” isolation/purification. Also, the composition of recovered EVs vary vastly according to the protocols used (6–8). In particular, polymer-based methods to precipitate EVs (used by some commercial kits) do not exclusively isolate EVs, and are likely to co-isolate other molecules, including RNA–protein complexes. Consequently, there is a need to determine the distinct contribution of EVs in any experiment that describes the molecular content or the functional consequences of the isolated material.
We recognize that different experimental systems, sources of biological specimens, investigator's experience and instrumentation used contribute to the heterogeneity of published protocols and the interpretation of results. A framework for providing data and attributing functions to EVs was discussed by the Executive Committee of the International Society for Extracellular Vesicles (ISEV), a group of scientists with collective long-term expertise in the field of EV biology. Here, we propose a series of criteria, based on current best-practice, that represent the minimal characterization of EVs that should be reported by investigators. Adoption of these criteria should aid researchers in planning studies as well as reporting their results. In addition, we suggest appropriate controls that should be included in EV-related functional studies. These controls should support conclusions regarding the functions of EVs and their relationship to physiologic and pathologic mechanisms.
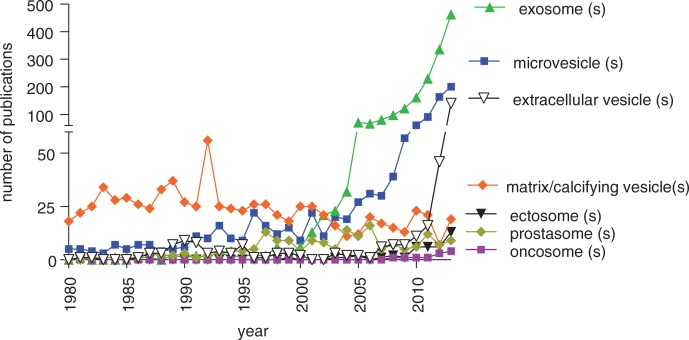
The term “exosomes” is the most commonly used word to designate any type of EV (Fig. 1), and this has become a “buzz term” for EV-related science. The actual meaning of this word, however, is not universally accepted [see letter by Gould and Raposo (9)]. Many publications specify that exosomes are formed in endosomal multivesicular compartments and are secreted when these compartments fuse with the plasma membrane. However, the isolated material generally studied contains a mixture of EVs. Unfortunately, the field of EV research has not matured to the point that we can propose a list of EV-specific “markers” that distinguish subsets of EVs from each other, for example, EVs produced via budding from the cell membrane or produced via endosomal compartments.
Fig. 1.
Comparative evolution of the use of different terms for EVs in the literature. An advanced search was performed in PubMed at the end of December 2013 to find, for each year of publication, all articles using the given term (singular or plural) as text word: exosome(s), microvesicles, oncosome(s), ectosome(s), prostasome(s), matrix/calcifying vesicle(s). Year of final publication (and not advanced online date) of articles in English (and not other languages) was taken into account. Manual elimination of articles describing non-EV-related work was performed for exosome(s) (RNA-excision machinery) and microvesicle(s) (intracellular secretory vesicles). Use of the term microparticle(s) could not be reliably evaluated, since it is massively used to refer to non-vesicle-related particles. Notably, from 2004 onwards, the term “exosome” has become the most often used in published articles describing EVs, whereas the term “extracellular vesicles,” chosen as generic term at creation of ISEV in September 2011, is steadily growing. This figure is not intended to show expansion of the EV field as compared to other fields, since numbers are not normalized to the total number of scientific medico-biological publications per year.
The criteria we provide here can be used by researchers to guide them in discriminating EV from non-EV components. These criteria will be updated with improvements in the “state of the art,” and we hope to eventually be able to provide specific markers and characteristics of EV subtypes. In the meantime, readers can also refer to 2 detailed Position Papers of ISEV published in 2013, listing recommendations on EV isolation (10) and EV/exRNA analysis (11).
Minimal requirements to claim the presence of EVs in isolates
One of the first criteria to define EVs is that they are isolated from extracellular fluids, that is, from conditioned cell culture medium or body fluids. Importantly, collection of the EV-containing fluid must be gentle, limiting cell disruption. Mechanical disruption of cells or tissues can result in isolation of vesicles that originate from the intracellular compartments, which obviously would reduce the purity of EVs. Therefore, the term “EVs” may not be appropriate for materials isolated in such ways.
Since there is currently no consensus on a “gold-standard” method to isolate and/or purify EVs, it cannot be claimed that there is an “optimal” method that should be uniformly used. The reader should be aware that the methods that are most efficient probably depend on (a) the specific scientific question asked and (b) on the downstream applications used. However, we urge researchers to describe in detail the methods used for EV isolation, to allow interpretation and replication by other researchers. Further, we also suggest a format of characteristics of EVs that should be analyzed and then provided in publication.
General characterization of EVs
A general overview of the protein composition of each EV preparation should be provided, at least in a first publication, including description or quantitation of components not necessarily expected to be present on or in EVs (see Table I). Although numerous proteomic analyses have highlighted proteins commonly found in exosome preparations, it is becoming clear that these do not represent “exosome-specific” markers but rather “exosome-enriched” proteins, as different subsets of secreted EVs contain many common markers. However, the relative proportions of different proteins seem to vary in the different types of EVs. Therefore, we suggest that investigators report the amount of several proteins (3 or more) in at least a semi-quantitative manner in any EV preparation, including EV isolates from body fluids or obtained from secreting cells in vitro. The proteins described and characterized should be proteins expected to be present in the EVs of interest, especially transmembrane proteins and cytosolic proteins with membrane-binding capacity (Table I, groups 1 and 2). In addition, the level of presence of proteins not expected to be enriched in EVs of endosomal origin should also be determined (Table I, group 3). This description will cast light on the extent of co-isolation of EVs of different intracellular origins and nature in the isolates (Table I). Furthermore, investigators can compare their protein isolates with those described in other EVs, by searches within databases [EVpedia and Vesiclepedia (12,13)].
Table I.
Different categories of proteins and their expected presence in EV isolates, including some examples (non-exclusive)
| 1. Transmembrane or lipid-bound extracellular proteins | 2. Cytosolic proteins | 3. Intracellular proteins | 4. Extracellular proteins |
|---|---|---|---|
| Argues presence of a membrane in the isolate | With membrane- or receptor-binding capacity | Associated with compartments other than plasma membrane or endosomes | Binding specifically or non-specifically to membranes, co-isolating with EVs |
| Present or enriched in EVs/exosomes | Present or enriched in EVs/exosomes | Absent or under-represented in EVs/exosomes, but present in other types of EVs | Variable association with EVs |
| Examples: Tetraspanins (CD9, CD63, CD81) Integrins (ITG**) or cell adhesion molecules (CAM*) Growth factor receptors Heterotrimeric G proteins (GNA**) Phosphatidylserine-binding MFGE8/lactadherin |
Examples: Endosome or membrane-binding proteins (TSG101, annexins=ANXA*, Rabs=RAB*) Signal transduction or scaffolding proteins (syntenin) |
Examples: Endoplasmic reticulum (Grp94=HSP90B1, calnexin=CANX) Golgi (GM130) Mitochondria (cytochrome C=CYC1) Nucleus (histones=HIST*H*) Argonaute/RISC complex (AGO*) |
Examples: Acetylcholinesterase (ACHE) Serum albumin Extracellular matrix (fibronectin=FN1, collagen=COL*A*) Soluble secreted proteins (cytokines, growth factors, matrix metalloproteinases =MMP*) |
At least one protein of each category 1, 2 and 3 should be quantified in the EV preparations. EV association of proteins of category 4 should be demonstrated by other means.
Italics: official gene names;
denotes different possible family members.
Analytic approaches can include Western blots (WB), (high resolution) flow cytometry (FACS) or global proteomic analysis using mass spectrometry techniques to identify e.g. transmembrane proteins. We recommend that analyses should be performed in a semi-quantitative manner, for example, using intensity analysis of Western blot signals or specific mean fluorescence intensity as compared to isotype control in FACS. When EVs secreted in vitro by cultured cells are analyzed, their composition should ideally be compared with that of the secreting cells, to determine level of enrichment of the EV components. This is not possible for biological fluid-derived EVs, as these are produced by a vast array of cells in the tissues. In that case, we recommend that reports include the relative proportion of different EV-associated proteins.
Table I lists the different categories and examples of proteins whose presence/absence should be simultaneously analyzed. Caution should be taken when using the enzymatic activity of proteins to indirectly determine the concentration of vesicles in any sample. An example of this is acetylcholinesterase (ACHE), a GPI-anchored protein localized in the membrane of reticulocytes, which is present in multiple membrane-anchored and non-membrane-anchored secreted forms also in other cells (14). While the activity of ACHE has been used as a marker of EVs released by reticulocytes, the use of this (or any other proteins in which activity can be measured) requires confirmation of the presence of the protein by Western blotting or functional inhibition by a specific enzyme inhibitor, as well as the recognition that these do not represent specific markers of EVs or exosomes (Table I, group 4). Therefore, their use should be restricted to cases where it is not possible to use other quantitative measures as described above, and the reasons for using them should be clearly justified.
Given the variable quality of commercial and home-made antibodies used for quantitation studies, appropriate negative controls should also be used and their results should be presented. These controls are best provided in the first reports using these antibodies. Ideally, the signal obtained in EVs should be compared to signals obtained from the biological fluid or conditioned medium depleted of EVs (i.e. recovered after the isolation procedure) and/or from complete medium non-conditioned by cells but processed for EV purification as conditioned medium. The reader should be aware that the supernatant, for example, after a 70-minute post-ultracentrifugation, still contains significant quantities of remaining EVs (15). The Methods section of reports should also contain details of the antibodies used (source, catalogue number and dilution) and conditions of preparations of the samples (e.g. reducing/non-reducing conditions for Western blot, an important issue to analyze some tetraspanins).
Characterization of single vesicles
We recommend characterization of single vesicles within a mixture to be performed, to provide an indication of the heterogeneity of the EV preparation studied. As a general rule, at least 2 different technologies should be used to characterize individual EVs. For electron microscopy (Transmission EM) or atomic force microscopy (AFM), images should show a wide field encompassing multiple vesicles in addition to close-up images of single vesicles. For larger vesicles such as apoptotic bodies, cytospins and/or immunofluorescent images may be presented to provide an overview of vesicles isolated, again not focusing on a single vesicle. Size distribution measurements of EVs, such as nanoparticle-tracking analysis, dynamic light scattering, or resistive pulse sensing provide diameters of a large number of vesicles. However, the values acquired with these techniques should be compared with TEM, AFM or other microscopy techniques, since they do not distinguish membrane vesicles from co-isolated non-membranous particles of similar size.
Studies of the functional activity of EVs: recommendations for controls
When in vitro functional studies are performed with isolated EVs, a quantitative analysis of the dose–function relationship should be presented. This dose–response curve should be supplemented by data on the volume of starting fluid and/or the number of producing cells used to isolate the range of functional EVs.
It is important to make use of systematic negative controls which should exhibit minimal functional effects. These may include “mock” EVs obtained from culture medium that has not been conditioned by the cells of interest (but incubated at 37°C as if used in culture) or the fluid remaining after the EV isolation (for body fluids and conditioned medium). These controls provide insights into the “background” functional activity or signal and possibly the proportion of functional “activity” present in the soluble versus EV-associated components of the isolated fluid. Clearly, there is value to negative controls being performed at concentrations of negative EVs approximating those of functional EVs. Foetal calf serum EVs and their protein and RNA cargo can influence measurements (16,17). Thus, there should be efforts to perform studies in the absence of the serum-derived EVs. In this regard, it should be noted that 70 minutes of high speed centrifugation is insufficient to remove EV RNA cargo in foetal calf serum (18).
The ISEV Executive Committee remains concerned about the future reporting of functional changes ascribed to specific single or small clusters of molecules (protein, RNA or other) associated with EVs. This will increasingly be important, as EV biomarkers, EV therapeutics and fundamental mechanisms of EV function are brought to clinical utility or claimed in patent protection drafts. Demonstration of association of these molecules to EVs should therefore be provided for such use. Some proteins (Table I, group 4), but also different RNA species (5), have been variably described as co-isolated with EVs, but may not necessarily be harboured in EVs. For instance, MMP9 has been described as secreted with EVs (19,20) or, conversely, as a soluble non-EV-associated molecules (21).
A direct approach to prove association of these molecules to EVs can be fractionation of the EV preparation using density gradients. Separation of EVs from other particulate material can be guaranteed only by floatation (=upward displacement). However, for some other separations, sedimentation (=downward displacement) may be more appropriate. Such separation should be followed by qPCR or other biochemical detection methods, and the functional moiety and/or biomarker cluster should be co-fractionated with the transmembrane or EV-enriched cytosolic protein used to characterize EVs (Table I). Thus, the functional activity should be resident within defined density gradient fractions specifically containing the EV proteins. Importantly, we are aware that some density gradients often used may alter or impede functional tests performed.
An alternative approach to link functional activity, or specific molecules, with isolated EVs may be based upon antibody-mediated capture or depletion of EVs from the biofluid or conditioned medium. The antibodies used should be specific to the transmembrane protein of the characterized EVs. In these studies, depleted preparations will have lost functional activity, whereas the antibody-captured EVs should retain it (if proper and non-destructive elution from the antibody-coated beads used for capture is technically possible). We realize that EVs with functional activity but without the transmembrane protein also exist, and thus would not be depleted nor captured with this approach.
Another approach would include the use of fluorescent labels of EVs incubated with target cells. Unstained EVs and non-EV dye materials and aggregates must be eliminated with appropriate technology when this method is used. As EVs elicit their function by binding to, fusing with or being uptaken into recipient cells, it could be possible to determine a functional activity in fluorescent cells (EV-associated cells) versus non-fluorescent cells.
In the absence of any of the above proposed controls, investigators may still conclude that an extracellular functional activity exists and affects recipient cells, but the specific EV nature of this function should not be claimed.
Conclusion
The EV field is rapidly expanding and becoming increasingly complex, especially as it overlaps with the even newer field of exRNA-mediated communication. A generic biological standard of EVs, or of “exosomes,” would be very useful as a baseline to compare EV preparations obtained by individual laboratories, and we are aware that European and US networks of researchers are working towards establishing such standards. When available, these standards may provide comparative EV preparative data and also could support inter-laboratory comparisons. However, such standards are not yet available, due to the lack of universal or unequivocally specific markers of EVs, a situation linked to the fact that the content of EVs is probably highly context-dependent. Such tools will therefore only become available with increased knowledge of the core composition and, perhaps, core functions of EVs recovered from diverse sources. Nonetheless, to harmonize research practice in the field of EV research, and to ensure an acceptable level of data comparability, we herein propose that technical and experimental information is provided in significant detail in any published scientific article, and that the characterization includes a minimal set of proofs of the EV relationship to the observations reported. We hope that the minimal requirements presented in this editorial therefore will increase awareness of all researchers for potential confounders in their EV-related results, and thus help editors and reviewers of journals other than J Extracell Vesicles, less specialized in EVs, to better assess and promote advances in the exceptionally promising field of EV research.
Jan Lötvall
Krefting Research Centre
University of Gothenburg, Göteborg, Sweden
Andrew F. Hill
Department of Biochemistry and Molecular Biology
Bio21 Molecular Science and Biotechnology Institute
University of Melbourne, Parkville, Australia
Fred Hochberg
Department of Neurosurgery
University of California at San Diego, San Diego, CA, USA
Edit I. Buzás
Department of Genetics, Cell- and Immunobiology
Semmelweis University, Budapest, Hungary
Dolores Di Vizio
Cedars-Sinai Medical Center, Los Angeles, CA, USA
Christopher Gardiner
Nuffield Department of Obstetrics and Gynaecology
University of Oxford, Oxford, UK
Yong Song Gho
Department of Life Sciences
Pohang University of Science and Technology, Pohang
Republic of Korea
Igor V. Kurochkin
Bioinformatics Institute, Singapore, Singapore
Suresh Mathivanan
Department of Biochemistry
La Trobe University, Melbourne, Australia
Peter Quesenberry
The Warren Alpert Medical School of Brown University
Providence, RI, USA
Susmita Sahoo
Cardiovascular Research Center
Icahn School of Medicine at Mount Sinai, New York, NY, USA
Hidetoshi Tahara
Department of Cellular and Molecular Biology
Hiroshima University Institute of Biomedical & Health Sciences
Hiroshima, Japan
Marca H. Wauben
Department of Biochemistry and Cell Biology
Faculty of Veterinary medicine
Utrecht University, Utrecht, The Netherlands
Kenneth W. Witwer
Department of Molecular and Comparative Pathobiology
Johns Hopkins School of Medicine, Baltimore, MD, USA
Clotilde Théry
INSERM U932, Institut Curie
26 rue d'Ulm, 75005, Paris, France
Correspondence to: clotilde.thery@curie.fr
Acknowledgements
We thank J. Kowal, C. Lässer, C. Neri, W. Stoorvogel and M. Tkach for critical comments on this editorial.
Declaration of interest and/or relationship
JL: Co-owner of patents from 2006 to 2012 (approved and pending) for using exosomes as therapeutics. AFH: Co-owner of patent (pending) for using exosomal miRNA as a biomarker; shareholder in D-Gen Ltd. DDV: Inventor of 2 patents on EVs as circulating biomarkers of cancer (pending). YSG: Inventor of patents for using EVs as therapeutics, diagnostics and vaccines; founder of Aeon Medix and own stock in the company; sponsored research agreements with AmorePacific. IK: Co-inventor of a technology for purification of exosomes licensed to Cell Guidance Systems Ltd that uses it for manufacturing Exo-spin™ kits. SS: Co-inventor of a patent (pending) on the use of exosomes as therapeutics in cardiovascular diseases. Sponsored research agreements with Baxter Healthcare and NeoStem. HT: Founder of MiRTeL, and own stock in the company. MHW: Collaborative research agreement with Friesland Campina on bovine milk EVs and with BD Biosciences on high resolution flow cytometry of EVs, and partnership between Technology Foundation STW, Utrecht University and Danone Nutricia research on EV-based biomarker profiling in human breast milk. KWW: Scientific research agreement with AgriSciX, Inc. Other authors declare no significant relationships or conflicts.
References
- 1.Mittelbrunn M, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–35. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boon RA, Vickers KC. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:186–92. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Grein SG, Nolte-'t Hoen EN. “Small Talk” in the innate immune system via RNA-containing extracellular vesicles. Front Immunol. 2014;5:542. doi: 10.3389/fimmu.2014.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13:3354–64. doi: 10.1002/pmic.201300282. [DOI] [PubMed] [Google Scholar]
- 7.Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24858. 24858, doi: http://dx.doi.org/10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zonneveld MI, Brisson AR, van Herwijnen MJ, Tan S, van de Lest CH, Redegeld FA, et al. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24215. 24215, doi: http://dx.doi.org/10.3402/jev.v3.24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20389. 20389, doi: http://dx.doi.org/10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20360. 20360, doi: http://dx.doi.org/10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill AF, Pegtel DM, Lambertz U, Leonardi T, O'Driscoll L, Pluchino S, et al. ISEV position paper: extracellular vesicle RNA analysis and bioinformatics. J Extracell Vesicles. 2014;2 doi: 10.3402/jev.v2i0.22859. 22859, doi: http://dx.doi.org/10.3402/jev.v2i0.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics. 2014 doi: 10.1093/bioinformatics/btu1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meshorer E, Soreq H. Virtues and woes of AChE alternative splicing in stress-related neuropathologies. Trends Neurosci. 2006;29:216–24. doi: 10.1016/j.tins.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Cvjetkovic A, Lotvall J, Lasser C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.23111. 23111, doi: http://dx.doi.org/10.3402/jev.v3.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 17.Ochieng J, Pratap S, Khatua AK, Sakwe AM. Anchorage-independent growth of breast carcinoma cells is mediated by serum exosomes. Exp Cell Res. 2009;315:1875–88. doi: 10.1016/j.yexcr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shelke GV, Lasser C, Gho YS, Lotvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24783. 24783, doi: http://dx.doi.org/10.3402/jev.v3.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181:1573–84. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginestra A, Monea S, Seghezzi G, Dolo V, Nagase H, Mignatti P, et al. Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. J Biol Chem. 1997;272:17216–22. doi: 10.1074/jbc.272.27.17216. [DOI] [PubMed] [Google Scholar]
- 21.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–30. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]