Abstract
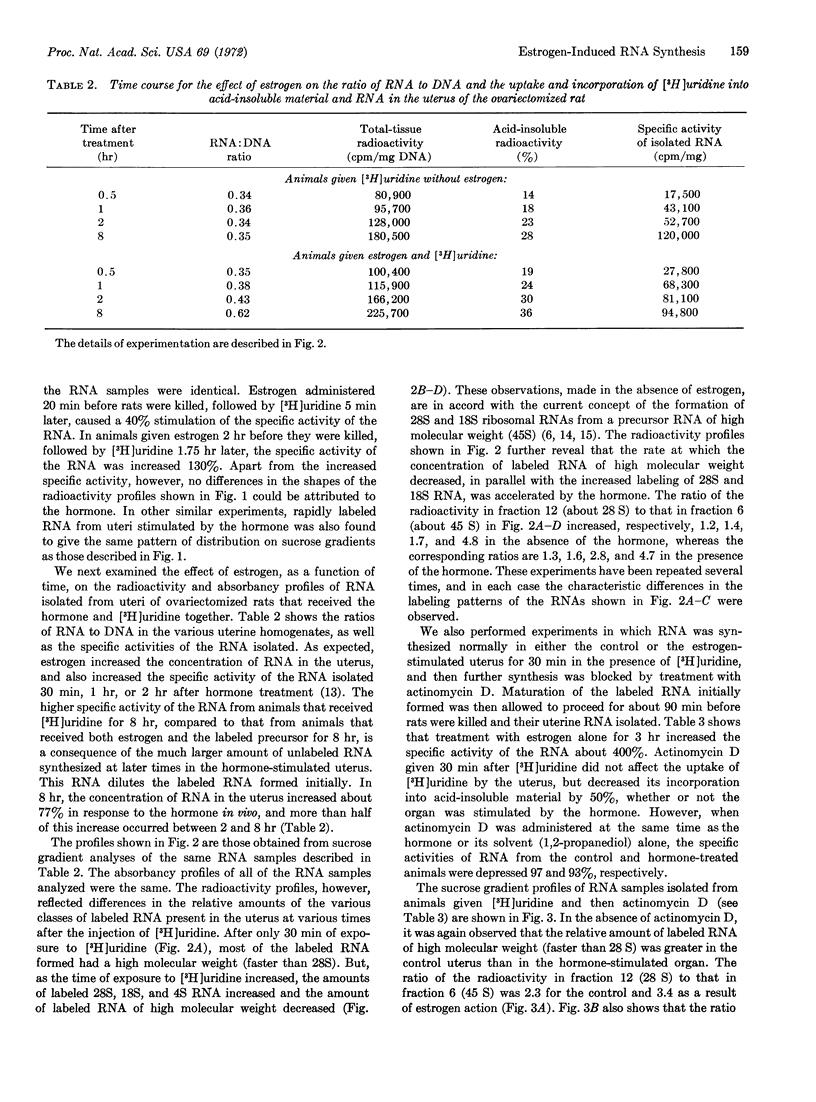
Samples of RNA, isolated from uteri of ovariectomized adult rats treated with estrogen, have been analyzed on sucrose gradients. Treatment with estrogen either for 20 min or 2 hr increased the specific activity of all classes of uterine RNA, but produced no significant alteration in the distribution of radioactivity in the gradients, when animals received [3H]uridine intraperitoneally 15 min before they were killed. After labeling periods of 30 min, 1 hr, or 2 hr, however, the RNAs isolated from animals treated with estrogen had a smaller percentage of rapidly sedimenting (faster than 28S) species of RNA than did RNA from animals not treated with the hormone. The decreased percentage of high molecular weight RNA correlated with increases in both the specific activity of 28S and 18S RNA and the concentration of RNA in the whole organ. The labeled RNA of high molecular weight was also demonstrated, by the use of actinomycin D in vivo, to have a more rapid turnover rate in the estrogen-stimulated uterus. Our results indicate that estrogen increases not only the rate of synthesis of ribosomal RNA in the uterus of the ovariectomized adult rat, but also the rate or efficiency of processing of precursor RNA species of high molecular weight.
Keywords: estradiol-17β, RNA isolation, uterus, ribosomal RNA precursors, actinomycin D
Full text
PDF

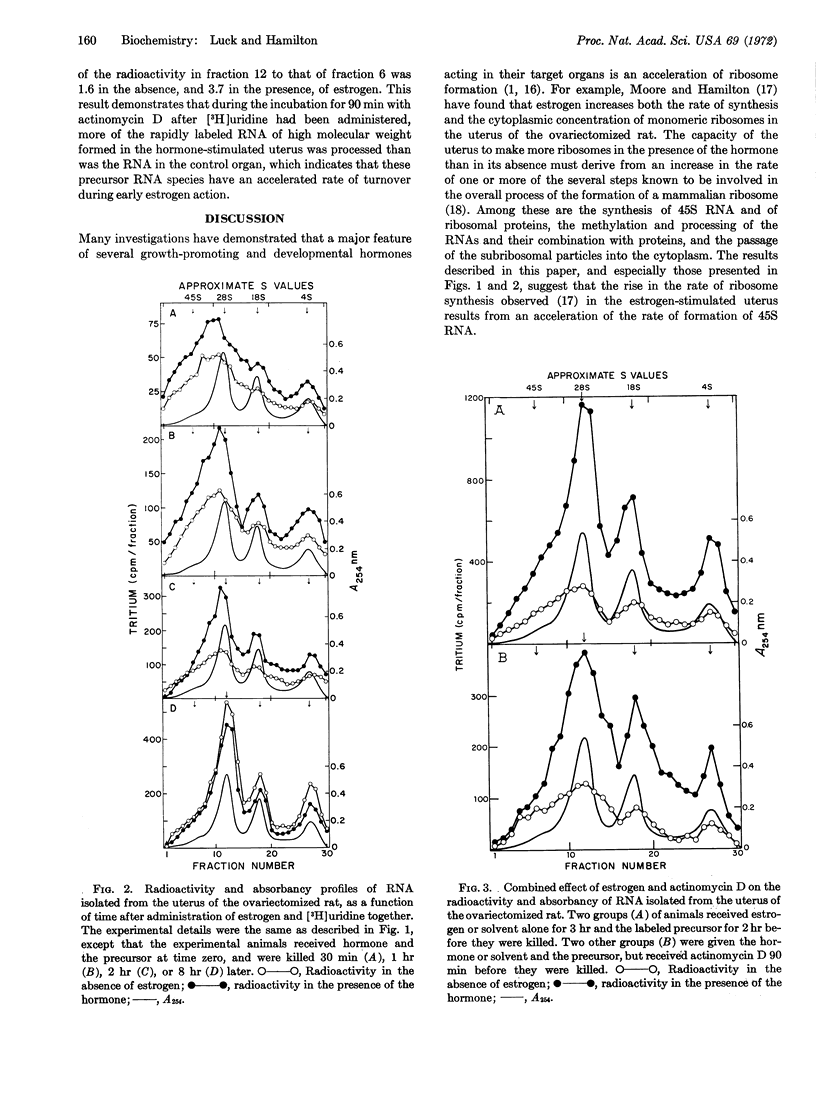

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri S., Lieberman I. Control of ribosome syntesis in normal and regenerating liver. J Biol Chem. 1968 Jan 10;243(1):29–33. [PubMed] [Google Scholar]
- Cooper H. L. Control of synthesis and wastage of ribosomal RNA in lymphocytes. Nature. 1970 Sep 12;227(5263):1105–1107. doi: 10.1038/2271105a0. [DOI] [PubMed] [Google Scholar]
- DIGIROLAMO A., HENSHAW E. C., HIATT H. H. MESSENGER RIBONUCLEIC ACID IN RAT LIVER NUCLEI AND CYTOPLASM. J Mol Biol. 1964 Apr;8:479–488. doi: 10.1016/s0022-2836(64)80005-x. [DOI] [PubMed] [Google Scholar]
- GORSKI J., NELSON N. J. RIBONUCLEIC ACID SYNTHESIS IN THE RAT UTERUS AND ITS EARLY RESPONSE TO ESTROGEN. Arch Biochem Biophys. 1965 May;110:284–290. doi: 10.1016/0003-9861(65)90120-7. [DOI] [PubMed] [Google Scholar]
- Green M. R., Bunting S. L., Peacock A. C. Changes in labeling pattern of ribonucleic acid from mammary tissue as a result of hormone treatment. Biochemistry. 1971 Jun 8;10(12):2366–2371. doi: 10.1021/bi00788a029. [DOI] [PubMed] [Google Scholar]
- Hamilton T. H., Widnell C. C., Tata J. R. Synthesis of ribonucleic acid during early estrogen action. J Biol Chem. 1968 Jan 25;243(2):408–417. [PubMed] [Google Scholar]
- Joel P. B., Hagerman D. D. Extraction of RNA from rat uterus. Biochim Biophys Acta. 1969 Dec 16;195(2):328–339. doi: 10.1016/0005-2787(69)90640-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOORE R. J., HAMILTON T. H. ESTROGEN-INDUCED FORMATION OF UTERINE RIBOSOMES. Proc Natl Acad Sci U S A. 1964 Aug;52:439–446. doi: 10.1073/pnas.52.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Perry R. P. On ribosome biogenesis. Natl Cancer Inst Monogr. 1966 Dec;23:527–545. [PubMed] [Google Scholar]
- Petri W. H., Fristrom J. W., Stewart D. J., Hanly E. W. The in vitro synthesis and characteristics of ribosomal RNA in imaginal discs of Drosophila melanogaster. Mol Gen Genet. 1971;110(3):245–262. doi: 10.1007/BF00337837. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- STEELE W. J., OKAMURA N., BUSCH H. EFFECTS OF THIOACETAMIDE ON THE COMPOSITION AND BIOSYNTHESIS OF NUCLEOLAR AND NUCLEAR RIBONUCLEIC ACID IN RAT LIVER. J Biol Chem. 1965 Apr;240:1742–1749. [PubMed] [Google Scholar]
- Tata J. R. Hormonal regulation of growth and protein synthesis. Nature. 1968 Jul 27;219(5152):331–337. doi: 10.1038/219331a0. [DOI] [PubMed] [Google Scholar]
- WILSON J. D. The nature of the RNA response to estradiol administration by the uterus of the rat. Proc Natl Acad Sci U S A. 1963 Jul;50:93–100. doi: 10.1073/pnas.50.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Processing of 45 s nucleolar RNA. J Mol Biol. 1970 Jan 28;47(2):169–178. doi: 10.1016/0022-2836(70)90337-2. [DOI] [PubMed] [Google Scholar]


