Abstract
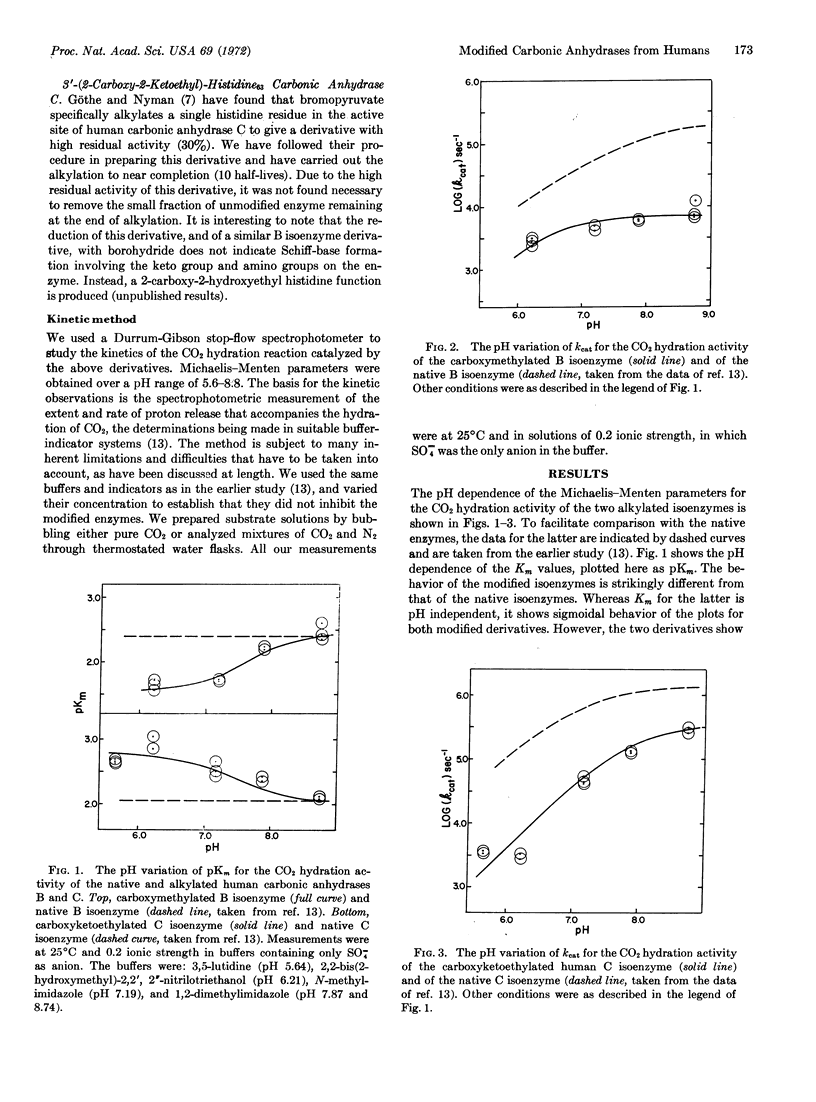
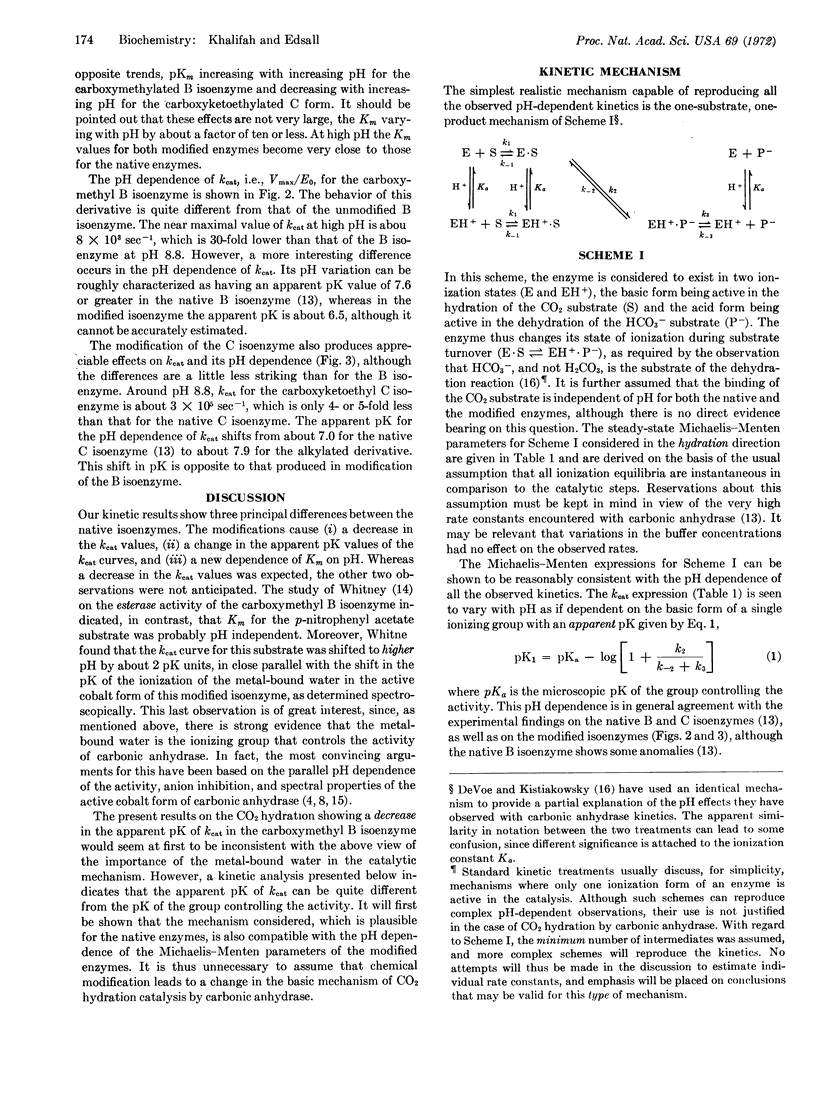
A stop-flow kinetic study was performed on the carbon dioxide hydration activity of the human carbonic anhydrase B isoenzyme carboxymethylated at its histidine200, and of the human C isoenzyme carboxyketoethylated at its histidine63. The Michaelis-Menten parameters determined between pH 5.6 and 8.7 showed striking differences between the native and the alkylated enzymes, as well as between the modified enzymes themselves. The alkylations caused: (i) a decrease in the kcat values, particularly marked for the carboxymethylated B isoenzyme, (ii) a change in the apparent pK of the kcat curves, and (iii) a dependence of Km on pH, for the alkylated enzymes, in contrast to the pH-independent Km values of the native enzymes. The CO2 hydration and esterase activities of the carboxymethyl B isoenzyme differ markedly in their pH dependence. A kinetic mechanism, which is found to be compatible with all the present observations, is proposed. The results indicate that the modifiable histidine residues do not play an essential role in the catalytic mechanism of the native carbonic anhydrases, but they may well influence the enzyme activity in a secondary role.
Keywords: metalloenzymes, isoenzymes, active sites, mechanism
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. M., Myers D. V., Verpoorte J. A., Edsall J. T. Purification and properties of human erythrocyte carbonic anhydrases. J Biol Chem. 1966 Nov 10;241(21):5137–5149. [PubMed] [Google Scholar]
- Bradbury S. L. The carboxymethylation of human carbonic anhydrase B. I. The nature of the reaction. J Biol Chem. 1969 Apr 25;244(8):2002–2009. [PubMed] [Google Scholar]
- Bradbury S. L. The carboxymethylation of human carbonic anhydrase B. II. The amino acid sequence around a reactive histidine residue. J Biol Chem. 1969 Apr 25;244(8):2010–2016. [PubMed] [Google Scholar]
- Henkart P., Dorner F. The dinitrophenylation of human carbonic anhydrase B. J Biol Chem. 1971 Apr 25;246(8):2714–2720. [PubMed] [Google Scholar]
- Kandel M., Gornall A. G., Wong S. C., Kandel S. I. Some characteristics of human, bovine, and horse carbonic anhydrases as revealed by inactivation studies. J Biol Chem. 1970 May 10;245(9):2444–2450. [PubMed] [Google Scholar]
- Khalifah R. G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971 Apr 25;246(8):2561–2573. [PubMed] [Google Scholar]
- Laurent G., Charrel M., Luccioni F., Autran M. F., Derrien Y. Sur les anhydrases carboniques erythrocytaires humaines. I. Isolement et purification. Bull Soc Chim Biol (Paris) 1965;47(6):1101–1124. [PubMed] [Google Scholar]
- Lindskog S. Interaction of cobalt(II)--carbonic anhydrase with anions. Biochemistry. 1966 Aug;5(8):2641–2646. doi: 10.1021/bi00872a023. [DOI] [PubMed] [Google Scholar]
- Nyman P. O., Strid L., Westermark G. Carboxyl-terminal region of human and bovine erythrocyte carbonic anhydrases. I. Amino acid sequences of terminal cyanogen bromide fragments. Eur J Biochem. 1968 Nov;6(2):172–189. doi: 10.1111/j.1432-1033.1968.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Storm D. R. The catalytic versatility of erythrocyte carbonic anhydrase. IV. Kinetic studies of enzyme-catalyzed hydrolyses of para-nitrophenly esters. Biochemistry. 1968 Mar;7(3):1202–1214. doi: 10.1021/bi00843a042. [DOI] [PubMed] [Google Scholar]
- RICKLI E. E., GHAZANFAR S. A., GIBBONS B. H., EDSALL J. T. CARBONIC ANHYDRASES FROM HUMAN ERYTHROCYTES. PREPARATION AND PROPERTIES OF TWO ENZYMES. J Biol Chem. 1964 Apr;239:1065–1078. [PubMed] [Google Scholar]
- Taylor P. W., King R. W., Burgen A. S. Influence of pH on the kinetics of complex formation between aromatic sulfonamides and human carbonic anhydrase. Biochemistry. 1970 Sep 29;9(20):3894–3902. doi: 10.1021/bi00822a007. [DOI] [PubMed] [Google Scholar]
- Wang J. H. Facilitated proton transfer in enzyme catalysis. It may have a crucial role in determining the efficiency and specificity of enzymes. Science. 1968 Jul 26;161(3839):328–334. doi: 10.1126/science.161.3839.328. [DOI] [PubMed] [Google Scholar]
- Whitney P. L., Fölsch G., Nyman P. O., Malmström B. G. Inhibition of human erythrocyte carbonic anhydrase B by chloroacetyl sulfonamides with labeling of the active site. J Biol Chem. 1967 Sep 25;242(18):4206–4211. [PubMed] [Google Scholar]
- Whitney P. L. Inhibition and modification of human carbonic anhydrase B with bromoacetate and iodoacetamide. Eur J Biochem. 1970 Sep;16(1):126–135. doi: 10.1111/j.1432-1033.1970.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Whitney P. L., Nyman P. O., Malmström B. G. Inhibition and chemical modifications of human erythrocyte carbonic anhydrase B. J Biol Chem. 1967 Sep 25;242(18):4212–4220. [PubMed] [Google Scholar]


