Abstract
The pathophysiology of atypical haemolytic-uraemic syndrome (aHUS) occurring de novo after renal transplantation may include genetic mutations of regulators of complement activation, but they are still rarely determined. A 41-year-old female renal transplant recipient presented two very different episodes of thrombotic microangiopathy. The first episode was associated with antibody-mediated rejection and the second was an isolated, acute aHUS, successfully treated with eculizumab. The diagnosis included a genetic analysis and we found a synonymous variant in the Complement Factor H (CFH) gene, c2634C>T (p.His878=) and low factor H (FH) activity during both events. In conclusion, the diagnosis of aHUS should be considered when TMA is associated with an AMR episode. In this setting, a silent polymorphism of factor H may be responsible for these rare cases of “de novo” aHUS after transplantation.
Background
The incidence of de novo post-transplant atypical haemolytic uraemic syndrome (aHUS) is 1–5%. Since the diagnosis of the disease is rarely established before transplantation, and while the prognosis is so reserved,1 it is necessary to better understand the pathology. Genetic mutations of alternative complement pathway regulators are determined in a small percentage of these patients compared with patients without kidney transplant (30% vs 60–70%).2 3 In addition to the mutation, an environmental trigger is required to induce the disease, which also depends on the dysregulation of the alternative complement pathway.
We report two clinically different episodes of thrombotic microangiopathy (TMA) that our patient developed during the first year of transplantation.
Case presentation
A 41-year-old woman with a history of hypertension (diagnosed in 2002, during a twin pregnancy) developed proteinuria and mild renal failure in 2007 and then end-stage renal disease, starting dialysis in August 2009. There was no family history of renal disease. Renal biopsy showed unspecific angiosclerotic lesions. Complement-dependent cytotoxicity tests were always negative for anti-human leucocyte antigen (HLA) antibodies. Luminex testing conducted in 2010 and 2011, more than 1 year before renal transplantation, detected anti-HLA antibodies in 2 of 15 samples, anti-A02 (median fluorescence intensity (MFI) max: 1784; significant threshold: ≥1500), anti-A68 and anti-A69. Subsequently, eight sera were consecutively negative. The patient was transplanted with a kidney from a 43-year-old deceased (after cardiac death) male donor in November 2012 (HLA A02, A31, B44, B14, DR12, DR13, DQ3). The number of HLA incompatibilities was 1 for each locus (A, B, DR). The cross-match was negative. The immunosuppressive induction regimen included thymoglobulin, tacrolimus, mycophenolate and methylprednisolone. The histology of the graft on the first day was normal. The patient was fine until 13 days after kidney transplantation when she presented with diarrhoea, abdominal pain and vomiting. Blood analyses revealed an increase in serum creatinine, from 106 to 292 µmol/L (normal value: 44–88) along with low platelet count, anaemia, a negative Coombs test and haemolysis signs: high lactate dehydrogenase values, the presence of schizocytes (count: 26/1000 red cells) and haptoglobin values that were below the limit of detection. Considering an underlying dysregulation of the complement system, we quickly analysed complement factors. Assays for ADAMTS13 and complement function (CH50 activity, serum levels of C3, C4, FH, FI (factor I), expression of MCP (membrane cofactor protein)/CD46) were within reference intervals except for factor H (FH) activity, which was found to be decreased to 21% (normal value, 86–103%) and C3d/C3 ratio, which was elevated to 1.7 (normal value <1.4). Other laboratory values are shown in table 1. Anti-FH antibody was absent. We also detected two different HLA donor-specific antibodies (HLA-DSA), anti-A2 and anti-DQ3, with high MFI values of 11 700 and 4100, respectively. Stool cultures were negative and, specifically, no shiga-toxigenic Escherichia coli was detected. As the kidney allograft biopsy revealed the diagnosis of TMA-associated acute antibody-mediated rejection (AMR; figure 1), the patient received a treatment regimen consisting of high-dose steroids, along with three consecutive daily plasma exchanges with 1.5 L of fresh frozen plasma, and intravenous immunoglobulins (IvIgs; 2 g/kg in 5 days); we considered that the TMA was a consequence of humoral rejection. Laboratory values returned to normal within 1 month and IvIgs were then administered monthly (0.4 g/kg).
Table 1.
Laboratory data*
| Variable | Reference range, adults | One week after renal transplant† | During 1st event | One month after 1st event | Interval | During 2nd event | One month after 2nd event | One year after renal transplant |
|---|---|---|---|---|---|---|---|---|
| White cell count (×103/mm3) | 4.2–11.4 | 4.2 | 7.2 | 3.6 | 6.3 | 2.6 | 1.7 | 3.0 |
| Haematocrit (%) | 35.3–46.1 | 27.2 | 18.7 | 34.2 | 37.5 | 26 | 27.1 | 39.7 |
| Haemoglobin (g/dL) | 11.8–15.5 | 9.0 | 6.4 | 11.8 | 12.4 | 8.6 | 8.6 | 13.3 |
| Platelet count (×103/ mm3) | 155–346 | 187 | 65 | 259 | 252 | 62 | 195 | 187 |
| Creatinine (μmol/L) | 44–88 | 106 | 292 | 106 | 141 | 168 | 141 | 141 |
| Lactate dehydrogenase (U/L) | <214 | 185 | 685 | 228 | 288 | 700 | 222 | ND |
| Bilirubin (mg/dL) | <1.2 | 0.2 | 1.5 | 0.31 | 0.45 | 1.1 | 0.22 | 0.37 |
| Haptoglobin (mg/dL) | 30–200 | ND | <5 | 43 | 37 | <5 | 46 | ND |
| Schizocyte count (per 1000 RC) | 0 | ND | 26 | 15 | 6 | 20 | 3 | 0 |
| FH activity (%) | 86–103 | ND | 21 | 65 | 67 | 42 | ND | ND |
*To convert the values for haemoglobin to millimoles per litre, multiply by 0.6206. To convert the values for bilirubin to micromoles per litre, multiply by 17.1. To convert the values for haptoglobin to grams per litre, multiply by 0.01.
†The day of renal transplantation was 15 November 2012. The first event occurred 13 days after transplantation. The second event occurred 7 months after transplantation.
FH, factor H; ND, not determined; RC, red cells.
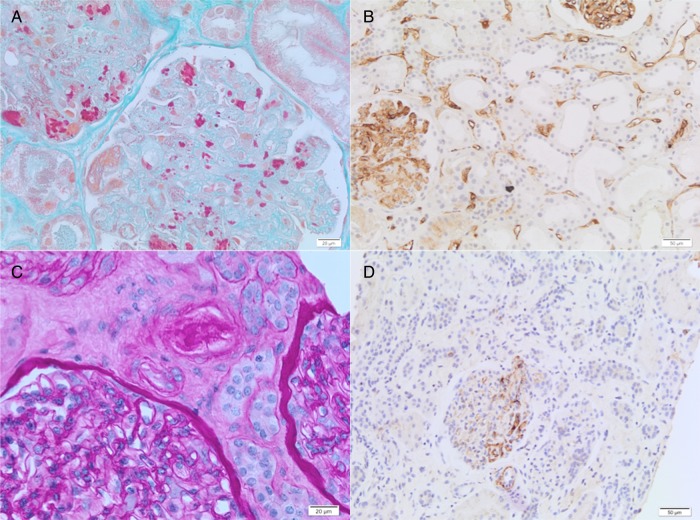
Figure 1.
Renal transplant biopsies: thrombotic microangiopathy (TMA), first event (A–B) and second event (C–D). (A) The glomerulus displays features of acute TMA, including marked glomerular capillary congestion, endothelial swelling and necrosis, and glomerular capillary thrombosis with entrapment of erythrocytes (Masson’ trichrome; ×400). (B) C4d immunohistochemistry (DB Biotech, clone 02A3) showing C4d deposition along peritubular capillaries highly suggestive of acute humoral rejection (×200). (C) The lumen of an arteriole is severely narrowed by intimal deposit of eosinophilic material suggestive of fibrin. Endothelial swelling is observed in the glomerulus; no glomerular thrombus noted (Periodic Acid Schiff; ×400). (D) C4d immunohistochemistry (DB Biotech, clone 02A3) reveals negative immune-staining for C4d (×200).
Six months after successful treatment of the AMR, the patient presented at the hospital with haemolytic anaemia and thrombocytopenia again, with a negative microbiological work up accompanied by low FH complement activity (42%). Her serum creatinine increased over the next 4 days (table 1). PCR tests of her blood for cytomegalovirus, parvovirus B19 and human herpesvirus 6 were negative. Interestingly, no HLA-DSA was detected. A second allograft biopsy performed at that time showed TMA features but, surprisingly, no sign of rejection and, in particular, negative immune staining for C4d (figure 1). A diagnosis of aHUS was made and genetic screening was started. After 1 week of plasma exchanges, we started eculizumab, 900 mg weekly for 5 weeks, followed by 900 mg every 2 weeks, as recommended for a body weight of 39 kg. Two days before the first doses of eculizumab, the patient received a tetravalent vaccine against Neisseria meningitides (A,C,Y,W135), together with prophylactic antibiotics (ciprofloxacin), that were continued because the vaccine against the most prevalent serogroup (B) was still unavailable. Haemolysis was inhibited immediately after the first injection of eculizumab, allowing for discontinuation of plasma exchanges.
Investigations
Genetic screening undertaken since the eculizumab therapy revealed a heterozygous single nucleotide polymorphism (SNP), c.2634C>T, on the short consensus repeat 15 (SCR15) of complement factor H (CFH). This synonymous change has been reported as a very rare variant with an average heterozygosity of 0.008 (dbSNP, rs35292876). Furthermore, we found no mutation for CFI (complement factor I), MCP/CD46, CFB (complement factor B), C3, thrombomodulin or complement factor H related (CFHR1)/3.
Outcome and follow-up
Two months later, a further fall of haptoglobin levels, but without anaemia or thrombocytopenia (results not shown), had been resolved by adjusting the dose of eculizumab to 1200 mg every 15 days. Twelve months after the initiation of eculizumab, laboratory values are still normal except for plasma creatinine 141 µmol/L (table 1).
Discussion
aHUS is a rare disease related to uncontrolled activation of the alternative complement pathway. Thus far, it has been assumed that the pathophysiology of post-transplant ‘de novo’ aHUS is due to a mild genetic susceptibility that, when associated with the occurrence of endothelial damage, may cause TMA.4 This type of trigger, such as acute rejection, which is still common, is prone to occur after renal transplantation, occurring in 10–15% of renal transplant recipients. Further, during episodes of AMR, TMA has been found in over 40% of biopsies.5 6 However, even in that setting, diagnose of aHUS is difficult to accurately establish; indeed, only 7.5% of post-transplant ‘de novo’ TMAs have exhibited biochemical characteristics of haemolytic anaemia.4 Our present case experienced a first TMA presentation that was considered linked to an acute humoral rejection at the time. Treatment included plasma exchanges that were rapidly able to control the TMA-associated AMR. The contribution of plasma would have boosted the activity of FH, nevertheless, we concluded that the argument was insufficient for the diagnosis of aHUS in this first episode. As a precaution, in the interval preceding the second episode, we continued to look for biological signs of haemolysis, which persisted, such as the presence of schizocytes and a relatively low haptoglobin level (table 1), but without anaemia or thrombocytopenia. Together with a lower activity of FH, although higher than in the acute attack, these laboratory findings could account for the persistence of the formation of microthrombi, though not enough to cause a clinical expression. Indeed, the patient had no symptoms and the graft function was stable, so we did not use a biopsy control. Also, during the interval of remission, eculizumab was not available. When the second event of TMA occurred, we could not identify any trigger for its outbreak and, in particular, there was no acute rejection. Considering other TMA triggers, such as immunosuppression treatment, calcineurin inhibitor and mammalian target of rapamycin inhibitor have been associated with the occurrence of post-transplant aHUS.1 In our case, blood levels of tacrolimus were within normal ranges, and maintenance treatment did not prevent disease remission during management of the aHUS episodes. With respect to the role of viral infections, parvovirus B19 and cytomegalovirus have also been implicated as triggers.7 8 These viruses were absent in our patient as attested by PCR assays. We witnessed recurrent disease with widely varying presentation. The first aHUS episode occurred in the context of AMR while the second developed without a clear triggering mechanism. If we go back to the period of pregnancy, we suspect that the hypertension developed by the patient was a rough first sign of the disease, which would be manifested quietly until end-stage renal disease, when the renal pathology revealed only terminal damage.
Finally, we found an SNP, c.2634C>T, on the SCR15 of CFH, coding for the same amino acid (p.His878=), a so-called ‘silent mutation’. Indeed, silent SNPs have been associated with structural and functional changes in the properties of proteins and synonymous codon substitution may lead to different kinetics of mRNA translation.9–12 FH is composed of 20 SCR domains. The complement regulatory region is located in the N-terminus, SCR1 to SCR4. The C-terminal region is the site of cell binding recognition.13 SCR15 mutations have been previously associated with aHUS disease.14 As His 878 is a random coil area of the structure, the c.2634C>T SNP could be responsible for modifying the conformation, and performance of CFH as has been proposed for other proteins, such as ADAMTS13.15 In fact, the activity of FH was repeatedly decreased during the crisis, in the absence of anti-FH antibodies, and normalised after treatment.
Until recently, treatment of aHUS relied only on plasma exchanges that are thought to replace dysfunctional circulating regulators of the alternative complement pathway and/or eliminate anti-FH antibodies, if any are present. However, in cases of membrane-anchored complement regulator defects, the response to plasma exchanges remains poor. Eculizumab is a humanised anti-C5 monoclonal antibody that prevents the cleavage of the C5 complement factor into C5a and C5b, hence inhibiting the formation of the terminal membrane-attack complex and the cytolytic action of the complement cascade.16 It has been shown to be effective in de novo as well as recurrent post-transplant aHUS.17 18 Following treatment with eculizumab, our patient experienced tremendous biological improvement. Eculizumab does not resolve the chronic alternative complement deregulation but prevents endothelial cell death. The tissue areas affected by TMA are ischaemic and will be irreversibly replaced by fibrosis. Therefore, the ability of a treatment to reverse renal damage depends on how early treatment is initiated.19 In our patient, despite rapid initiation of treatment with eculizumab, plasma creatinine decreased very little but has since remained steady. Indeed, full recovery of baseline renal function was observed in only 31% of patients treated with eculizumab after transplantation.20
Learning points.
We hypothesise that a rare silent change in the complement factor H (CFH) gene, c2634C>T, could be responsible for de novo post-transplant atypical haemolytic uraemic syndrome (aHUS), in association with other stress factors such as initial humoral alloreactivity, although the presence of other unknown genetic changes cannot be ruled out.
Furthermore, we suggest that any manifestation of thrombotic microangiopathy after renal transplantation should be rapidly and carefully investigated in order not to overlook the diagnosis of aHUS.
In this case, eculizumab treatment should be administered as soon as possible to prevent irreversible allograft failure.
Acknowledgments
The authors are grateful to Dr Annick Massart, Professor Lidia Ghisdal, Professor Fabrice Gankam, Dr Maria Kyriakopoulou and Dr Ahmed Goubella for participation in the medical care, and Professor Alain Le Moine and Professor Pierre Vereerstraeten for valuable comments, and Mrs Fatiha Ouanda for secretarial assistance.
Footnotes
Contributors: ENB treated the patient together with the team, conceived, designed and wrote the article. PS analysed and interpreted the immunobiological data and, revised the article. SR realised and interpreted the anatomopathology, and revised the article. KD realised and interpreted the genetic data, and revised the article.
Competing interests: ENB received fees from Alexion Pharmaceuticals for training seminars (aHUS disease).
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Reynolds JC, Agodoa LY, Yuan CM et al. Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis 2003;42:1058–68. 10.1016/j.ajkd.2003.07.008 [DOI] [PubMed] [Google Scholar]
- 2.Le Quintrec M, Lionet A, Kamar N. Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant 2008;8:1694–701. 10.1111/j.1600-6143.2008.02297.x [DOI] [PubMed] [Google Scholar]
- 3.Noris M, Remuzzi G. Atypical haemolytic-uremic syndrome. N Engl J Med 2009;361:1676–87. 10.1056/NEJMra0902814 [DOI] [PubMed] [Google Scholar]
- 4.Zuber J, Le Quintrec M, Sberro-Soussan R et al. New insights into postrenal transplant hemolytic uremic syndrome. Nat Rev Nephrol 2011;7:23–35. 10.1038/nrneph.2010.155 [DOI] [PubMed] [Google Scholar]
- 5.Nickeleit V, Zeiler M, Gudat F et al. Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol 2002;13:242–51. [DOI] [PubMed] [Google Scholar]
- 6.Lefaucheur C, Nochy D, Hill GS et al. Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant 2007;7:832–41. 10.1111/j.1600-6143.2006.01686.x [DOI] [PubMed] [Google Scholar]
- 7.Murer L, Zacchello G, Bianchi D et al. Thrombotic microangiopathy associated with parvovirus B19 infection after renal transplantation. J Am Soc Nephrol 2000;11:1132–7. [DOI] [PubMed] [Google Scholar]
- 8.Olie KH, Goodship TH, Verlaak R et al. Post-transplantation cytomegalovirus-induced recurrence of atypical haemolytic uremic syndrome: successful treatment with intensive plasma exchanges and gancyclovir. Am J Kidney Dis 2005;45:e12–15. 10.1053/j.ajkd.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 9.Komar AA. Silent SNPs: impact on gene function and phenotype. Pharmacogenomics 2007;8:1075–80. 10.2217/14622416.8.8.1075 [DOI] [PubMed] [Google Scholar]
- 10.Kimchi-Sarfaty C, Oh JM, Kim IW et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007;315:525–8. 10.1126/science.1135308 [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Ignatova Z. Generic algorithm to predict the speed of translational elongation: implications for protein biogenesis. PLoS ONE 2009;4:e5036 10.1371/journal.pone.0005036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders R, Deane CM. Synonymous codon usage influences the local protein structure observed. Nucleic Acids Res 2010;38:6719–28. 10.1093/nar/gkq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jozsi M, Heinen S, Hartmann A et al. Factor H and atypical haemolytic uremic syndrome: mutations in the C-terminus cause structural changes and defective recognition functions. J Am Soc Nephrol 2006;17:170–7. 10.1681/ASN.2005080868 [DOI] [PubMed] [Google Scholar]
- 14.Dragon-Durey M, Frémaux-Bacchi V, Loirat C et al. Heterozygous and homozygous factor H deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol 2004;15:787–95. 10.1097/01.ASN.0000115702.28859.A7 [DOI] [PubMed] [Google Scholar]
- 15.Edwards NC, Hing ZA, Perry A et al. Characterization of coding synonymous and non-synonymous variants in ADAMTS13 using ex vivo and in silico approaches. PLoS ONE 2012;7:e38864 10.1371/journal.pone.0038864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidtko J, Peine S, El-Housseini Y et al. Treatment of atypical hemolytic uremic syndrome and thrombotic microangiopathies: a focus on eculizumab. Am J Kidney Dis 2013;61:289–99. 10.1053/j.ajkd.2012.07.028 [DOI] [PubMed] [Google Scholar]
- 17.Chatelet V, Frémaux-Bacchi V, Lobbedez T et al. Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome. Am J Transplant 2009;9:2644–5. 10.1111/j.1600-6143.2009.02817.x [DOI] [PubMed] [Google Scholar]
- 18.Nürnberger J, Philipp T, Witzke O et al. Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med 2009;360:542–4. 10.1056/NEJMc0808527 [DOI] [PubMed] [Google Scholar]
- 19.Legendre CM, Licht C, Muus P et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 2013;368:2169–81. 10.1056/NEJMoa1208981 [DOI] [PubMed] [Google Scholar]
- 20.Zuber J, Fakhouri C, Roumenina LT et al. Use of eculizumab for atypical haemolytic ureamic and C3 glomerulopathies. Nat Rev Nephrol 2012;8:643–57. 10.1038/nrneph.2012.214 [DOI] [PubMed] [Google Scholar]