Abstract
The theory that the αβ dimer is the functional unit of cooperativity in hemoglobin has been tested by determination of the oxygen equilibrium curve of stable deoxy dimers, obtained by the addition of 0.9 M MgCl2 to human des-Arg 141α-hemoglobin. Cooperativity was absent in this medium, but was regained on transfer of the hemoglobin to a dilute phosphate buffer, where tetramers reformed. X-ray analysis of crystals of oxy- and deoxy-des-Arg hemoglobins showed that the removal of Arg 141α would leave the structure of αβ dimers unchanged. Nonreactivity of the sulfhydryl groups at 112β proved that the subunits in deoxy dimers form the same contact as in oxy dimers, namely α1β1, and that no significant dissociation into free subunits occurs in 0.9 M MgCl2. The absorption spectrum of the deoxy dimers corresponded to the sum of the spectra of the free deoxy α and β subunits, and was different from that of the deoxy tetramer, showing the constraining salt bridges formed by the C-terminal residues in the tetramer to be necessary for the spectral changes normally observed on association of the deoxy subunits.
Keywords: human, dissociation, equilibrium, sulfhydryl, absorption, x-ray analysis
Full text
PDF

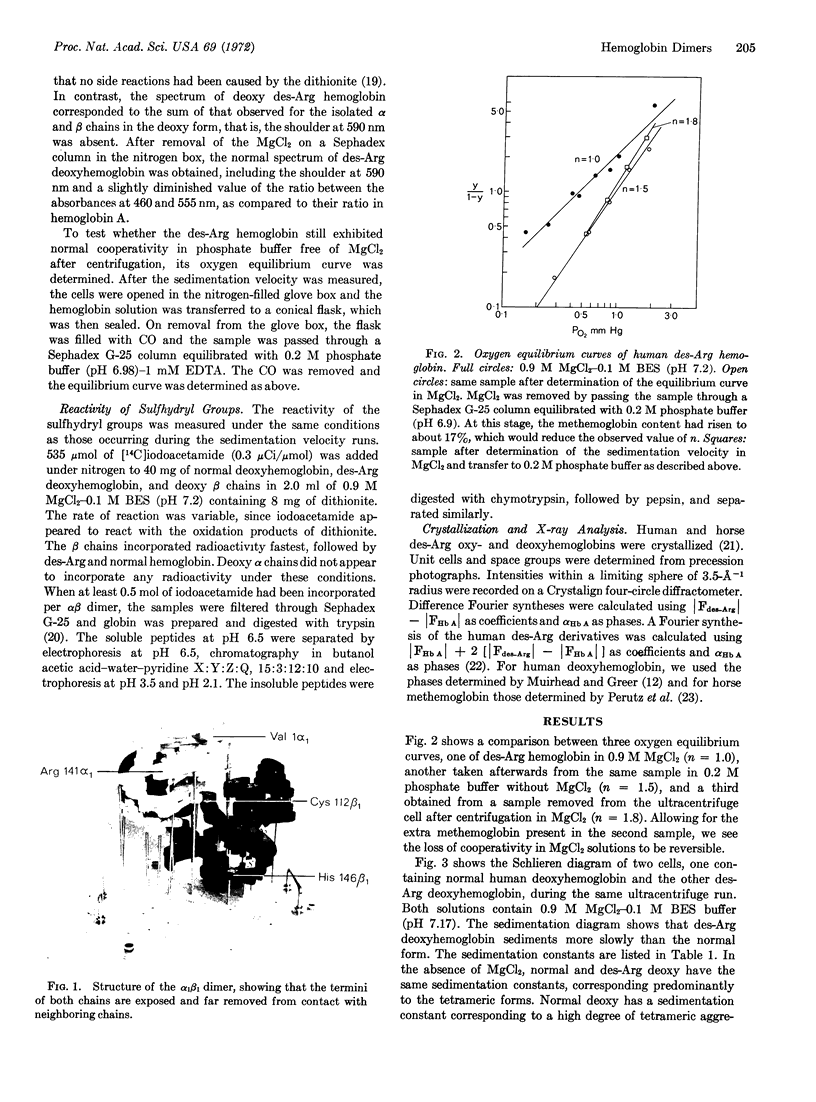
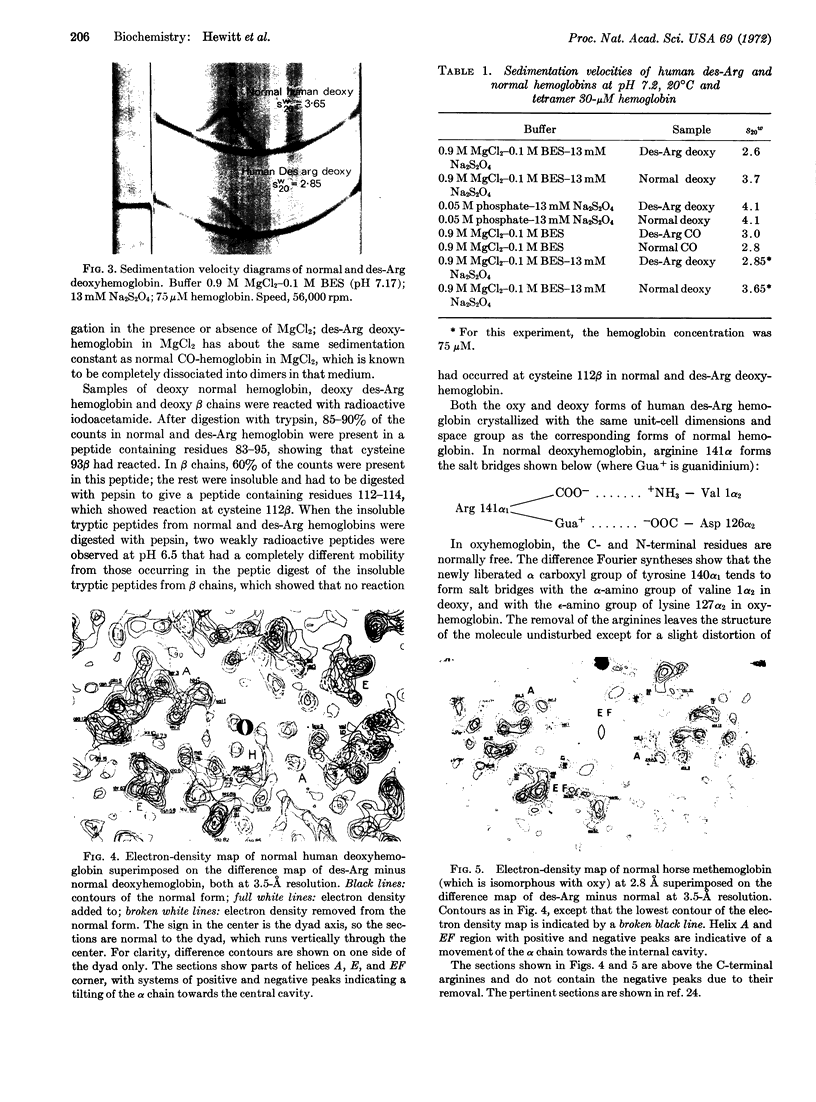

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. E., Moffat J. K., Gibson Q. H. The kinetics of ligand binding and of the association-dissociation reactions of human hemoglobin. Properties of deoxyhemoglobin dimers. J Biol Chem. 1971 May 10;246(9):2796–2807. [PubMed] [Google Scholar]
- Antonini E. Hemoglobin and its reaction with ligands. Science. 1967 Dec 15;158(3807):1417–1425. doi: 10.1126/science.158.3807.1417. [DOI] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- DALZIEL K., O'BRIEN J. R. Side reactions in the deoxygenation of dilute oxyhaemoglobin solutions by sodium dithionite. Biochem J. 1957 Sep;67(1):119–124. [PMC free article] [PubMed] [Google Scholar]
- GUIDOTTI G., CRAIG L. C. Dialysis studies. VIII. The behavior of solutes which associate. Proc Natl Acad Sci U S A. 1963 Jul;50:46–54. doi: 10.1073/pnas.50.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIDOTTI G., KONIGSBERG W., CRAIG L. C. ON THE DISSOCIATION OF NORMAL ADULT HUMAN HEMOGLOBIN. Proc Natl Acad Sci U S A. 1963 Oct;50:774–782. doi: 10.1073/pnas.50.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Parkhurst L. J. Kinetic evidence for a tetrameric functional unit in hemoglobin. J Biol Chem. 1968 Oct 25;243(20):5521–5524. [PubMed] [Google Scholar]
- Kellett G. L. Dissociation of hemoglobin into subunits. Ligand-linked dissociation at neutral pH. J Mol Biol. 1971 Aug 14;59(3):401–424. doi: 10.1016/0022-2836(71)90307-x. [DOI] [PubMed] [Google Scholar]
- Kellett G. L., Gutfreund H. Reactions of haemoglobin dimers after ligand dissociation. Nature. 1970 Aug 29;227(5261):921–926. doi: 10.1038/227921a0. [DOI] [PubMed] [Google Scholar]
- Kellett G. L. Ligand-free haemoglobin dimers. Nat New Biol. 1971 Dec 8;234(49):189–191. doi: 10.1038/newbio234189a0. [DOI] [PubMed] [Google Scholar]
- Kellett G. L., Schachman H. K. Dissociation of hemoglobin into subunits. Monomer formation and the influence of ligands. J Mol Biol. 1971 Aug 14;59(3):387–399. doi: 10.1016/0022-2836(71)90306-8. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Clegg J. B. Amino-acid replacements in horse haemoglobin. Nature. 1967 Jan 21;213(5073):269–271. doi: 10.1038/213269a0. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. The binding of carbon dioxide by horse haemoglobin. Biochem J. 1971 Aug;124(1):31–45. doi: 10.1042/bj1240031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead H., Greer J. Three-dimensional Fourier synthesis of human deoxyhaemoglobin at 3.5 Angstrom units. Nature. 1970 Nov 7;228(5271):516–519. doi: 10.1038/228516a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Miurhead H., Cox J. M., Goaman L. C., Mathews F. S., McGandy E. L., Webb L. E. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: (1) x-ray analysis. Nature. 1968 Jul 6;219(5149):29–32. doi: 10.1038/219029a0. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Studies on the relations between molecular and functional properties of hemoglobin. I. The effect of salts on the molecular weight of human hemoglobin. J Biol Chem. 1961 Feb;236:391–396. [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E., CAPUTO A. Studies on the relations between molecular and functional properties of hemoglobin. II. The effect of salts on the oxygen equilibrium of human hemoglobin. J Biol Chem. 1961 Feb;236:397–400. [PubMed] [Google Scholar]
- Rosemeyer M. A., Huehns E. R. On the mechanism of the dissociation of haemoglobin. J Mol Biol. 1967 Apr 28;25(2):253–273. doi: 10.1016/0022-2836(67)90141-6. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Henderson R., Blow D. M. Structure of crystalline alpha-chymotrypsin. 3. Crystallographic studies of substrates and inhibitors bound to the active site of alpha-chymotrypsin. J Mol Biol. 1969 Dec 14;46(2):337–348. doi: 10.1016/0022-2836(69)90426-4. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HUTCHINSON W. D. Carbon-14 labelled hybrids of haemoglobin. Nature. 1960 Jul 16;187:216–218. doi: 10.1038/187216a0. [DOI] [PubMed] [Google Scholar]