Abstract
X-linked retinoschisis (XLRS) is a retinal disease caused by mutations in the gene encoding the protein retinoschisin (RS1) and one of the most common causes of macular degeneration in young men. Currently, no FDA-approved treatments are available for XLRS and a replacement gene therapy could provide a promising strategy. We have developed a novel gene therapy approach for XLRS, based on the administration of AAV8-scRS/IRBPhRS, an adeno-associated viral vector coding the human RS1 protein, via the intravitreal route. On the basis of our prior study in an Rs1-KO mouse, this construct transduces efficiently all the retinal layers, resulting in an RS1 expression similar to that observed in the wild-type and improving retinal structure and function. In support of a clinical trial, we carried out a study to evaluate the ocular safety of intravitreal administration of AAV8-scRS/IRBPhRS into 39 New Zealand White rabbits. Two dose levels of vector, 2e10 and 2e11 vector genomes per eye (vg/eye), were tested and ocular inflammation was monitored over a 12-week period by serial ophthalmological and histopathological analysis. A mild ocular inflammatory reaction, consisting mainly of vitreous infiltrates, was observed within 4 weeks from injection, in both 2e10 and 2e11 vg/eye groups and was likely driven by the AAV8 capsid. At 12-week follow-up, ophthalmological examination revealed no clinical signs of vitreitis in either of the dose groups. However, while vitreous inflammatory infiltrate was significantly reduced in the 2e10 vg/eye group at 12 weeks, some rabbits in the higher dose group still showed persistence of inflammatory cells, histologically. In conclusion, intravitreal administration of AAV8-scRS/IRBPhRS into the rabbit eye produces a mild and transient intraocular inflammation that resolves, at a 2e10 vg/eye dose, within 3 months, and does not cause irreversible tissue damages. These data support the initiation of a clinical trial of intravitreal administration of AAV8-scRS/IRBPhRS in XLRS patients.
Introduction
X-linked retinoschisis (XLRS) is a neurodevelopmental retinal abnormality caused by mutations in the gene encoding the protein retinoschisin (RS1). It affects 1:5,000 to 1:25,000 males worldwide and is one of the most common causes of vision loss from macular degeneration in young men (George et al., 1995). RS1 gene expression is mainly localized to photoreceptors (Grayson et al., 2000; Molday et al., 2001; Takada et al., 2004) and to neurons in the inner nuclear retinal layer (Molday et al., 2001; Takada et al., 2004), and it is thought to play a critical role for cell adhesion during the normal development and maintenance of retinal structure (Wu et al., 2005). An absent or mutated RS1 protein in the retina of patients with XLRS leads to abnormalities in the normal laminar structure of the retina, resulting in impaired visual acuity and increased propensity to retinal detachment (Sieving et al., 2009). Although various experimental treatment strategies have been tested, including the use of topical and systemic carbonic anhydrase inhibitors (Ghajarnia and Gorin, 2007; Genead et al., 2010), to date, no proven treatment is available for XLRS.
The eye represents a promising target for gene therapy. Many forms of retinal degeneration are the consequence of inherited monogenic mutations, and a gene replacement strategy holds great promises in the treatment of these diseases (Berger et al., 2010). Because of its unique anatomical properties, the eye offers the opportunity to visualize and access directly the intraocular compartments, so that the viral vector can be easily delivered in situ. Moreover, the existence of ocular immune privilege (Streilein, 2003) may reduce the likelihood of developing an immune response against the virus or its product, increasing thereby the effectiveness of the gene transfer.
Adeno-associated viral (AAV) vectors have gained an increasing acceptance as potential gene delivery vehicles for many inherited and acquired human diseases (Bakay et al., 2007; Bainbridge et al., 2008; Cideciyan et al., 2008; Maguire et al., 2008; Marks et al., 2010; Nathwani et al., 2011). Recently, the results from three separate clinical trials for Leber's congenital amaurosis (LCA) showed the safety of AAV2 in human patients (Bainbridge et al., 2008; Cideciyan et al., 2008; Maguire et al., 2008). An AAV2 vector expressing RPE65 was administered by subretinal injection into nine subjects with LCA. The majority of the subjects showed evidence of improvement in retinal function and, importantly, no vector-related adverse events were described. In addition, in a phase 1/2 clinical trial, six patients affected with choroideremia were treated with subretinal injections of an AAV2 vector encoding the Rab escort protein-1. Mean retinal sensitivity increased in five out of six eyes and maximal retinal sensitivity improved in all the treated eyes. Two patients with the most advanced choroideremia had substantial gains in visual acuity and no safety concerns were reported (MacLaren et al., 2014). These encouraging results provided the basis for the clinical development of an AAV vector-based therapeutics for the treatment of XLRS.
Administering the viral vector in the subretinal space proved to be effective and safe in patients with LCA and choroideremia. However, this approach may not be indicated for patients affected by XLRS whose retina is extremely fragile and susceptible to retinal detachment and hemorrhage. Even if associated with a high efficiency of photoreceptor transduction, subretinal injection involves the surgical manipulation of retinal tissue, a procedure that could have a detrimental impact on the retina, especially if it is affected by a degenerative disease like XLRS. Administering the viral vector into the vitreous represents a potential alternative. Intravitreal injection is indeed a safer and less invasive procedure than subretinal injection, and is a routine clinical procedure used to deliver different pharmacological agents into the human eye.
A previous study using an XLRS mouse model showed ability of an AAV8 construct to transduce with high efficiency all the retinal layers after intravitreal injection, resulting in a retinoschisin expression similar to that observed in a wild-type mouse, and in the improvement of the retinal structure and function (Park et al., 2009).
Although the vitreous is generally considered immunologically privileged, there is evidence that a preexisting systemic immunity to the AAV capsid could limit the vector expression when the virus is administered into the vitreous cavity (Bainbridge et al., 2008). In this setting, the use of a viral vector associated with a low antibody neutralizing activity could reduce the chance of developing an immune response in the recipient's eye, increasing the chance of therapeutic success. AAV8 was isolated in 2002 from rhesus-monkey tissues (Gao et al., 2002), and since its discovery many experimental studies have shown high efficiency in transducing a wide range of tissues, including the retina (Wang et al., 2005; Lebherz et al., 2008).
Moreover, when compared with human-derived AAVs, such as AAV2, for which the human population shows a preexisting immunity, the neutralizing activity against AAV8 in the human sera appears to be very low (Boutin et al., 2010).
Since AAV8 has yet to enter human clinical testing for an ocular indication, we sought to assess the safety of an intravitreal administration of an AAV8-based vector in a laboratory study.
The rabbit eye was selected based on its size, volume, and its extensive use in pharmacokinetic and toxicity testing of investigational drugs injected into the vitreous cavity (Dierks et al., 2005; Albini et al., 2007; Lang et al., 2007). The use of a large-eye animal model like the rabbit offers many advantages over animals such as small rodents. A larger eye, and particularly a larger vitreous cavity, reduces the technical difficulties associated with the intravitreal injection in a small animal eye and allows for a more detailed clinical examination of the intraocular structures, factors that are both critical when the tested article is delivered directly into the vitreous.
Previously, we (Park et al., 2009) demonstrated that intravitreal administration of an AAV8 vector expressing the human RS1 gene is able to restore the retinal function in a murine model of XLRS. The present study was designed to assess the safety and tolerability of intravitreal injection of AAV8-scRS/IRBPhRS in the rabbit eye prior to clinical testing in humans.
Results and Discussion
Clinical trial
The two AAV8-scRS/IRBPhRS vector doses used in this study, 2e10 and 2e11 vg/eye, resulted in minimum to mild vitreous inflammation that resolved completely by 3 months for the lower dose. These preliminary results support the advancement of AAV8-scRS/IRBPhRS for testing in a phase I clinical trial in patients with XLRS. The planned clinical trial would utilize good manufacturing practice-grade material of the same construct and administered intravitreally into XLRS patients with verified RS1 mutations. The primary outcome would be the safety of ocular AAV8-scRS/IRBPhRS as determined from assessment of retinal function, ocular structure, laboratory tests, and occurrence of adverse events. Secondary outcomes would include changes in visual function, electroretinogram responses, retinal imaging with optical coherence tomography and visual field measurements.
Objectives and study design
The objective of the present study was to evaluate the intraocular safety profile of an AAV8 vector for an XLRS gene transfer construct containing the human RS1 gene, by administration to rabbits via intravitreal injections. More precisely, this study aimed to investigate (1) the consequences of intravitreal administration of an AAV8-scRS/IRBPhRS construct both clinically and histologically, (2) to characterize the inflammatory cell types present, (3) to understand which part of the vector was responsible for the ocular inflammation, and (4) to characterize the systemic antibody response to intraocular exposure to AAV8.
Twenty-one New Zealand White (NZW) rabbits received a single intravitreal injection into the right eye of AAV8-scRS/IRBPhRS solution at two different doses, 2e10 vector genomes per eye (vg/eye) (n=7) and 2e11 vg/eye (n=7), or vehicle (n=7). The onset and the intensity of an ocular inflammatory response were evaluated in all animals with ophthalmological examination at 2, 4, 8, and 12 weeks, and by a histopathological analysis of the ocular structures. The latter was performed in a first group of 9 animals sacrificed at 4 weeks and in a second group of 12 animals that were sacrificed at the end of the 12-week follow-up period (Table 1). In order to understand which component of the vector contributed to the observed ocular inflammatory response, an additional experiment was conducted. Two groups of six NZW rabbits were intravitreally injected in one eye with a dose of 2e11 vg/eye of either AAV8-scRS/IRBPhRS vector or AAV8-null vector, and were followed for 4 weeks. A third group of rabbits was used as control and received an intravitreal injection of vehicle (Table 1). The follow-up time was selected based on the results of the 12-week study described above, in which the vector-injected eyes developed a maximum ocular inflammatory response between 2 and 4 weeks. For this study, we selected to administer the higher vector dose of 2e11 vg/eye. We reasoned that, at the higher dose, we may detect more pronounced differences in ocular responses between eyes injected with the AAV8 vector containing the RS1 transgene versus the AAV8 vector minus transgene.
Table 1.
Study Design
| Design of the 12-week toxicology study | |||||
|---|---|---|---|---|---|
| Group | Article | Dose level (vg/eye) | Number of animals | 4-week sacrifice | 12-week sacrifice |
| 1 | AAV8-scRS/IRBPhRS | 2 e10 | 7 | 3 | 4 |
| 2 | AAV8-scRS/IRBPhRS | 2 e11 | 7 | 3 | 4 |
| 3 | Vehicles | (−) | 7 | 3 | 4 |
| Design of the 4-week null-vector study | ||||
|---|---|---|---|---|
| Group | Article | Dose level (vg/eye) | Number of animals | 4-week sacrifice |
| 1 | AAV8-scRS/IRBPhRS | 2 e11 | 6 | 6 |
| 2 | AAV8-null-vector | 2 e11 | 6 | 6 |
| 3 | Vehicles | (−) | 6 | 6 |
Summary of data
Twelve-week ocular safety study
Intravitreal administration of AAV8-scRS/IRBPhRS resulted in a mild and transient vitreous inflammation at ophthalmological examination
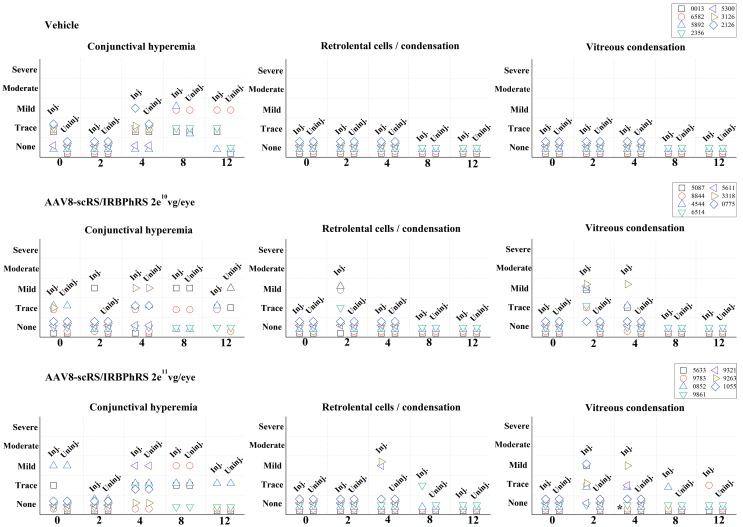
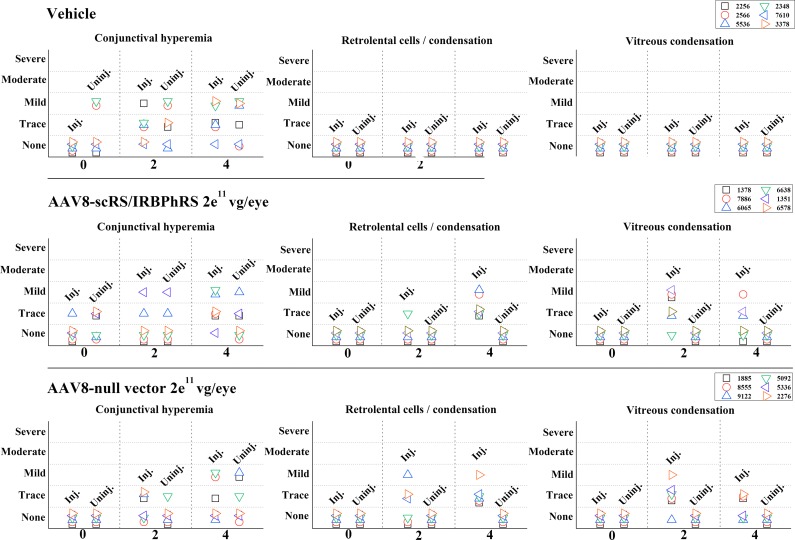
Clinical examination results of ocular structures during the 12-week period are summarized in Fig. 1.
FIG. 1.
Ophthalmological findings in rabbits intravitreally injected with two doses (2e10 and 2e11 vg/eye) of AAV8-scRS/IRBPhRS and vehicle. Findings from both the injected (inj.) and uninjected (uninj.) eye are displayed for each animal at five time points (0, 2, 4, 8, and 12 weeks) during the study. Clinical changes were graded at five levels of severity. *Rabbit #9783 showed trace of optic disc edema at 4-week follow-up that resolved at 8 weeks.
At early time points (2 and 4 weeks), the main clinical finding in the injected eye of both 2e10 and 2e11 vg/eye dosing groups was the onset of posterior vitreous abnormalities. These included trace or mild prepapillary and central vitreous condensation, and were observed in 6 out of 7 rabbits (86%) in each dosing group. Concomitant signs of anterior vitreous inflammation, consisting in retrolental cells infiltration and/or opacities, were found in 3 animals (43%; #4544, #6514, and #8844) in the low-dose group and in 2 animals (29%; #9321 and #9263) in the high-dose group. A mild optic disc edema associated with trace prepapillary vitreous condensation was observed in one rabbit (#9783) in the 2e11 dosing group.
At late time points (8 and 12 weeks), vitreous inflammation decreased in both dosing groups. In the 2e10 vg/eye group, two (#3318 and #5611) of the six animals that had showed vitreous alterations at early time points were sacrificed at 4 weeks for histopathological analysis. The other four animals showed no sign of residual anterior or posterior vitreous alterations. In the 2e11 vg/eye dosing group, three (#9321, #9263, and # 1055) of the six rabbits that had showed vitreous alterations at early time points were sacrificed at 4 weeks for histopathological examination. Of the remaining three animals, two (#5633 and #0852) showed no signs of posterior vitreous condensation at 12 weeks, while one (#9783) showed a resolution of optic disc edema at 8 weeks even though traces of vitreous condensation were still detectable at 12 weeks. One rabbit (#9861) showed no inflammatory changes during the 12-week follow-up period, except for the late onset of trace of anterior vitreous inflammation at 8 weeks that was not observed at 12 weeks. No animals in either dosing group showed any vitreous abnormality in the untreated eye during the 12-week period. No generalized or severe vitritis was noted in any eye at any time point.
A variable, focal, limbal conjunctival hyperemia, ranging in intensity from trace to mild, was observed at different time points (including baseline examination prior to the injection) in both the treated and untreated eyes of rabbits injected with vector solution and vehicle. This suggests that conjunctival hyperemia was neither related to the injection procedure, nor the result of an inflammatory response to the vector. No other ophthalmic abnormalities were observed in the rabbits that received an intravitreal injection of vehicle.
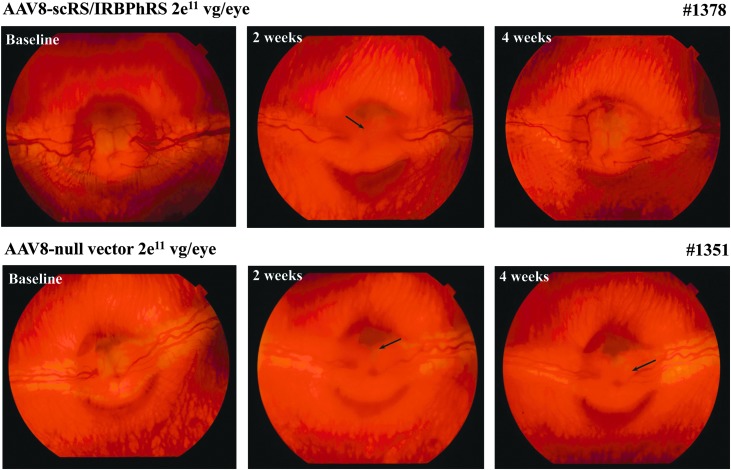
The observed ocular inflammatory response was overall mild and transient in both the low- and the high-dosing groups. Focal areas of vitreous condensation, suggestive of modest cellular infiltration, represented the major clinical finding and were primarily localized anteriorly to the optic nerve head (see fundus photos; Fig. 5). In a few animals, these extended toward the middle of the vitreous cavity, or manifested instead as mild cellular infiltration of the anterior vitreous cavity.
FIG. 5.
Ophthalmoscopic findings in rabbits intravitreally injected with 2e11 vg/eye of AAV8-scRS/IRBPhRS or AAV8-null vector, at baseline, and after 2 and 4 weeks from the injection. Two weeks after injection, both animals showed signs of a mild vitreous condensation localized on the surface of the optic nerve head (ONH) (arrows). At 4 weeks from injection, animal #1378 showed a complete resolution of the vitreous inflammatory signs. Traces of vitreous condensation were still detected at 4 weeks on the surface of the ONH of rabbit #1351 (arrow).
Histopathogical analysis
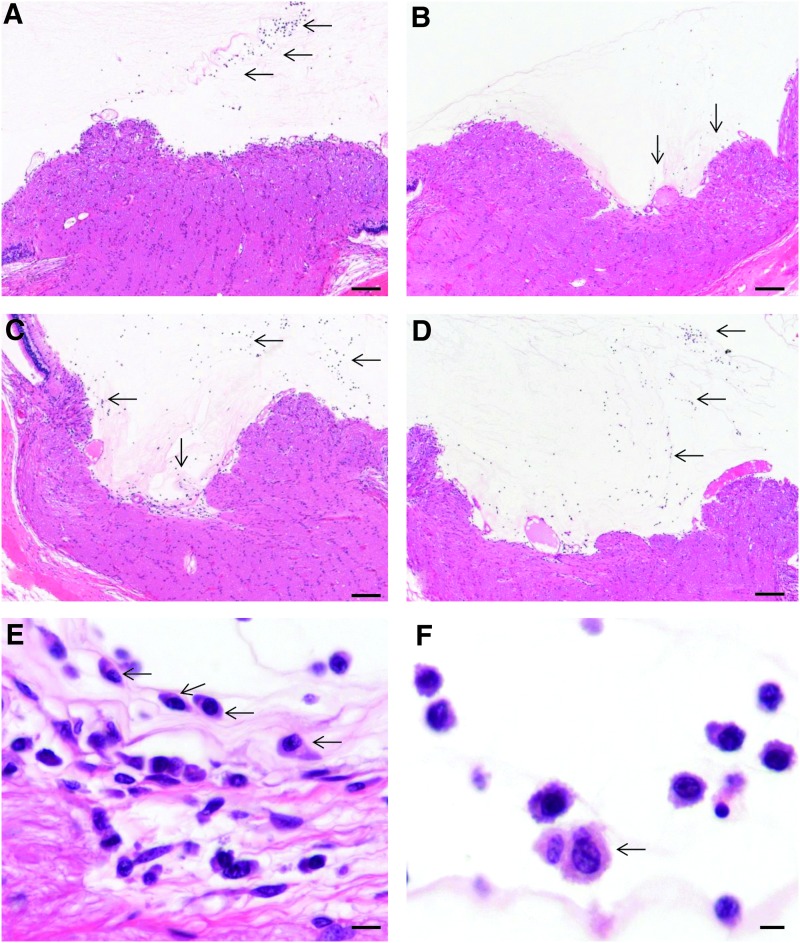
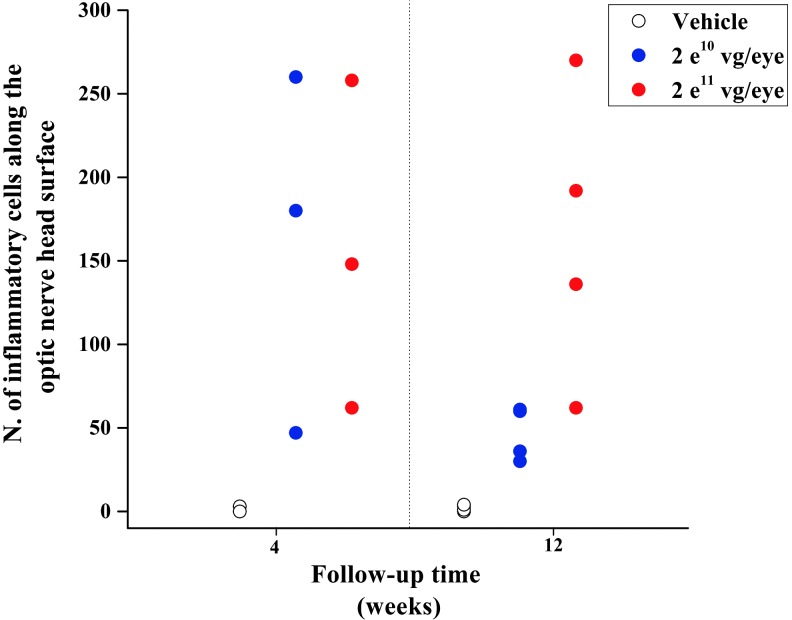
At the 4-week interim sacrifice, all rabbits in both 2e10 and 2e11 vg/eye dosing groups displayed similar histopathological changes in the injected eye. The inflammatory reaction was mild, mainly limited to the vitreous cavity, and was qualitatively and quantitatively similar between the two dose groups. It consisted of lymphocytes, macrophages, and plasma cells adjacent and adherent to the surface of the optic nerve head (Fig. 2A and C), along the margin of the peri-optic nerve inner retina and within the inferior vitreous cavity. Along the surface of the optic nerve head, where the inflammatory infiltrate was more prominent, the number of the inflammatory cells was similar between the two vector dose groups (Fig. 3). Animal #5611 in the 2e10 vg/eye dosing group and animal #9321 in the 2e11 vg/eye dosing group had rare plasma cells in the filtration angle. No other rabbit showed any sign of inflammation in the anterior segment.
FIG. 2.
Histopathological findings in rabbits intravitreally injected with AAV8-scRS/IRBPhRS. At 4 weeks after injection, a various number of inflammatory cells adhered to the optic nerve head (ONH) surface in rabbits treated with both 2e10 (A, arrows) and 2e11 (C, arrows) vg/eye doses. At 12 weeks, the number of inflammatory cells infiltrating the vitreous in front of the ONH decreased significantly in the rabbits injected with 2e10 vg/eye (B, arrows). Conversely, a number of inflammatory cells similar to that observed at 4 weeks were still detectable along the ONH in rabbits treated with the 2e11 vg/eye dose (D, arrows). Inflammatory cells near the optic nerve head consisted of plasma cells (E, arrows) with fewer lymphocytes and macrophages. Similar cell types occupied the vitreous chamber; however, large activated macrophages (F, arrow) are more prominent. Both (E) and (F) are images taken from the rabbit in (A). Hematoxylin and eosin; scale bar=100 μm (A–D); 10 μm (E); 5 μm (F).
FIG. 3.
Number of inflammatory cells infiltrating the vitreous in front of the optic nerve head at 4 and 12 weeks. Inflammatory cells were counted within an area of 2.35 mm2 centered on the optic nerve head. At 4-week follow-up, the extent of the vitreous inflammatory reaction was similar between the two vector dosing groups, as indicated by a similar number of inflammatory cells in rabbits that received AAV8-scRS/IRBPhRS at either 2e10 or 2e11 vg/eye. At 12-week follow-up, while the vitreous inflammatory infiltrate was noticeably reduced in the 2e10 vector dose group, rabbits in the 2e11 group still retained a number of inflammatory cells similar to that observed at 4 weeks.
The only other histopathological finding related to the vector injection was observed in the treated eye of one animal (#9321, in 2e11 dosing group) in which two focal areas of retinal detachment, outer nuclear layer thinning, subretinal microglial migration, and choroidal inflammation were present. Other histopathological findings observed at 4 weeks included a focal area of inner retinal swelling in the injected eye of rabbit #0775 and in the uninjected eye of rabbit #5611, both treated with the 2e10 dose.
At 12-week follow-up, the intensity of the inflammatory cell infiltrate in the vitreous of the injected eye differed between the two dosing groups (Fig. 3). Inflammation along the surface of the optic nerve head was reduced in eyes that had received a vector dose of 2e10 vg/eye when compared with 4-week follow-up (Fig. 2B). Conversely, rabbits that were injected with a 2e11 vg/eye viral dose showed a vitreous inflammatory infiltrate that was similar in intensity to that observed at 4 weeks (Fig. 2D). Unlike the rabbits that were treated with the viral vector, animals that received an injection of vehicle showed no or rare inflammatory cells (macrophages only) at the optic nerve surface and in the vitreous of their injected and uninjected eyes at both 4 and 12 weeks.
Two additional histopathological findings observed at 12 weeks were the presence of choroidal inflammation and focal areas of photoreceptor loss. These findings did not segregate clearly with vector injection and were noted in both the injected and uninjected eyes of rabbits treated with either 2e10 or 2e11 vg/eye dose, as well as in animals that received a vehicle injection. Various degrees of choroidal inflammation, consisting of nests of heterophils mixed with plasma cells, macrophages, and lymphocytes, were observed in rabbits injected with both vector doses. In the 2e10 vg/eye dosing group, this change was present in the injected eyes of two rabbits (#5087 and #8844) and in the uninjected eyes of two other rabbits (#4544 and #6514), while in the 2e11 vg/eye dosing group it was observed in both eyes of animal #0852 and in the uninjected eye of rabbit #5633. Similarly, focal areas of photoreceptor atrophy were observed in both the injected and uninjected eyes of rabbits that were treated with either 2e10 or 2e11 vg/eye dose. Focal areas of photoreceptor loss were observed in the inferior retina of both treated and untreated eyes of animal #4544, in the injected eye of animal #0852, and in the uninjected eye of animal #5633.
Similar histopathological alterations were observed in rabbits treated with vehicle injection. Specifically, signs of choroidal inflammation were observed at 4 weeks in the injected eye of animal #2126, while focal areas of photoreceptor atrophy were observed at 12 weeks in both eyes of one animal (#0013) and in the uninjected eye of two animals (#5892 and #6582).
Four-week null-vector study
Intravitreal injection AAV8-null vector resulted in an intraocular inflammatory response similar to those observed in the AAV8-scRS/IRBPhRS treated animals
Similar to the 12-week study, the most relevant clinical finding in this group of animals at 2 and 4 weeks after intravitreal vector delivery was the presence of posterior vitreous condensation, accompanied by retrolental cells infiltration and/or vitreous condensation (Fig. 4 and Fig. 5). Notably, no significant differences, in location or intensity of the inflammatory response, were observed between the animals that received an injection of AAV8-scRS/IRBPhRS or those that were injected with the AAV8-null vector. Both groups of animals showed inflammatory changes in the anterior vitreous that ranged in intensity from trace to mild. Similarly, a posterior vitreous condensation of analogous intensity ranging from trace to mild was observed in five out of six rabbits in each treatment group.
FIG. 4.
Ophthalmological findings in rabbits intravitreally injected with AAV8-scRS/IRBPhRS at 2e11 vg/eye, AAV8-null vector at 2e11 vg/eye, and vehicle. Findings from both the injected (inj.) and uninjected (uninj.) eyes are displayed for each animal at 3 time points over a 4-week period. Clinical changes were graded at five levels of severity.
Consistently with the clinical findings, 1-month histopathological analysis revealed mild ocular inflammatory changes in rabbits that received an injection of either AAV8-null vector or AAV8-scRS/IRBPhRS. Inflammation was mainly limited to the posterior segment and was characterized by accumulation of macrophages, lymphocytes, and plasma cells adherent to the surface of the optic disc and by small numbers of macrophages and plasma cells spreading into the inferior vitreous. The number of inflammatory cells along the optic nerve head surface was similar between the two treatment groups. Except for rabbit #1351, which had a focal area of subacute to chronic optic neuritis in the eye treated with AAV8-scRS/IRBPhRS, no other animals had an inflammatory infiltrate in the optic nerve substance.
Subtle inner retinal changes consisting of cytoplasmic eosinophilic material prolapsing through a periodically disrupted inner limiting membrane were evident in all the injected eyes in both treatment groups. Similar cytoplasmic swelling and accumulation of eosinophilic material often surrounded neurons or occurred in the nerve fiber layer. The same degenerative alteration was, however, observed in the rabbits treated with vehicle, both in the injected eye (#2258 and #3378) as well as in the untreated one (#3378 and #7610), and in the untreated eye of two animals (#1351 and #6578) that received either AAV8-scRS/IRBPhRS or the null vector. One rabbit treated with AAV8-scRS/IRBPhRS (#1378) had a focal area of photoreceptor loss in the injected eye. The same alteration was, however, observed also in the treated eye of a rabbit that received a vehicle injection (#2566).
As both clinical and histopathological examinations showed a similar inflammatory response between the two groups and as the AAV8 capsid is the major common immunogenic component between the two vectors, it is therefore reasonable to consider that the capsid itself was the primary reason for the observed inflammation.
Intravitreal injection of either AAV8-scRS/IRBPhRS or AAV8-null vector produced a systemic humoral immune against the viral capsid
At 4 weeks from injection, high titers of antibodies to the AAV8 viral capsid were detected in the serum of animals treated with either AAV8-scRS/IRBPhRS or AAV8-null vector, showing the onset of a systemic humoral immune response against the viral vector. No AAV8 capsid antibodies were found in the serum of rabbits treated with vehicle injection (Table 2).
Table 2.
Titer of AAV8 Neutralizing Antibodies in Rabbit Sera
| Rabbit No. | Treatment | Before treatment | After treatment |
|---|---|---|---|
| 2258 | Vehicle | <100 | <100 |
| 2348 | Vehicle | <100 | <100 |
| 2566 | Vehicle | <100 | <100 |
| 3378 | Vehicle | <100 | <100 |
| 5530 | Vehicle | <100 | <100 |
| 7610 | Vehicle | <100 | <100 |
| 6065 | AAV8-scRS/IRBPhRS | <100 | 800 |
| 1378 | AAV8-scRS/IRBPhRS | <100 | 1600 |
| 7886 | AAV8-scRS/IRBPhRS | <100 | 1600 |
| 1351 | AAV8-scRS/IRBPhRS | <100 | >3200 |
| 6638 | AAV8-scRS/IRBPhRS | <100 | >3200 |
| 6578 | AAV8-scRS/IRBPhRS | <100 | >3200 |
| 1885 | AAV8-null vector | <100 | 800 |
| 9122 | AAV8-null vector | <100 | 1600 |
| 5336 | AAV8-null vector | <100 | 3200 |
| 8555 | AAV8-null vector | <100 | 3200 |
| 2276 | AAV8-null vector | <100 | >3200 |
| 5092 | AAV8-null vector | <100 | >3200 |
Prevalence of neutralizing antibody to AAV8 in rabbit serum. Serum samples from rabbits treated with AAV8-scRS/IRBPhRS at 2e11 vg/eye, AAV8-null vector at 2 e11 vg/eye, or vehicle were collected on preinjection day and 30 days after injection, and were tested using a luciferase assay system. The luciferase activity was measured in 293 cells transduced with AAV-CMV-Luc in the absence (negative control) or presence of rabbit serum samples (dilution range 1:100–1:3200). The neutralization titer for each serum sample was defined as the serum dilution at which the relative light unit (RLU) was reduced by 50% compared with positive control wells after subtraction of the background RLU in nonvirus control wells. Serum from rabbits that were administered AAV8-scRS/IRBPhRS and AAV8-null vector showed both high titers, thus indicating the presence of antibodies to AVV8 capsid.
Conclusions
Our results showed that the intravitreal administration of the AAV8-scRS/IRBPhRS vector into the rabbit eye induced a vitreous inflammation that developed and reached an observable maximum intensity within 4 weeks. Clinical manifestations consisted mainly of localized areas of mild vitreous condensation on the surface of the optic nerve head or in the central vitreous cavity, associated in some animals to mild signs of anterior vitreous involvement. The histopathological analysis confirmed the clinical findings, showing mixed infiltration of lymphocytes, macrophages, and plasma cells in proximity to the optic nerve head and in the inferior vitreous cavity.
The finding of similar levels of inflammation in animals injected with the AAV8-null vector and in those that received the AAV8-scRS/IRBPhRS suggests that it may be the AAV8 capsid, and not the expression of the RS1 protein, the causative agent for the observed ocular immune response. When comparing the two dosing groups at the 2- and 4-week time points, both clinical and histopathological analysis showed no significant difference in the intensity of the inflammatory response between rabbits that received a dose of 2e10 or 2e11 vg/eye. At 12 weeks both dosing groups showed an overall resolution of the vitreous condensations that were clinically observed at early time points. However, while rabbits that received a 2e10 vg/eye dose showed a decrease of the inflammatory infiltrate, rabbits treated with the higher dose still showed some persistence of an immune cell infiltrate at the optic nerve head and in the inferior vitreous cavity. These findings suggest that an increase of the viral dose above 2e10 vg/eye may not result in a corresponding augmentation in the intensity of inflammatory response, but may contribute to a greater duration of the vitreous inflammation, possibly related to the persistence of the vector in the vitreous.
It is relevant to note that except for one rabbit in the higher dose group, that is, 2e11 vg/eye, that displayed two focal areas of retinal detachment, not appreciated on clinical examination, no other animal showed any sign of tissue damage potentially related to the vector administration. Other histological findings, such as inner retinal swelling or areas of photoreceptor atrophy, were observed also in eyes that were untreated or that received a vehicle injection, and therefore are very unlikely to be a consequence of the vector administration.
The choice to use two different vector doses, 2e10 and 2e11 vg/eye, derives from previous unpublished efficacy studies in Rs1-KO mice that were treated with an AAV8 viral vector expressing RS1. A dose of 2e10 vg/eye (when scaled to the mouse eye based on the retinal surface area and vitreous volume) was the minimum dose that proved to be effective in recovering the retinal function in the Rs1-KO mouse model. The reason for testing a viral vector dose of 2e11 vg/eye, 10-fold higher than the minimum effective dose, was to assess the potential ocular toxicity of AAV8 with a greater safety margin.
Although data on the safety of AAV-mediated gene transfer in animal models have been reported (Stieger et al., 2009), this is the first study to describe the ocular response after the intravitreal injection of an AAV8 vector in a large animal eye. Safety studies on AAV8 were previously conducted in animals that received an injection of the vector in the subretinal space. In nonhuman primates, the subretinal administration of an AAV8 vector expressing GFP was well tolerated at doses lower than 1011 vg/eye. Retinal infiltrates and retinal degeneration were reported only in some of the animals injected with a dose of 1011 genome copies, and were likely caused by the immune responses to the specific transgene and by retinal bleeding during the injection procedure (Vandenberghe et al., 2011). Another study on dogs and rats reported dissemination of vector genomes to the brain, and transgene expression in the retina and in the distal neurons of the geniculate nucleus following subretinal injection of an AAV2/8 vector driven by the CMV promoter (Stieger et al., 2008). In our study we did not investigate the biodistribution of AAV8-scRS/IRBPhRS. However, as retinoschisin is only expressed in the retina and pineal gland (Takada et al., 2006), and the vector we used in this study encodes the gene-specific retinoschisin promoter, transgene expression outside these areas is unlikely.
Intravitreal administration for gene therapy offers different advantages over the subretinal delivery. Besides the possibility of introducing a larger volume of viral vector, the intravitreal administration is a less invasive and risky technique, a factor that may be particularly important in the setting of offering gene transfer to patients with XLRS, in which the retina is known to be fragile and predisposed to retinal detachment and hemorrhage. A recent study (Igarashi et al., 2013) comparing different routes of intraocular administration of AAV8 showed that contrary to the intravitreal approach, mice that received a subretinal injection had reduced electroretinographic responses 3 months after the injection, supporting the potential detrimental effects of this approach on retinal function.
On the other hand, there are two main potential impediments for intravitreal delivery of an AAV vector: potential low transduction levels of the retina and possible generation of an immune response against the viral vector. Previous studies showed that among natural occurring AAVs, only serotype 2 and 8 can infect retinal cells from the vitreous and that transduction is limited to the inner retina (Lebherz et al., 2008). As a consequence, the intravitreal approach has been reserved so far to target the retinal ganglion cells rather than the outer retinal cells. A possible barrier to the virus penetration through the retinal tissue seems to be represented by the inner limiting membrane or cellular barriers (Dalkara et al., 2009). However, recently, a novel engineered capsid-modified AAV2 virus proved to transduce sufficiently photoreceptors after intravitreal delivery (Dalkara et al., 2013).
RS1 is a retinal structural protein that is predominantly expressed as a surface protein in photoreceptors and bipolar cells. In its absence, the retina of patients affected by XLRS loses its integrity and can constitute a potential target for a gene therapy based on AAV intravitreal administration. The lack of RS1 protein alters indeed the structural properties of the retina, increasing its porosity and permeability. AAVs are able to penetrate and transduce all the layers of the degenerated retinas, as it was demonstrated in Rs1-KO mice that were injected in the vitreous with AAV types 2 and 8 (Zeng et al., 2004).
The other aspect to be considered when delivering an AAV vector through the vitreous is the potential onset of an immune response. Although the vitreous is considered an immune-privileged space, there are reports that intravitreal administration of AAV vectors generates humoral immune responses against the capsid (Li et al., 2008). A possible explanation could be the proximity of the vitreous to the vascular system, through which vector antigens can be presented to the immune system. In addition, there is evidence that the resident cells in the vitreous, the hyalocytes, may have an antigen-presenting ability, and are therefore able to trigger an inflammatory response when activated by foreign antigens (Sonoda et al., 2005). The presence of a natural immunity against the viral vector can further exacerbate the immune response against the virus and block its expression. It has been demonstrated indeed that even low levels of neutralizing antibodies can completely halt transduction with high titers of vectors (Jiang et al., 2006; Scallan et al., 2006).
Recently, an AAV2 vector expressing sFLT01, an anti-vascular endothelial growth factor for the treatment of age-related macular degeneration, was tested in cynomolgus monkeys. When administered into the vitreous, AAV2-sFLT01 was well tolerated, as it produced a moderate but self-resolving vitreal inflammation (MacLachlan et al., 2011). However, a preexisting humoral immunity against AAV2 is present in almost 60% of the human population (Boutin et al., 2010), an aspect that could limit significantly the efficacy of AAV2 when delivered directly into the human vitreous. In this respect, AAV8 could represent a valid alternative. Deriving from a nonhuman primate, the sero-prevalence of neutralizing antibodies against AAV8 in the human population is 18%, much lower when compared with AAV2 (Boutin et al., 2010).
Since a large-eye animal model with retinoschisis is not currently available, we characterized the ocular response to the AAV8 vector using a wild-type rabbit. It is known that the wild-type retina is not transduced to the same extent as the retinoschisin knockout mouse by the AAV8-RS1 vector (Park et al., 2009). This suggests that the disruption of the retinal architecture in an animal or a human with retinoschisis affects the distribution of the vector in the retina, and may also influence the extent or nature of the ocular response.
In conclusion, this pilot study shows that the administration of an AAV8 vector into the vitreous of the rabbit eye at a 2e10 vg/eye dose is a well-tolerated procedure that produces a mild and transient intraocular inflammatory state and does not lead to any irreversible tissue damage to the injected eyes. Together with the evidence of efficient and retinal-specific expression of retinoschisin and restoration of retinal function in an XLRS mouse model, the results of this study further support intravitreal injection of AAV8 as a gene therapy approach to XLRS disease.
For Materials and Methods section, see Supplementary Data (available online at www.liebertpub.com/humc).
Supplementary Material
Acknowledgments
We thank Dr. Ginger Tansey, Bunea Irina, and Parker Denise of Veterinary Research and Resources Section of National Eye Institute for their technical assistance with the animals.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Deafness and Other Communication Disorders, and the National Eye Institute.
Author Disclosure Statement
None of the authors have any actual or potential conflicts of interest related to this study.
References
- Albini T.A., et al. (2007). Long-term retinal toxicity of intravitreal commercially available preserved triamcinolone acetonide (Kenalog) in rabbit eyes. Invest. Ophthalmol. Vis. Sci. 48, 390–395 [DOI] [PubMed] [Google Scholar]
- Bainbridge J.W., et al. (2008). Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358, 2231–2239 [DOI] [PubMed] [Google Scholar]
- Bakay R., et al. (2007). Analyses of a phase 1 clinical trial of adeno-associated virus-nerve growth factor (CERE-110) gene therapy in Alzheimer's disease. Neurosurgery 61, 216 [Google Scholar]
- Berger W., Kloeckener-Gruissem B., and Neidhardt J. (2010). The molecular basis of human retinal and vitreoretinal diseases. Prog. Retin. Eye Res. 29, 335–375 [DOI] [PubMed] [Google Scholar]
- Boutin S., et al. (2010). Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 21, 704–712 [DOI] [PubMed] [Google Scholar]
- Cideciyan A.V., et al. (2008). Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. USA 105, 15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D., et al. (2009). Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 17, 2096–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D., et al. (2013). In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 189, 189ra76. [DOI] [PubMed] [Google Scholar]
- Dierks D., Lei B., Zhang K., and Hainsworth D.P. (2005). Electroretinographic effects of an intravitreal injection of triamcinolone in rabbit retina. Arch. Ophthalmol. 123, 1563–1569 [DOI] [PubMed] [Google Scholar]
- Gao G.P., et al. (2002). Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA. 99, 11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genead M.A., Fishman G.A., and Walia S. (2010). Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with X-linked retinoschisis. Arch. Ophthalmol. 128, 190–197 [DOI] [PubMed] [Google Scholar]
- George N.D., Yates J.R., and Moore A.T. (1995). X linked retinoschisis. Br. J. Ophthalmol. 79, 697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajarnia M., and Gorin M.B. (2007). Acetazolamide in the treatment of X-linked retinoschisis maculopathy. Arch. Ophthalmol. 125, 571–573 [DOI] [PubMed] [Google Scholar]
- Grayson C., et al. (2000). Retinoschisin, the X-linked retinoschisis protein, is a secreted photoreceptor protein, and is expressed and released by Weri-Rb1 cells. Hum. Mol. Genet. 9, 1873–1879 [DOI] [PubMed] [Google Scholar]
- Grimm D., et al. (2003). Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood 102, 2412–2419 [DOI] [PubMed] [Google Scholar]
- Igarashi T., et al. (2013). Direct comparison of administration routes for AAV8-mediated ocular gene therapy. Curr. Eye Res. 38, 569–577 [DOI] [PubMed] [Google Scholar]
- Jiang H., et al. (2006). Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 108, 3321–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Y., Zemel E., Miller B., and Perlman I. (2007). Retinal toxicity of intravitreal kenalog in albino rabbits. Retina 27, 778–788 [DOI] [PubMed] [Google Scholar]
- Lebherz C., et al. (2008). Novel AAV serotypes for improved ocular gene transfer. J. Gene Med. 10, 375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., et al. (2008). Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol. Vis. 14, 1760–1769 [PMC free article] [PubMed] [Google Scholar]
- MacLachlan T.K., et al. (2011). Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol. Ther. 19, 326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren R.E., et al. (2014). Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 383, 1129–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M., et al. (2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano R.P., et al. (2008). Ocular toxicity of intravitreous adalimumab (Humira) in the rabbit. Graefes Arch. Clin. Exp. Ophthalmol. 246, 907–911 [DOI] [PubMed] [Google Scholar]
- Marks W.J. Jr., et al. (2010). Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 9, 1164–1172 [DOI] [PubMed] [Google Scholar]
- Molday L.L., et al. (2001). Expression of X-linked retinoschisis protein RS1 in photoreceptor and bipolar cells. Invest. Ophthalmol. Vis. Sci. 42, 16–25 [PubMed] [Google Scholar]
- Nathwani A.C., et al. (2011). Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T.K., et al. (2009). Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther. 16, 916–926 Erratum in: Gene Ther. 16, 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan C.D., et al. (2006). Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 107, 1810–1817 [DOI] [PubMed] [Google Scholar]
- Sieving P.A., MacDonald I.M., Meltzer M.R., and Smaoui N. (2009). X-linked juvenile retinoschisis. In GeneReviews® [Internet]. Pagon R.A., Adam M.P., Ardinger H.H., et al., eds. (University of Washington–Seattle, Seattle, WA: ), pp. 1993–2014 [Google Scholar]
- Sonoda K.H., et al. (2005). The analysis of systemic tolerance elicited by antigen inoculation into the vitreous cavity: vitreous cavity-associated immune deviation. Immunology 116, 390–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger K., et al. (2008). Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain. Mol. Ther. 16, 916–923 [DOI] [PubMed] [Google Scholar]
- Stieger K., Lhériteau E., Moullier P., and Rolling F. (2009). AAV-mediated gene therapy for retinal disorders in large animal models. ILAR J. 50, 206–224 [DOI] [PubMed] [Google Scholar]
- Streilein J.W. (2003). Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 11, 879–889 [DOI] [PubMed] [Google Scholar]
- Takada Y., et al. (2004). A retinal neuronal developmental wave of retinoschisin expression begins in ganglion cells during layer formation. Invest. Ophthalmol. Vis. Sci. 45, 3302–3312 [DOI] [PubMed] [Google Scholar]
- Takada Y., et al. (2006). Retinoschisin expression and localization in rodent and human pineal and consequences of mouse RS1 gene knockout. Mol. Vis. 12, 1108–1116 [PubMed] [Google Scholar]
- Vandenberghe L.H., et al. (2011) Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci. Transl. Med. 3:88ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., et al. (2005). Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 23, 321–328 [DOI] [PubMed] [Google Scholar]
- Wu W.W., Wong J.P., Kast J., and Molday R.S. (2005). RS1, a discoidin domain-containing retinal cell adhesion protein associated with X-linked retinoschisis, exists as a novel disulfide-linked octamer. J. Biol. Chem. 280, 10721–10730 [DOI] [PubMed] [Google Scholar]
- Zeng Y., et al. (2004). RS-1 gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Invest. Ophthalmol. Vis. Sci. 45, 3279–3285 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.