Abstract
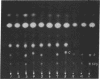
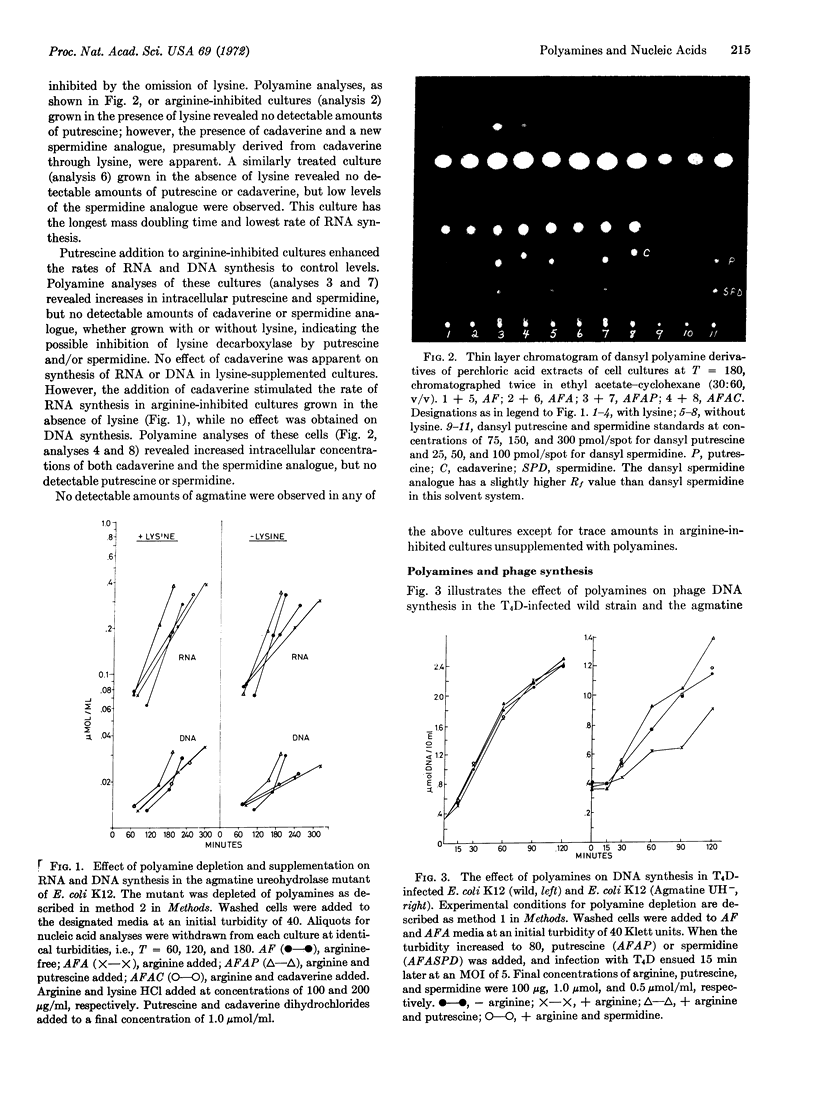
The addition of arginine to cultures of Escherichia coli K12 deficient in agmatine ureohydrolase (EC 3.5.3.7) results in polyamine depletion and a striking inhibition of nucleic acid accumulation and growth. The omission of lysine from these cultures leads to a further decrease in growth rate and nucleic acid synthesis. In arginine-inhibited cells the addition of putrescine or spermidine, in the presence or absence of lysine, restores the control rate of growth and nucleic acid accumulation. Under the same conditions of arginine inhibition in the absence of lysine, the addition of cadeverine alone stimulates growth rate and RNA synthesis. The addition of lysine to polyamine-depleted cultures results in cadaverine production and in the appearance of a new spermidine analogue, containing lysine carbon. The new compound has been identified as N-3-aminopropyl-1,5-diaminopentane.
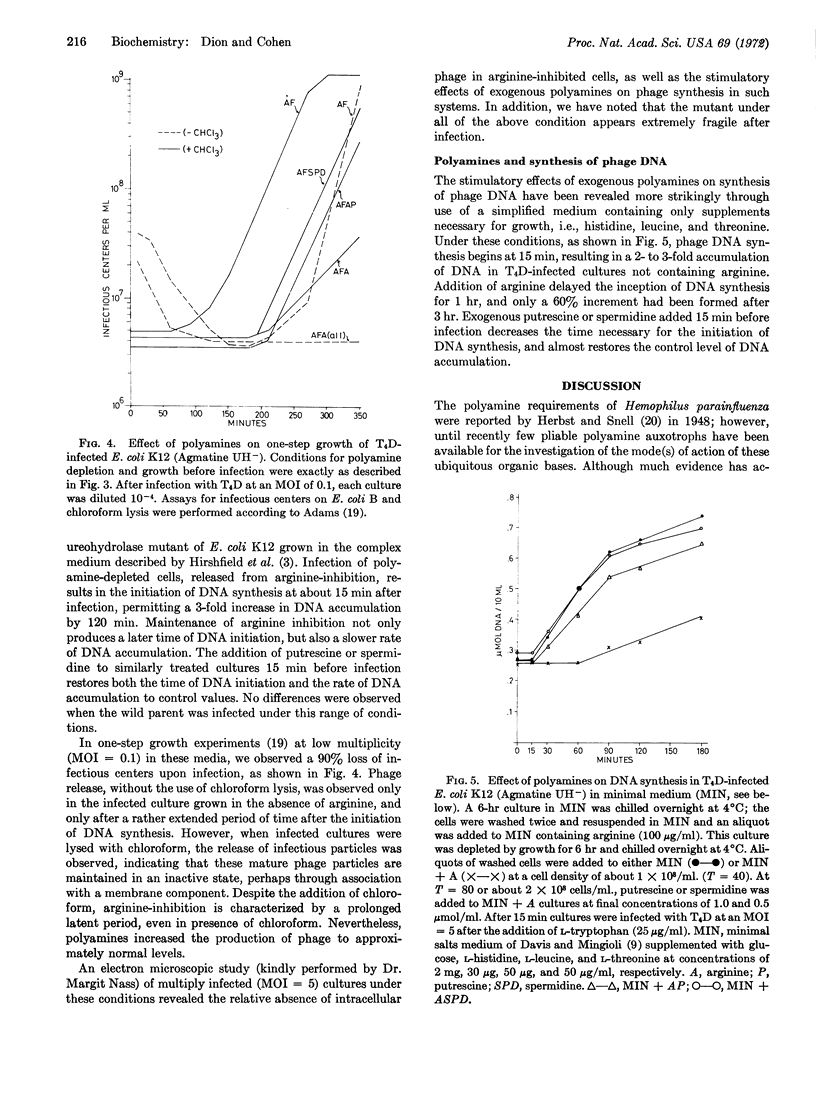
Infection of this arginine-inhibited, polyamine-depleted mutant with T4D results in markedly decreased amounts of DNA accumulation, as compared to infected cells uninhibited by arginine. Supplementation of arginine-inhibited infected cells by putrescine or spermidine restores DNA synthesis to the uninhibited level.
Keywords: arginine, agmatine ureohydrolase, putrescine, spermidine, lysine, cadaverine
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T., ROSENTHAL S. M. Presence of polyamines in certain bacterial viruses. Science. 1958 Apr 11;127(3302):814–815. doi: 10.1126/science.127.3302.814-a. [DOI] [PubMed] [Google Scholar]
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BROCK M. L. THE EFFECTS OF POLYAMINES ON THE REPLICATION OF T4RII MUTANTS IN ESCHERICHIA COLI K-12 (LAMBDA). Virology. 1965 Jun;26:221–227. doi: 10.1016/0042-6822(65)90049-8. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer S. V., Kosuge T. Spermidine and spermine--polyamine components of turnip yellow mosaic virus. Virology. 1970 Apr;40(4):930–938. doi: 10.1016/0042-6822(70)90139-x. [DOI] [PubMed] [Google Scholar]
- Buller C. S., Astrachan L. Replication of T4rII bacteriophage in Escherichia coli K-12 (lambda). J Virol. 1968 Apr;2(4):298–307. doi: 10.1128/jvi.2.4.298-307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D. Some minor components of bacteriophage T2 particles. Virology. 1957 Oct;4(2):237–264. doi: 10.1016/0042-6822(57)90061-2. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Rosenfeld H. J., Leifer Z., Maas W. K. Isolation and characterization of a mutant of Escherichia coli blocked in the synthesis of putrescine. J Bacteriol. 1970 Mar;101(3):725–730. doi: 10.1128/jb.101.3.725-730.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L. Urea production and putrescine biosynthesis by Escherichia coli. J Bacteriol. 1967 Nov;94(5):1516–1519. doi: 10.1128/jb.94.5.1516-1519.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. A biosynthetic ornithine decarboxylase in Escherichia coli. Biochem Biophys Res Commun. 1965 Sep 22;20(6):697–702. doi: 10.1016/0006-291x(65)90072-0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Olenick J. G., Hahn F. E. Reactions of quinine, chloroquine, and quinacrine with DNA and their effects on the DNA and RNA polymerase reactions. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1511–1517. doi: 10.1073/pnas.55.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer S. Differential effects of putrescine, cadaverine, and glyoxal-bis-(guanylhydrazone) ON DNA- and nucleothistone-supported DNA synthesis. Biochim Biophys Acta. 1968 Aug 23;166(1):251–254. doi: 10.1016/0005-2787(68)90510-8. [DOI] [PubMed] [Google Scholar]
- Shalitin C. Selective gene transcription in bacteriophage T4 by putrescine. J Virol. 1967 Jun;1(3):569–575. doi: 10.1128/jvi.1.3.569-575.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]