Highlights
-
•
Dealing with acute pancreatitis in pregnancy is a challenging problem.
-
•
Even in the presence of reassuring NST and biophysical profile assessment, an unpredictable fetal loss can occur during the medical management of the pregnancies complicated with mild acute pancreatitis.
-
•
Acute pancreatitis (AP) is a potentially life threatening inflammatory condition of the pancreas with a high mortality and morbidity rates.
-
•
We report a complicated case of mild acute pancreatitis induced by gallbladder sludge in a pregnant woman whose pregnancy ended up with unexpected fetal demise at 34 weeks of her gestation.
Abbreviations: AP, acute pancreatitis; ICU, intensive care unit; NST, nonstress test
Keywords: Acute pancreatitis, Unexpected fetal demise, Pregnancy complication
Abstract
INTRODUCTION
Dealing with acute pancreatitis in pregnancy is a challenging problem due to unexpected nature of the disease.
PRESENTATION OF CASE
We report a complicated case of a 29-year-old pregnant woman with a mild acute pancreatitis whose pregnancy ended up with an unexpected fetal demise at her 34th gestational week. This unfortunate outcome led us reconsider our obstetrical approach to acute pancreatitis during pregnancy.
CONCLUSION
Based on this unfortunate event, we now think that obstetricians should keep in mind that even in the presence of reassuring NST and biophysical profile assessment, an unpredictable fetal loss can occur during the medical management of the pregnancies complicated with mild acute pancreatitis.
DISCUSSION
The subject patient of this case report was diagnosed with mild AP and underwent conservative medical management. Since the patient was stable and fetal well-being was confirmed with BPP and NST, the termination of pregnancy was out of question at that time. The occurrence of unexpected fetal death despite assuring parameters led us reconsider the approach to the pregnant women with mild AP.
1. Introduction
Acute pancreatitis (AP) is a potentially life threatening inflammatory condition of the pancreas with a high mortality and morbidity rates. While the annual incidence of AP is 5–80 per 100,000 of the general population,1 the incidence of AP in pregnancy varies between 1 in 1000 and 3 in 10,000 births.2,3 The spectrum of AP in pregnancy ranges from a mild self-limiting condition to severe rapidly progressive disease associated with pancreatic abscesses, necrosis, multiple organ dysfunction as well as poor fetal and maternal outcomes.4 Herein we report a complicated case of mild acute pancreatitis induced by gallbladder sludge in a pregnant woman whose pregnancy ended up with unexpected fetal demise at 34 weeks of her gestation, despite the fact that she had reactive nonstress test (NST) described in the guideline5 3 h prior to fetal death, and we discuss the management of this condition during pregnancy.
2. Presentation of case
A 29-year-old primigravida was admitted to emergency department at the 34th gestational week complaining of upper abdominal pain lasting more than a week, radiating to the right flank and back. The pain was accompanied by nausea and vomiting. Her blood analysis showed white blood cell count: 7.12 K/μL (reference interval (RI) 4.3–1.3 K/μL), hemoglobin: 14.5 g/dL (RI: 13.6–17.2 g/dL), platelet: 249 K/μL(RI: 156–373 K/μL), aspartate transaminase: 86 U/L (RI: 0–32 U/L), alanine transaminase: 108 U/L (RI: 0–32 U/L), total bilirubin: 0.7 mg/dL (RI: 0–1.1 mg/dL), direct bilirubin: 0.437 mg/dL (RI: 0–0.3 mg/dL), potassium 2.87 mmol/L (RI: 3.3–5.4 mmol/L), calcium: 9 mmol/L (RI: 8.6–10.2 mmol/L), gamma-glutamil transferase: 47 U/L (RI: 8–51 U/L), amilase: 188 U/L (RI: 25–100 U/L), lipase: 291 U/L (RI: 13–50 U/L), C-reactive protein (CRP): 7.98 mg/dL (0–0.8 mg/dL). Other blood parameters were observed as in normal ranges. While hepatic ultrasound was normal, gallbladder sludge was identified on biliary ultrasound. The patient was hospitalized by the department of general surgery with the diagnosis of mild acute pancreatitis according to the Ranson criteria. At the time of hospitalization, vital signs of the patient were detected as stable and fever was not significant. Obstetrical assessment of the patient revealed 34 weeks pregnancy with excellent biophysical profile (BPP: 10), reactive fetal nonstress test (NST). NST was planned to be performed three times a day and assessment of the test was made by obstetrics clinics. Medical treatment of the patient was performed accordingly by department of general surgery. She was evaluated in obstetrics clinic for routine daily NST. NST was interpreted as reactive without uterine contractions. The regular follow-up procedures were maintained for 3 days with no observed deterioration in general conditions and laboratory results of the patient.
Three hours after her daily assessment on the third day of her hospitalization, the condition of the patient suddenly started to deteriorate. An emergency cesarean section was performed due to continuing deterioration of her clinical and biochemical parameters. The 2300 g male fetus was delivered dead with conspicuous generalized peeling skin lesions (Picture 1) which were especially located on his neck. Generalized ecchymotic lesions were also visualized on his skin. The accumulation of 3000 cc of intra-abdominal yellowish green ascitic fluid was drained during the surgery. Intestines, ovaries and the uterus were observed as edematous and congested with scattered necrotic lesions on them. Operation was ended after the participation of the general surgeon. The patient was transferred to the intensive care unit (ICU) for postoperative follow up. Postmortem assessment of the fetus did not reveal any major congenital anomalies and signs of infection related demise. Pathological investigation of the fetus confirmed that skin lesions were occurred as a result of pancreatic lytic enzymes released into the circulation. No signs of infection related to fetal demise were detected during the post-mortem examination of fetus. The patient stayed in the ICU for 36 days and later she was discharged 40 days after admission to the hospital.
Picture 1.
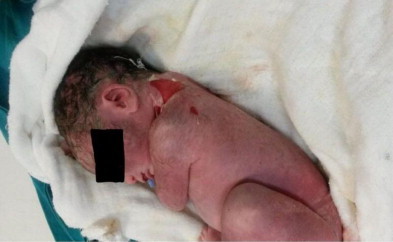
A dead fetus with scalded skin lesions on his neck and back.
3. Discussion
Despite the excellent biophysical profile performed 3 days ago, and the reactive NST maximum 3 h prior to intrauterine fetal death, unexpected antepartum stillbirth occurred at 34 weeks of gestation. The patient was diagnosed as having mild pancreatitis and treatment was made accordingly by department of general surgery. This shocking outcome let us reconsider the management of mild acute pancreatitis in pregnancy. Acute pancreatitis in pregnancy is first described in 1818,6 and the management of this disease during the pregnancy has been the subject of obstetrics care of high risk pregnancies due to challenging nature of the disease, and maternal and fetal considerations.
Cholelithiasis is the most common cause of AP during pregnancy, with an incidence of 2.5–4.2%.7 While 40–66% of acute pancreatitis in pregnancy occurs as a result of gallstone or biliary sludge which was considered as having better prognosis than the non-biliary pancreatitis,8 10–50% of AP in pregnancy is related to hypertriglyceridemia.9 In this group pre-existing abnormalities in lipid metabolism usually accompany to AP.10 Hypertriglyceridemia induced AP in pregnancy is tend to be more severe with an increased risk of concomitant complications (multiple organ failure, shock, infections).11,12 Idiopathic reasons, alcohol consumption, pre-eclampsia, and the use of certain drugs such as tetracycline, thiazides (not common in pregnancy) are responsible for the rest of the AP cases in pregnancy.8,13
Clinical manifestations of AP include, anorexia, nausea, vomiting, and midepigastric pain, left upper quadrant pain radiating to the back, decreased bowel sounds, fever, and associated pulmonary findings. Diagnosis of AP is supported by increased serum amylase and lipase, as well as triglyceride levels, calcium levels, and a complete blood count. Serum amylase level is usually considered as a reliable marker of AP in pregnant women. However, patients with necrotic pancreatitis, or hypertriglyceridemia induced AP may have normal serum levels of serum amylase, and this result can be interpreted as not having AP in pregnancy. After diagnosing of AP in pregnancy, certain tests such as blood tests, urine tests, and imaging methods should be performed in order to determine the severity of the diseases which will totally change the way of approaching to the patient. The goal of the treatment in AP is to maintain both maternal and fetal well-being.
Conservative medical management of pancreatitis can be summarized as intravenous fluids, fat restriction with total parenteral nutrition, total parenteral nutrition if necessary, nasogastric suctioning, bowel rest, use of analgesics, antispasmodics, and antibiotics, management of underlying cause.14
In this current case report, amylase and lipase levels were mildly increased, and serum triglyceride, calcium levels were in normal ranges. Hepatobiliary ultrasound revealed nothing rather than biliary sludge. The patient did not give any history of any kind of disease which predisposed AP.
Several studies have demonstrated the relationship between biliary sludge and AP.19–21 The mechanism for biliary sludge causing acute pancreatitis can be explained as biliary and pancreatic sphincter dysfunction by mechanical irritation of microcrystals and subsequent inflammation.
The patient subject to this case report, was classified as having mild AP and conservative medical management was made subsequently. Since the patient was stable and fetal well-being was confirmed with BPP and NST, the termination of pregnancy was out of question at that time. This unexpected event what fetal death was occurred, despite those assurance parameters, let us reconsider the approach to the pregnant woman with mild AP. The most widely cited causes of fetal demise in AP are severe metabolic disturbance, acidosis and placental abruption. However, none of these conditions was observed in our patient. Furthermore, the male fetus demonstrated generalized ecchymotic lesions on his skin, and scattered skin peeling especially located on his neck. These skin lesions were considered as a sign of affected fetus, despite having mild AP.
The majority of cases with mild AP in pregnancy can be cured by the conventional therapy with a good prognosis. But the requirement of surgery in this group remains controversial. Severe AP is considered as having pancreatic abscess, necrosis, infection, or deterioration of biochemical and clinical parameters despite the effective conservative medical treatment of 48–72 h.
Severe AP usually occurs in the third trimester of pregnancy, and pregnant women are more prone to developing serious condition that results in a higher risk of intrauterine fetal death.22 In this group, pregnancy must be terminated in order to prevent further damage to the mother and the fetus. Another study demonstrated that mild AP in pregnancy is not absolutely a sign for neither pregnancy termination nor cesarean section but deteriorating severe AP is.23 However, in this Chinese study they reported early pregnancy termination in pregnant woman with mild AP, since couples decided to terminate the pregnancies, due to China's national single child per couple policy.
In this study induced abortion was performed in 5 cases of 22 mild AP patients during 12–34 weeks. Cesarean section was performed in another 4 mild AP cases during 33–37 weeks due to developing severe AP. They report only one case of fetal death due to severe AP. In the study of Vilallonga et al. they identified 19 cases of AP in pregnancy without fetal losses.
They concluded that AP in pregnant women usually has a benign course with proper treatment.25
Based on the research, we can conclude that AP in pregnancy should be managed with conservative medical treatment except in pancreatic abscess, necrosis, infection, large intra-abdominal exudates, infected effusion; associated with other serious complications such as gastrointestinal perforation; the situation deteriorates after active treatment of 48–72 h.15–18,24 However, obstetricians should always keep in mind that even in the presence of reassuring NST and biophysical profile assessment, an unpredictable fetal loss may occur during the medical management of the pregnancies complicated with mild acute pancreatitis.
4. Conclusion
AP in pregnancy should be managed carefully in order to prevent further damage to the mother and the fetus.
Conflict of interest
No potential conflict of interest relevant to this article is reported.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contributions
M. Yildirim wrote the first draft of this paper. A.F. Avsar helped to revise this paper. All authors contributed to the intellectual context and approved the final version.
Key learning points.
-
•
Dealing with acute pancreatitis in pregnancy is a challenging problem due to unexpected nature of the disease. Obstetricians should keep in mind that even in the presence of reassuring NST and biophysical profile assessment, an unpredictable fetal loss can occur during the medical management of the pregnancies complicated with mild acute pancreatitis.
References
- 1.Pitchumoni C.S., Yegneswaran B. Acute pancreatitis in pregnancy. World J Gastroenterol. 2009;15:5641–5646. doi: 10.3748/wjg.15.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nanda S., Gupta A., Dora A., Gupta A. Acute pancreatitis: a rare cause of acute abdomen in pregnancy. Arch Gynecol Obstet. 2009;279:577–578. doi: 10.1007/s00404-008-0755-8. [DOI] [PubMed] [Google Scholar]
- 3.McKay A.J., O’Neill J., Imrie C.W. Pancreatitis, pregnancy and gallstones. Br J Obstet Gynaecol. 1980;87:47–50. doi: 10.1111/j.1471-0528.1980.tb04425.x. [DOI] [PubMed] [Google Scholar]
- 4.Tang S.J., Rodriguez-Frias E., Singh S., Mayo M.J., Jazrawi S.F., Sreenarasimhaiah J. Acute pancreatitis during pregnancy. Clin Gastroenterol Hepatol. 2010;8:85–90. doi: 10.1016/j.cgh.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 5.PROTOCOL, IV FETAL ASSESSMENT. “Nonstress Test (NST) Guideline.” (12/01/2007) University of Michigan Health System.
- 6.Schmitt W.J. Wimmer; Vienna, Austria: 1818. Sammlung zweifelhafter Schwangerschaftsfalle nebst einer kritischen Einleitung uber die Methode des Untersuchens, zum Gebrauche fur angehende Geburtschelfer; p. 172. [Google Scholar]
- 7.Zeng Y.L., Li L.A. Clinical analysis of 6 cases of mid-pregnancy complicated with acute pancreatitis. Chin J Obstet Gynecol Pediatr. 2007;3:96–97. [in Chinese] [Google Scholar]
- 8.Eddy J.J., Gideonsen M.D., Song J.Y., Grobman W.A., O’Halloran P. Pancreatitis in pregnancy: a 10-year retrospective of 15 midwest hospitals. Obstet Gynecol. 2008;112:1075–1081. doi: 10.1097/AOG.0b013e318185a032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewald N., Hardt P.D., Kloer H.U. Severe hypertriglyceridemia and pancreatitis: presentation and management. Curr Opin Lipidol. 2009;20:497–504. doi: 10.1097/MOL.0b013e3283319a1d. [DOI] [PubMed] [Google Scholar]
- 10.Keilson L.M., Vary C.P., Sprecher D.L., Renfrew R. Hyperlipidemia and pancreatitis during pregnancy in two sisters with a mutation in the lipoprotein lipase gene. Ann Intern Med. 1996;124:425–428. doi: 10.7326/0003-4819-124-4-199602150-00007. [DOI] [PubMed] [Google Scholar]
- 11.Tsuang W., Navaneethan U., Ruiz L., Palascak J.B., Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol. 2009;104:984–991. doi: 10.1038/ajg.2009.27. [DOI] [PubMed] [Google Scholar]
- 12.Deng L.H., Xue P., Xia Q., Yang X.N., Wan M.H. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008;14:4558–4561. doi: 10.3748/wjg.14.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badja N., Troché G., Zazzo J.F., Benhamou D. Acute pancreatitis and preeclampsia–eclampsia: a case report. Am J Obstet Gynecol. 1997;176:707–709. doi: 10.1016/s0002-9378(97)70574-x. [DOI] [PubMed] [Google Scholar]
- 14.Swisher S.G., Hunt K.K., Schmit P.J., Hiyama D.T., Bennion R.S., Thompson J.E. Management of pancreatitis complicating pregnancy. Am Surg. 1994;60:759–762. [PubMed] [Google Scholar]
- 15.Pezzilli R., Zerbi A., Di Carlo V., Bassi C., Delle Fave G.F. Practical guidelines for acute pancreatitis. Pancreatology. 2010;10:523–535. doi: 10.1159/000314602. [DOI] [PubMed] [Google Scholar]
- 16.Hasibeder W.R., Torgersen C., Rieger M. Critical care of the patient with acute pancreatitis. Anaesth Intensive Care. 2009;37:190–206. doi: 10.1177/0310057X0903700206. [DOI] [PubMed] [Google Scholar]
- 17.Wada K., Takada T., Hirata K., Mayumi T., Yoshida M., Yokoe M. Treatment strategy for acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:79–86. doi: 10.1007/s00534-009-0218-z. [DOI] [PubMed] [Google Scholar]
- 18.Amano H., Takada T., Isaji S., Takeyama Y., Hirata K., Yoshida M. Therapeutic intervention and surgery of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:53–59. doi: 10.1007/s00534-009-0211-6. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox C.M., Varadarajulu S., Eloubeidi M. Role of endoscopic evaluation in idiopathic pancreatitis: a systematic review. Gastrointest Endosc. 2006;63:1037–1045. doi: 10.1016/j.gie.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Venneman N.G., Renooij W., Rehfeld J.F., VanBerge-Henegouwen G.P., Go P.M., Broeders I.A. Small gallstones, preserved gallbladder motility, and fast crystallization are associated with pancreatitis. Hepatology. 2005;41:738–746. doi: 10.1002/hep.20616. [DOI] [PubMed] [Google Scholar]
- 21.Houssin D., Castaingi D., Lemoine J., Bismuth H. Microlithiasis of the gallbladder. Surg Gynecol Obstet. 1983;157:20–24. [PubMed] [Google Scholar]
- 22.Sun L., Li W., Geng Y., Shen B., Li J. Acute pancreatitis in pregnancy. Acta Obstet Gynecol Scand. 2011;90:671–676. doi: 10.1111/j.1600-0412.2011.01072.x. [DOI] [PubMed] [Google Scholar]
- 23.Qihui C., Xipingi Z., Xianfeng D. Clinical study on acute pancreatitis in pregnancy in 26 cases. Gastroenterol Res Pract. 2012;2012:271925. doi: 10.1155/2012/271925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polydorou A., Karapanosi K., Vezakis A., Melemeni A., Koutoulidis V., Polymeneas G. A multimodal approach to acute biliary pancreatitis during pregnancy: a case series. Surg Laparosc Endosc Percutaneous Tech. 2012;22:429–432. doi: 10.1097/SLE.0b013e31825e38bb. [DOI] [PubMed] [Google Scholar]
- 25.Vilallonga R., Calero-Lillo A., Charco R., Balsells J. Acute pancreatitis during pregnancy, 7-year experience of a tertiary referral center. Cir Esp. 2014, March doi: 10.1016/j.ciresp.2013.12.016. pii: S0009-739X(14)00051-7 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


