Abstract
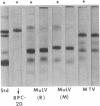
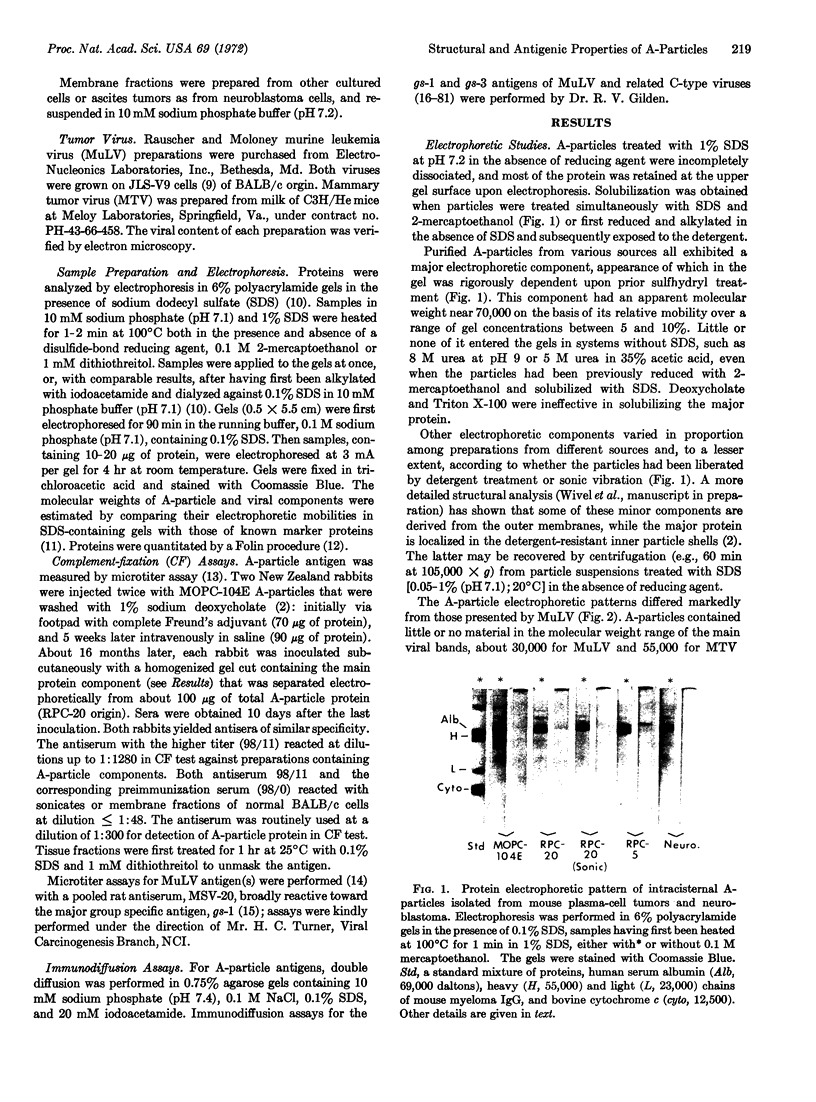
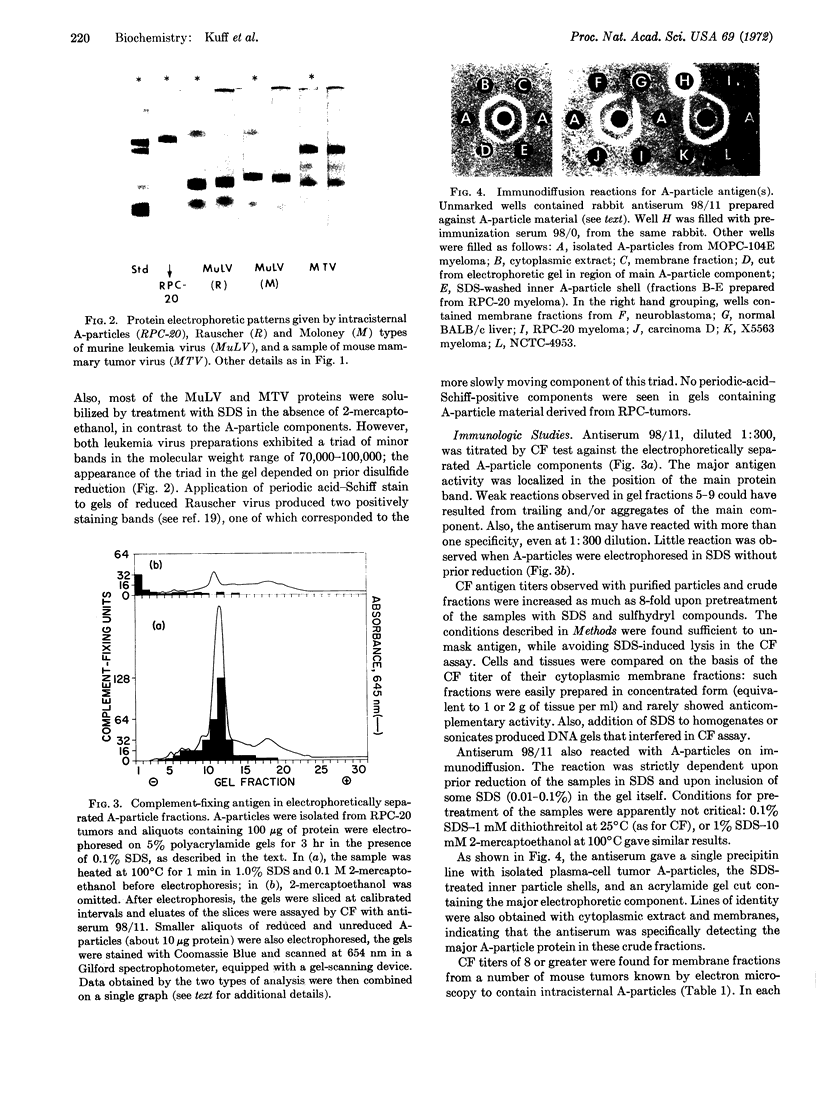
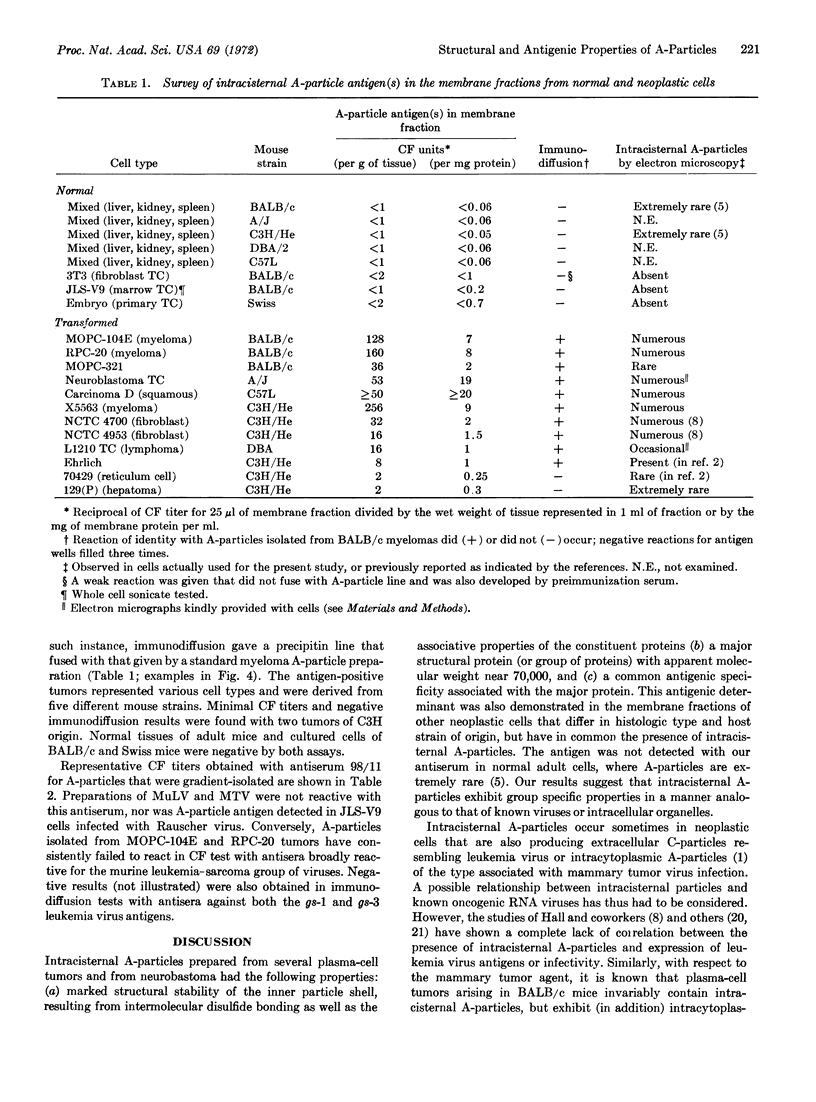
Intracisternal A-particles were isolated from three different myeloma lines in BALB/c mice and from cultured neuroblastoma cells of A/J origin. All preparations contained a major structural protein with an apparent molecular weight near 70,000 as estimated by electrophoretic mobility in sodium dodecyl sulfate-containing polyacrylamide gels. Solubilization of this component by sodium dodecyl sulfate was dependent on prior or concomitant treatment with sulfhydryl compounds. The size distribution of A-particle proteins was markedly different from that observed for extracellular murine leukemia and mammary tumor viruses. Rabbit antiserum was developed that reacted with the major A-particle protein in both complement fixation and immunodiffusion assays. The antigen was detected in isolated neuroblastoma A-particles, in cytoplasmic membrane fractions prepared from various mouse tumors known to contain intracisternal particles, but not in preparations from normal mouse cells, in samples of leukemia and mammary tumor virus, or in JLS-V9 cells infected with Rauscher leukemia virus. Conversely, isolated A-particles did not react in complement fixation or immunodiffusion assays with antisera against leukemia virus antigens.
Keywords: complement fixation, immunodiffusion, neuroblastoma, plasma-cell tumor
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUNHAM L. J., STEWART H. L. A survey of transplantable and transmissible animal tumors. J Natl Cancer Inst. 1953 Apr;13(5):1299–1377. [PubMed] [Google Scholar]
- Dalton A. J., Potter M. Electron microscopic study of the mammary tumor agent in plasma cell tumors. J Natl Cancer Inst. 1968 Jun;40(6):1375–1385. [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Officer J. E., Rongey R. W., Estes J. D., Turner H. C., Huebner R. J. C-type RNA tumour virus genome expression in wild house mice. Nature. 1971 Aug 27;232(5313):617–620. doi: 10.1038/232617a0. [DOI] [PubMed] [Google Scholar]
- Geering G., Hardy W. D., Jr, Old L. J., de Harven E., Brodey R. S. Shared group-specific antigen of murine and feline leukemia viruses. Virology. 1968 Dec;36(4):678–680. doi: 10.1016/0042-6822(68)90199-2. [DOI] [PubMed] [Google Scholar]
- Geering G., Old L. J., Boyse E. A. Antigens of leukemias induced by naturally occurring murine leukemia virus: their relation to the antigens of gross virus and other murine leukemia viruses. J Exp Med. 1966 Oct 1;124(4):753–772. doi: 10.1084/jem.124.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden R. V., Oroszlan S., Huebner R. J. Coexistence of intraspecies and interspecies specific antigenic determinants on the major structural polypeptide of mammalian C-type viruses. Nat New Biol. 1971 May 26;231(21):107–108. doi: 10.1038/newbio231107a0. [DOI] [PubMed] [Google Scholar]
- Gregoriades A., Old L. J. Isolation and some characteristics of a group-specific antigen of the murine leukemia viruses. Virology. 1969 Feb;37(2):189–202. doi: 10.1016/0042-6822(69)90198-6. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., TURNER H. C., LANE W. T. SPECIFIC ADENOVIRUS COMPLEMENT-FIXING ANTIGENS IN VIRUS-FREE HAMSTER AND RAT TUMORS. Proc Natl Acad Sci U S A. 1963 Aug;50:379–389. doi: 10.1073/pnas.50.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. T., Hartley J. W., Sanford K. K. Characteristics of and relationship between C particles and intracisternal A particles in cloned cell strains. J Virol. 1968 Mar;2(3):238–247. doi: 10.1128/jvi.2.3.238-247.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Isolation of naturally occurring viruses of the murine leukemia virus group in tissue culture. J Virol. 1969 Feb;3(2):126–132. doi: 10.1128/jvi.3.2.126-132.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda T., Roberts E., Suntzeff V. Electron microscopic study of methylcholanthrene-induced epidermal carcinogenesis in mice: mitochondrial dense bodies and intracisternal A-particles. Cancer Res. 1970 Apr;30(4):1011–1019. [PubMed] [Google Scholar]
- Kuff E. L., Wivel N. A., Lueders K. K. The extraction of intracisternal A-particles from a mouse plasma-cell tumor. Cancer Res. 1968 Oct;28(10):2137–2148. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oroszlan S., Foreman C., Kelloff G., Gilden R. V. The group-specific antigen and other structural proteins of hamster and mouse C-type viruses. Virology. 1971 Mar;43(3):665–674. doi: 10.1016/0042-6822(71)90290-x. [DOI] [PubMed] [Google Scholar]
- Paraf A., Moyne M. A., Duplan J. F., Scherrer R., Stanislawski M., Bettane M., Lelievre L., Rouze P., Dubert J. M. Differentiation of mouse plasmocytomas in vitro: two phenotypically stabilized variants of the same cell. Proc Natl Acad Sci U S A. 1970 Oct;67(2):983–990. doi: 10.1073/pnas.67.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N., Zambernard J., Lasher R., VanWoert M. H. Transmission of mouse neuroblastoma by a cell-free extract. Nature. 1970 Dec 5;228(5275):997–999. doi: 10.1038/228997a0. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer W., Anderer F. A., Bauer H., Pister L. Studies on mouse leukemia viruses. I. Isolation and characterization of a group-specific antigen. Virology. 1969 Jul;38(3):387–394. doi: 10.1016/0042-6822(69)90151-2. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Watson J., Ralph P., Sarkar S., Cohn M. Leukemia viruses associated with mouse myeloma cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):344–351. doi: 10.1073/pnas.66.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wivel N. A., Smith G. H. Distribution of intracisternal A-particles in a variety of normal and neoplastic mouse tissues. Int J Cancer. 1971 Jan 15;7(1):167–175. doi: 10.1002/ijc.2910070119. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]