Abstract
Vascular early response gene (Verge) is a novel immediate early gene that is highly expressed during developmental angiogenesis and after ischemic insults in adult brain. However, the role of Verge after neonatal injury is not known. In the present study, we investigated the hypothesis that Verge contributes to vascular remodeling and tissue repair after neonatal ischemic injury. The Rice–Vanucci model (RVM) was employed to induce neonatal stroke in both Verge knockout (KO) and wild-type (WT) postnatal day 10 (P10) mice. Histological and behavioral outcomes at acute (24 h), subacute (7 days) and chronic (30 days) phases were evaluated. Angiogenesis, neurogenesis, and glial scar formation were also examined in the ischemic brain. No significant differences in outcomes were found between WT and Verge mice at 24 h or 7 days after stroke. However genetic deletion of Verge led to pronounced cystic cavitation, decreased angiogenensis and glial scar formation in the ischemic hemisphere compared to WT mice at 30 days. Verge KO mice also had significantly worse functional outcomes at 30 days which was accompanied by decreased neurogenesis and angiogenesis in the ischemic hemisphere. Our study suggests that Verge plays an important role in the induction of neurogenesis and angiogenesis after ischemia, contributes to improved tissue repair, and enhances chronic functional recovery.
Keywords: Angiogenesis, Ischemic stroke, Neonate, Neurogenesis, Vascular early response gene
1. Introduction
Perinatal arterial ischemic stroke (PAIS), with an incidence of 1/2500 to 1/5000 term neonates, is an important cause of hemiplegic cerebral palsy and cognitive impairment in children (Cheong and Cowan, 2009). The underlying pathophysiology of PAIS is difficult to study in the human. It is critical to model neonatal stroke in animals to identify and develop therapeutic strategies to reduce brain injury in newborns with PAIS. Numerous experimental studies have examined potential therapies for neonatal stroke ranging from anti-excitotoxic, anti-oxidative, and anti-apoptotic therapies to stem cell replacement and hypothermia therapy (Kleman et al., 2010). However, due to the complexity of the response to ischemic injury in the brain at present no individual pharmacological agents have been proven effective (Lai and Yang, 2011). Novel therapeutic strategies are urgently needed for this devastating disease. The active developmental state of the early postnatal brain provides an exciting potential therapeutic opportunity, i.e. enhancement of angiogenesis. Angiogenesis, especially in a relatively young brain, could represent an effective way to repair the injured brain tissue and potentially lead to improved function and recovery. Importantly, discovering mechanisms by which repair can recapitulate normal development may have implications for adult brain injury as well.
Vascular early response gene (Verge), a novel immediate early gene (IEG), is expressed selectively in vascular endothelial cells and platelets (Regard et al., 2004). Verge mRNA and protein are rapidly and transiently induced in mature, quiescent vasculature in adult; however, they are constitutively expressed at high levels in the endothelium of developing tissues in association with angiogenesis in neonatal animals (Regard et al., 2004). Verge expression is responsive to local tissue conditions, as transient focal ischemia induced a threefold increase in Verge mRNA expression in the ischemic hemisphere. Previous work has shown that mice with Verge deletion develop normally, but exhibit reduced edema formation after experimental stroke in adulthood, an effect that was independent of infarct volumes (Liu et al., 2012c) which were equivalent in Verge KO and WT adult mice. In this study, we utilized the RVM (Vannucci and Perlman, 1997) in Verge KO and WT littermate mice to induce ischemia at post-natal day 10 (P10) to test the hypothesis that Verge activation increases angiogenesis and improves chronic recovery after neonatal stroke.
2. Materials and methods
2.1. Experimental animals
Verge KO (original breeding pairs from Johns Hopkins University as a gift from Dr. Paul Worley) and WT C57BL/6 littermates P10 mice were used. The sex ratio of mice was balanced by randomly putting equal numbers of male and female pups in each cohort. This study was conducted in accordance with NIH guidelines for the care and use of animals in research and under protocols approved by the Animal Care and Use Committee of the University of Connecticut Health Center.
2.2. RVM model
The RVM was modified for use in mice (Knox et al., 2013). Briefly, P10 mice were anaesthetized with 1.5% Isoflurane and placed in the supine position. Body temperature was maintained at 36.5 °C with an automated temperature control feedback system. The fur and skin of the frontal neck region were disinfected with 70% ethanol, and then a less than 0.5 cm long midline incision was made to expose the right common carotid artery (CCA). Double permanent ligation of CCA was made with 6–0 silk suture, and then the incision was closed and the pups were returned to their dams. Sham mice underwent the same procedure except ligation of the right CCA. 1 h after surgery, pups were placed in a 36 °C chamber containing 10% oxygen and 90% nitrogen for 45 min, 60 min or 90 min to induce ischemic injury. Mice were sacrificed 24 h (acute), 7 days (subacute), or 30 days (chronic) after stroke. Pups in the 30-day survival cohort were injected with 5-Bromo-2′-deoxyuridine (BrdU, Millipore) (100 mg/kg) i.p. twice a week after VRM modeling (Bartley et al., 2005). The corner test was performed by an investigator blinded to stroke/sham and genotype at 7 days and 30 days after stroke; the score of corner test was determined by the number of right turn/right plus left turns as previously described (Li et al., 2004).
2.3. Histological assessment
The infarct was quantified at both the acute and subacute endpoints by triphenyltetrazolium chloride (TTC) staining (Liu et al., 2009); brain atrophy was quantified at chronic endpoints stroke with cresyl violet (CV) staining as in (Liu et al., 2012a). Brain infarct volume (acute) or tissue loss (subacute and chronic endpoints) was calculated as previously described (Liu et al., 2009, 2012a). Hemispheric cyst formation was examined when brains were taken out of the skulls and confirmed by TTC or CV staining.
2.4. Immunohistochemistry
Pups were sacrificed at 7 days or 30 days after stroke; brains were perfused (Liu et al., 2012a) and stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, 1:1000, Invitrogen) for nuclei, Verge antibody (obtained as a gift from Dr. Paul Worley in John Hopkins University, 1:100) for Verge expression in the vessel, Endoglin (CD105, 1:20, Dako) and Von Willebrand factor (VWF, 1:100, Santa-Cruz) for angiogenesis (Starke et al., 2011; Tan et al., 2013), anti-BrdU (1:20, Sigma–Aldrich) was used to identify new-born cells (Biron et al., 2011), anti-NeuN for neurons (Liu et al., 2009), and glial fibrillary acidic protein (GFAP, 1:200, Dako) for glial scar formation (Bao et al., 2012). Secondary antibodies used were Alexa Fluor dyes 488 and 594 (1:1000, Invotrogen), complementary to the species of primary antibodies. The signal was visualized with a Zeiss Axiovert 200M microscope (Carl Zeiss) and captured with Zeiss LSM510 Meta software (Liu et al., 2012b). The fluorescent intensity of the signal was analyzed by ImageJ software (NIH).
2.5. Statistical analysis
Investigators were blinded to mouse genotype and treatment (sham vs. stroke) for all experiments. Mice with abnormal body weights and behavior (e.g. body shaking, limp, etc.) before VRM were excluded. Data from individual experiments were presented as Mean ± SEM and analyzed with t-test for two groups, and two-way ANOVA for multiple group comparison. The hemispheric cyst formation data was presented as the count of cysts/total count of samples in the group and analyzed with Fisher’s Exact Test. P < 0.05 was considered statistically significant for all data.
3. Results
3.1. Acute and subacute outcomes of 90-min neonatal stroke
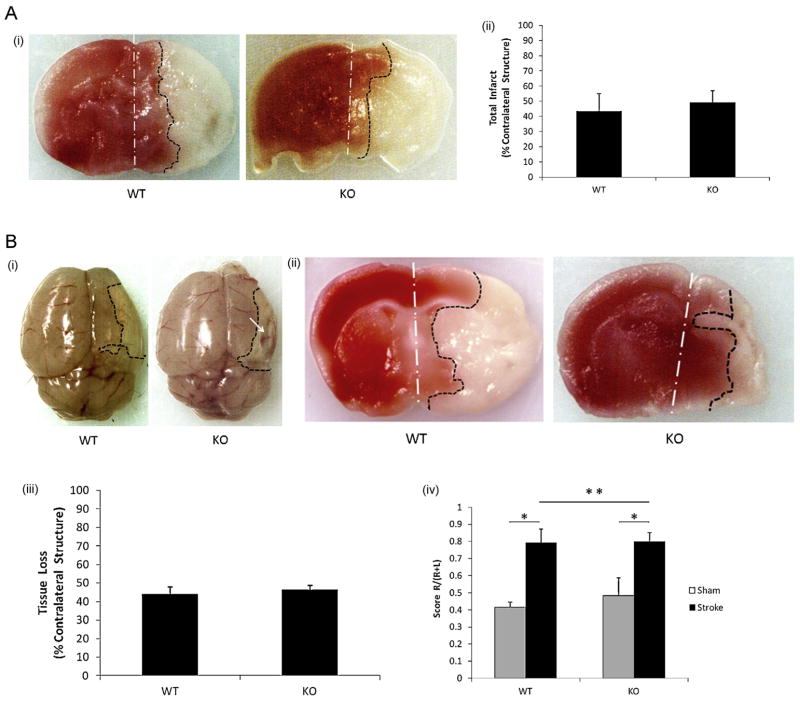
Brain injury varies in the mouse RVM depending on the time exposed to 10% oxygen with a range from 15 min (Jiang et al., 2008) to 2.5 h (Nakanishi et al., 2009) reported in the literature. We chose the median time (90 min) at the beginning of this study. 24 h after a 90 min 10% oxygen exposure, visible infarct was seen in the right (ipsilateral) hemisphere in both WT and KO pups (Fig. 1A-i); however, quantitative analysis revealed no difference in infarct volumes between the two strains (Fig. 1A-ii). Next we extended the survival time to 7 days to examine subacute stroke outcomes. Stroke mice of both strains had a significant preference in the corner test compared to sham mice. Surprisingly, we found significantly more gross hemispheric cyst formation in KO brains compared to WT mice (KO vs. WT: 6/6 vs. 2/7; P = 0.02) (Fig. 1B-i and ii); however quantification of either tissue loss (Fig. 1B-iii) or behavioral deficits in the corner test (Fig. 1B-iv) revealed no differences between genotypes.
Fig. 1.
Stroke outcomes in Verge KO and WT P10 mice subjected to 90-min stroke (RVM) followed by 24-h and 7-day survival. (A) Stroke outcomes in the 90 min stroke plus 24 h survival cohort. (A-i) Representative coronal brain sections of TTC staining: white dotted line shows the midline of the brain; black dotted line illustrates the borderline of the infarct; red color area: unaffected tissue; white color area: infarct tissue. (A-ii) quantification of infarct volumes; n = 6/group. (B) Stroke outcomes in the 90 min stroke plus 7 days survival cohort. (B-i) Gross examination of the cyst in the ischemic hemisphere: the white arrow indicates the cyst in the KO ipsilateral hemisphere. (B-ii) Representative coronal brain sections of TTC staining. (B-iii) Quantification of infarct volumes; n = 7 in WT group; n = 6, KO. (B-iv) Scores of Corner test; *P < 0.05 and **P > 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.2. Angiogenesis and glial scar formation after 7 days of 90 min neonatal stroke
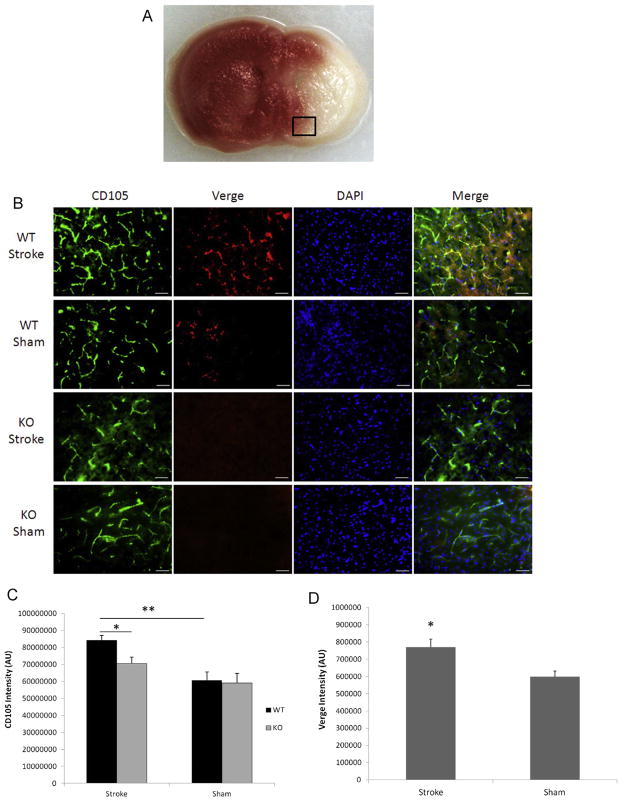
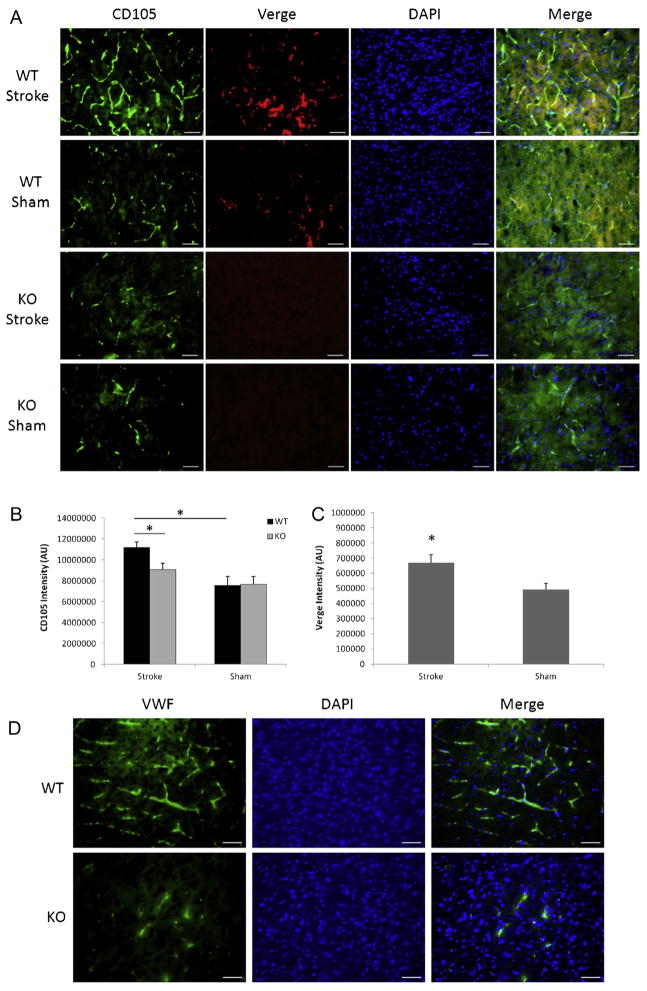
Surprised by the distinct phenotype of hemispheric cyst formation seen in Verge KO mice after 7 days of 90 min stroke, we hypothesized Verge may have a role in promoting post-stroke angiogenesis which can prevent cavitation in the ischemic injury (Leker et al., 2009). CD105 is an important marker for angiogenesis (Duff et al., 2003). We performed triple labeling of brain slices with CD105, Verge, and DAPI. Consistent with our previous findings in adult mice, Verge expression was increased after stroke in WT pups (Fig. 2B and D). Verge also co-localized with CD105 (Fig. 2B). Stroke induced a significant increase in CD105 expression only in WT mice, and KO pups expressed significantly less CD105 compared with their WT counterparts (Fig. 2B and C). The decreased angiogenesis in KO vs. WT brains after ischemia was further confirmed by VWF staining (Fig. 2E).
Fig. 2.
Angiogenesis and glial scar formation in the cohort of 90 min stroke plus 7 days survival. (A) The black box in a TTC-stained coronal brain section indicates the area of interest to do the immunofluorescent staining. (B) Representative pictures (20×) of CD105, Verge antibody and DAPI staining in KO and WT ipsilateral hemispheres; scale bar = 50 μm. (C) Quantification of CD105 fluorescent intensity. *P < 0.05, **P < 0.01; n = 6/group. (D) Quantification of Verge fluorescent intensity. *P < 0.05 vs. sham; n = 6/group. (E) Representative pictures (20×) of VWF antibody staining in KO and WT ipsilateral hemispheres; scale bar = 50 μm. (F) Representative pictures (10×) of GFAP, DAPI staining in KO and WT ipsilateral hemispheres; scale bar = 50 μm. In the GFAP/WT picture, the insert box (100×) indicates the same astrocyte as the one pointed by the arrow. (G) Quantification of GFAP fluorescent intensity. *P < 0.05 vs. WT group; n = 6/group.
Glial scars formed early in the ischemic brain have a beneficial role in curbing ischemic lesion (Beck et al., 2008). We used GFAP staining to identify glial scars and found dispersed, tangled GFAP positive signals in both WT and KO ipsilateral hemispheres (Fig. 2F). WT pups had significantly denser glial scars than KO pups (Fig. 2F and G). The astrocytes comprising the scar exhibited activated morphology of plump cell bodies and short processes (Fig. 2F: the insert). In sham mice, only a few GFAP positive astrocytes were seen near the edge of the cortex.
3.3. Acute and subacute outcomes after 60-min neonatal ischemia
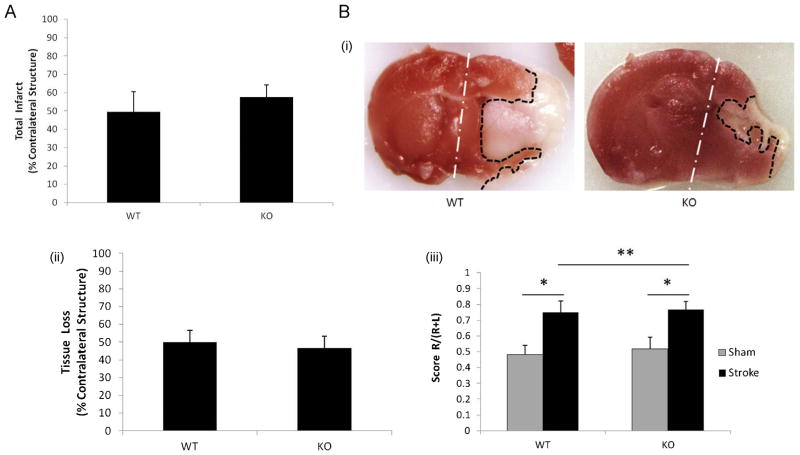
To exclude the possibility that the 90 min ischemic insult was too severe to reveal any difference in stroke outcomes between KO and WT pups due to the “ceiling effect”, we shortened the ischemic time to 60 min. Again, 24 h after stroke, no difference in infarct volumes was seen between the two cohorts (Fig. 3A); significantly more hemispheric cyst formation was seen in KO vs. WT brains after 7 days of stroke (KO vs. WT: 5/6 vs. 0/7; P = 0.004) (Fig. 3B-i), but similar to what was seen with a 90 min insult, there was no significant difference in tissue loss (Fig. 3B-ii) or in the results of corner testing (Fig. 3B-iii) between KO and WT pups. We further reduced the stroke time to 45 min in our model, but the infarct at day 7 was almost undetectable and non-measurable in most of the brains, and no difference in corner test scores was seen between sham and stroke mice (data not shown).
Fig. 3.
Stroke outcomes from the cohort of 60 min stroke + 24 h/7 days survival. (A) Total infarct volumes show no significant difference between KO and WT mice of 60 min stroke + 24 h survival cohort; n = 7/group. (B) Outcomes from the cohort of 60 min stroke + 7 days survival. (B-i) Representative coronal brain sections of TTC staining; (B-ii) quantification of infarct volumes showed no difference between KO and WT mice, n = 7 (WT), n = 6 (KO). (B-iii) Scores of Corner test; *P < 0.05 and **P > 0.05.
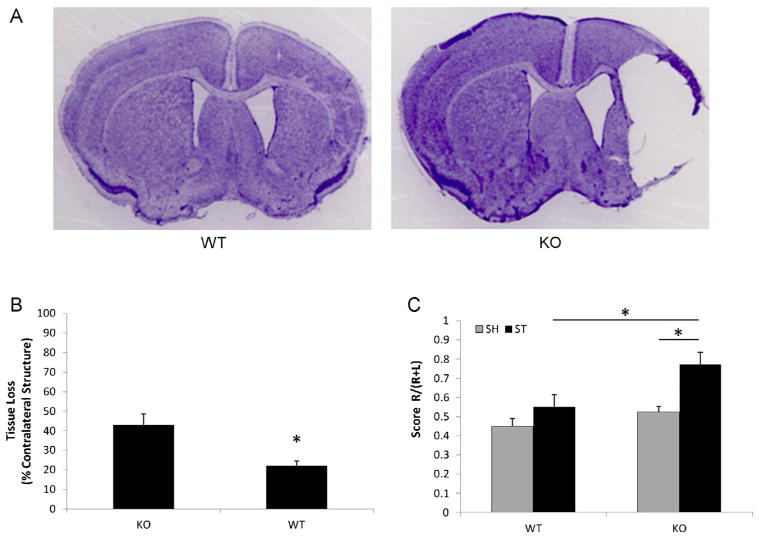
3.4. Chronic outcomes of 60-min neonatal stroke
The phenotype of hemispheric cyst development in KO pups was striking. We hypothesized that these early cysts may be important to more chronic functional and histological outcomes. Therefore another cohort of pups was examined that were subjected to a 60 min ischemic challenge and allowed to survive for 30 days. Histological assessment showed that the majority of KO brains (5/7) had visible cyst formation; however, the cavity was only seen in one WT brain (1/8). Evident atrophy was seen in the ipsilateral hemisphere of both KO and WT mice as indicated by an enlarged ipsilateral ventricle due to volume loss (Fig. 4A). Since atrophy could conceal the presence of a previously formed cavity, statistical analysis of cavity formation is not applicable for this chronic cohort. The quantitative analysis of remaining tissue in the ischemic brain showed that KO pups had significantly more ipsilateral tissue loss than WT mice (Fig. 4B). Correspondingly, KO mice had significantly higher corner test scores than their WT counterparts (Fig. 4C).
Fig. 4.
Chronic stroke outcomes from the cohort of 60 min stroke + 30 days survival. (A) Representative coronal brain sections of CV staining. The right ventricles of both WT and KO mice are larger compared to the left. In the WT brain, the right striatum and cortex are smaller than the ones on the left; there is cavity formation in the ipsilateral hemisphere of the KO brain. (B) Tissue loss in ipsilateral hemispheres of WT and KO mice. *P < 0.05 vs. KO group; n = 8(WT), n = 7(KO). (C) Scores of Corner test 30 days after 60 min stroke. *P < 0.05.
3.5. Angiogenesis, neurogenesis, and glial scar formation assessed at a chronic endpoint after neonatal stroke
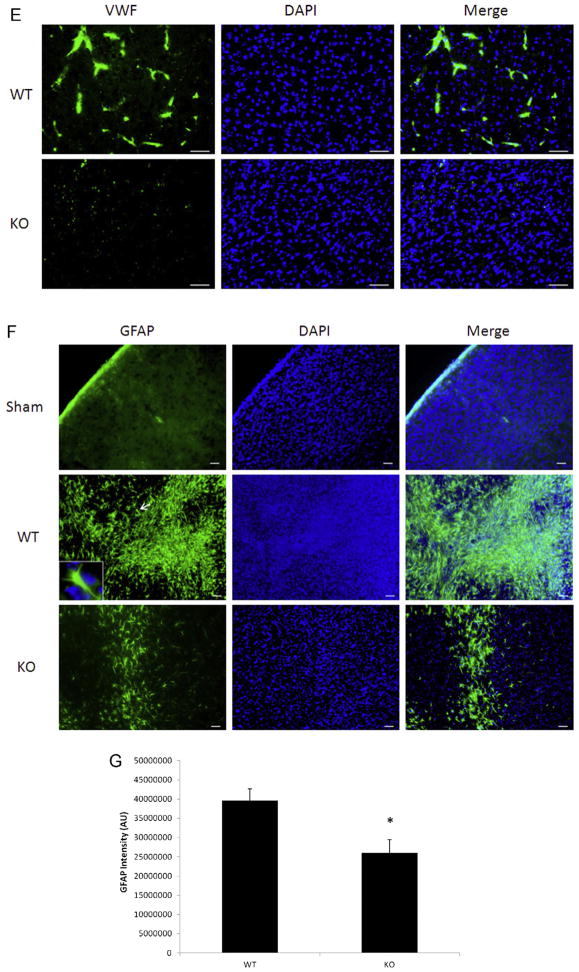
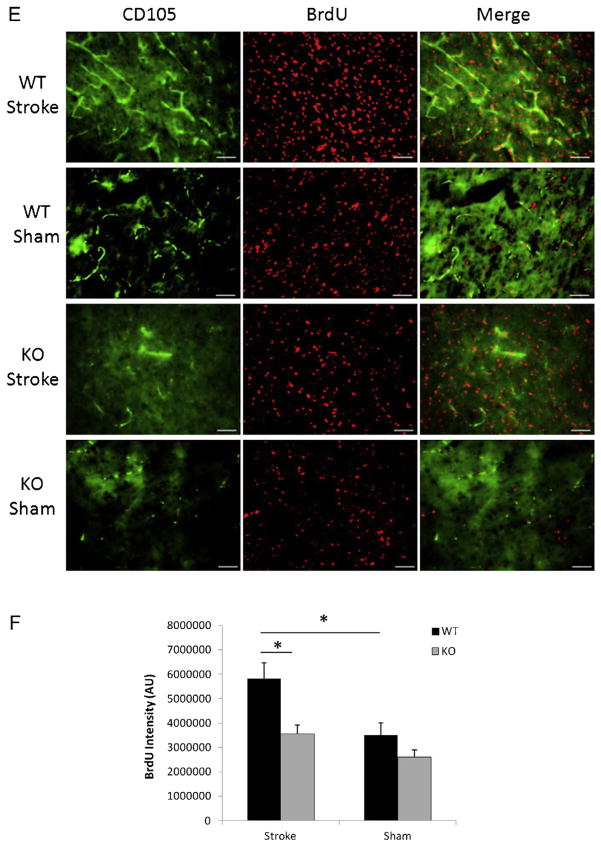
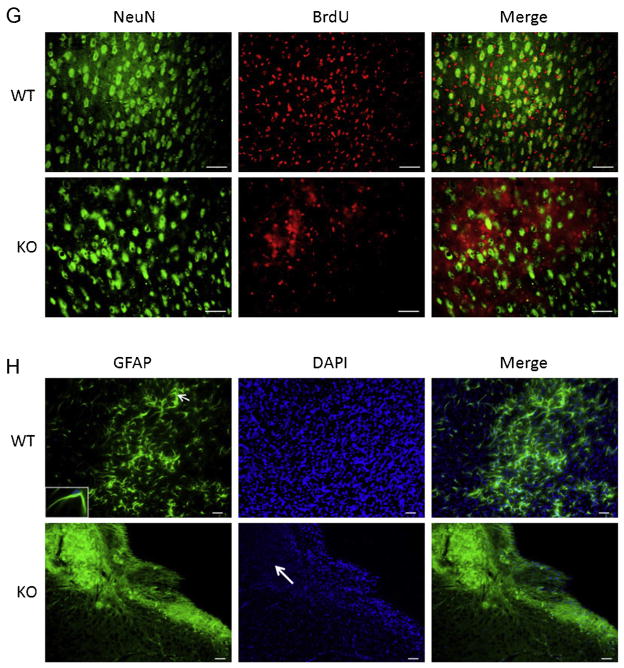
Next we examined CD105 and Verge expression at 30 days after a 60 min stroke. Surprisingly increased expression of Verge after stroke persisted at 30 days (Fig. 5A and C), accompanied by the same pattern of CD105 expression (Fig. 5A and B). Similar to the early time points (Fig. 2B), CD105 positive vessels also expressed Verge, suggesting a supportive role for Verge in angiogenesis. The decreased angiogenesis in KO vs. WT brains after ischemia was further confirmed by VWF staining (Fig. 2D). It has been demonstrated that increased angiogenesis leads to enhancement of neurogenesis (Jin et al., 2002; Taguchi et al., 2004; Wang et al., 2004; Zhang et al., 2003). In order to determine whether Verge also has an impact on neurogenesis post-ischemia, we injected both WT and KO mice in the chronic endpoint cohort with BrdU to examine the new-born cells. WT pups had significantly more BrdU positive cells in the ipsilateral hemisphere than KO mice (Fig. 5E and F). Almost all CD105 positive vessels were co-labeled with BrdU, indicating the presence of post-stroke angiogenesis. We also performed double staining with BrdU and NeuN, and found WT brains had more co-localized signals than KO brains 30 days after ischemia (Fig. 5G). Finally we examined chronic glial scar formation in the ipsilateral hemisphere of both WT and KO pups. Surprisingly a very distinct phenotype of chronic glial scar formation was observed between the two strains (Fig. 5H). In WT brains, dispersed GFAP signals were still seen in the ipsilataral hemisphere, although the astrocytes exhibited smaller bodies and finer processes (Fig. 5H: the insert) compared to those in early phase of stroke (Fig. 2F: the insert). However, very dense and thick GFAP signals were seen along the cavity wall in which DAPI staining was absent or attenuated, suggesting most of these GFAP signals are protein deposition (“aged” scars) rather than astrocytes.
Fig. 5.
Angiogenesis, neurogenesis, and glial scar formation in the cohort of 60 min stroke + 30 days survival. (A) Representative pictures (20×) of CD105, Verge antibody and DAPI staining in KO and WT ipsilateral hemispheres; scale bar = 50 μm. (B) Quantification of CD105 fluorescent intensity. *P < 0.05; n = 8(WT), n = 7(KO). (C) Quantification of Verge fluorescent intensity. *P < 0.05 vs. sham; n = 8/group. (D) Representative pictures (20×) of VWF antibody staining in KO and WT ipsilateral hemispheres; scale bar = 50 μm. (E) Representative pictures (20×) of CD105 and BrdU staining; scale bar = 50 μm. (F) Quantification of BrdU fluorescent intensity; *P < 0.05; n = 8(WT), n = 7(KO). (G) Representative pictures (20×) of BrdU and NeuN double staining in KO and WT ipsilateral hemispheres; scale bar = 50 μm. (H) Representative pictures (10×) of GFAP, DAPI staining in KO and WT ipsilateral hemispheres; scale bar = 50 μm. In GFAP/WT picture, the insert box (100×) indicates the same astrocyte as the one pointed by the arrow. In the DAPI/KO picture, the arrow indicates the area of no DAPI signals.
4. Discussion
Verge is an important IEG whose function remains unclear. Verge is very highly expressed in the vasculature and in platelets during development and is important in angiogenesis (Regard et al., 2004). We have previously shown that Verge is induced by stroke in the adult brain. However, as this is a developmentally regulated gene, and is only highly-expressed in select vessels after an ischemic injury in adulthood (Regard et al., 2004), we hypothesized that Verge may have a greater impact after a developmentally induced injury such as perinatal stroke. The present study is the first report of the effects of loss of Verge, after ischemic injury in the neonatal brain. Several important results were found. Firstly, acute and subacute stroke outcomes, both histological (infarct) and behavioral (corner turning preference) were not affected by Verge deletion. This is consistent with our previous study in adult animals (Liu et al., 2012c). However, loss of Verge led to significant differences in chronic outcome after an ischemic injury, suggesting that Verge signaling is important in stroke recovery. Secondly, loss of Verge led to a dramatic increase in ipsilateral cyst formation in the neonatal brain. Stroke-induced cyst formation was seen at subacute stage of ischemia (7 days), and was still evident 30 days after stroke in Verge KO mice. This was related to a reduction in both tissue repair and functional recovery. Thirdly, a significant difference in glial scar formation was found in WT versus KO pups. At an early stage post-ischemia, enhanced glial scar formation was seen in WT compared to KO brains; however, KO but not WT brains exhibited GFAP deposition along the cyst wall at chronic endpoints. Finally, neurogenesis and angiogenesis were compromised in the ischemic brain of Verge KO mice, which was closely related to the glial scar formation and stroke outcomes.
A previous study examined the role of Verge in experimental stroke induced by 90 min middle cerebral artery occlusion (MCAO) in adult mice and found loss of Verge did not affect acute stroke outcomes (i.e., infarct size) (Liu et al., 2012c). The lack of acute effects of Verge deletion extends to neonates as shown in this study. Infarct volumes early after stroke were measured by TTC staining, a marker of tissue dehydrogenase and mitochondrial dysfunction that occurs in both the core and penumbra (Benedek et al., 2006; Tureyen et al., 2004). The pathology of the penumbra is traditionally considered as a one-dimensional, linear progression from initial injury to final infarct; however it is now apparent that the penumbra may not passively die over time, but can also actively recover (Lo, 2008a, b). Therefore, outcomes at the early stage of stroke tend to be severer than that of the recovery stage, a time when neurological deficits reflect more closely the size and location of the structural lesion that is much smaller than the previous combination of core and penumbra (Liu et al., 2009). The dynamic progress of infarct typically occurs over a few days in ischemic brains of WT mice; however, in brains of Verge KO mice, the ischemic tissue developed much quicker and more completely into the core, and was more likely to form necrotic cyst. Interestingly these early differences were not reflected in either behavioral or histological assessments of tissue injury, however the lack of post-stroke angiogenesis at even 7 days of injury set the stage for cyst formation, later enhanced tissue loss, and poorer functional outcomes.
Our data provide mechanistic clues as to why KO pups develop more cystic cavitation after injury and resultantly lose more tissue in the ischemic hemisphere than WT mice at 30 days. Verge expression was significantly increased in the ipsilateral hemisphere at both early and chronic stages of ischemia, and this was associated with up-regulated expression of CD105 and VWF, increased glial scar formation (GFAP), and an enhancement in the number of BrdU and NeuN positive cells. These data suggest that active angiogenesis and neurogenesis occurs in WT ischemic brains after neonatal injury that is impaired in Verge KO mice. Verge has the potential to induce angiogenesis and thus improved formation of the neurogenic niche (Young et al., 2011). This may keep the penumbral area active in cell proliferation and tissue repair. In cell culture models using rat brain endothelial cells that express Verge, treatment with phorbol 12-myristate 13-acetate, a potent inducer of Verge expression, led to a reorganization of the actin cytoskeleton and the formation of paracellular gaps (Regard et al., 2004), suggesting a positive role for Verge in tissue repair. Neurogenesis and angiogenesis start to occur days after stroke (Lanfranconi et al., 2011) and have an effect in reducing necrotic cavity formation (Leker et al., 2009; Ohta et al., 2004; Osaka et al., 2010). Knockout of Verge suppresses angiogenesis so much so that ischemia could not even induce a significant increase in CD105 expression compared to Verge KO sham mice (Fig. 2B and C), probably due to the “floor effect”.
Glial scar formation may also have a protective effect early after ischemic injury in the brain. The glial scar protects healthy brain areas from exposure to toxic substances and cellular debris in the injured tissue, repairs the BBB, and prevents an excessive hyper-inflammatory response to the infarct (Beck et al., 2008; Kahle and Bix, 2013; Li et al., 2005). Therefore, glial scar formation early after stroke can help isolate injured tissue and limit necrotic cyst formation. The beneficial effects of glial scar formation correlated with the occurrence of necrotic cysts 7 days after neonatal injury. However, in the chronic phase of ischemic injury, the glial scar becomes a physical and chemical detriment to repair (Silver and Miller, 2004; Yiu and He, 2006). This was also reflected in the appearance of the glial scars 30 days after stroke, which were thicker, more disorganized and fibrillary along the cyst wall. This appears to form a mechanical obstacle to angiogenesis and neurogensis. Glial scar was also apparent in WT ischemic brains at chronic endpoints, but the morphology was very distinct from that of KO mice. The scar consists of quiescent astrocytes with smaller bodies and finer processes compared to those seen at early stage of ischemia. It is possible that this early scar in WT brains provides a tissue “scaffold” for angiogenesis and neurogenesis. When Verge is absent, less glial scar was formed in the early phase of ischemia and the scar that appears later after injury is likely detrimental in the chronic phase.
There are several caveats that need to be recognized when interpreting this data. We only compared the ischemic responses of Verge KO to WT by examining the effects of loss of Verge. Whether enhancement of Verge signaling has any effect on either ischemic damage or recovery is unknown and awaits future studies. We only examined angiogenesis and glial scar formation at 7 days and 30 days after ischemia; therefore how the tissue in the ischemic brain evolved between these two time points is unknown. The third caveat is that although we examined chronic recovery at 30 days, this is still not long enough to examine the effect of neonatal stroke and Verge manipulation on adult behavioral recovery, as these 40 day animals are considered “adolescent” (Harrison, 2011). However, it is most likely that the changes that were seen at 30 days would persist into adulthood with WT mice continuing to improve, and continued deficits in the KO mice. The cavitation seen in the KO brains 30 days after stroke is likely permanent and not amenable to repair. Finally, since the sex of animals was equally balanced in the present study, we cannot conclude if there was an effect of sex on either stroke outcomes or on angiogenesis or neurogenesis. The effect of Verge on neonatal stroke merits further investigation.
In summary, Verge KO mice develop more hemispheric cyst formation compared to their WT counterparts when subjected to ischemia. This was related to a significantly diminished level of angiogenesis/neurogenesis in the KO mice. Loss of Verge led to a decrease in early (7 day) glial scar formation, which was more robust in WT mice. However at 30 days, the scar was more dysmorphic, thicker and disorganized in the KO mice, which was correlated with cyst formation, tissue loss, and poorer behavioral outcomes. Knockout of Verge had no effect on acute and subacute stroke outcomes (as measured by infarct volumes); however, stroke recovery at more chronic stages is strikingly compromised in KO mice. Verge activation promotes neurogenesis and angiogenesis after ischemia, and enhances chronic stroke recovery, suggesting that investigating Verge signaling may have translational potential.
Acknowledgments
This work was supported by the NIH/NINDS (grants NS050505 and NS055215 to LDM), and by AHA (grant 12SDG9030000 to FL). We thank Dr. Paul F. Worley for the Verge KO mice and the Verge antibody.
Abbreviations
- BrdU
5-Bromo-2′-deoxyuridine
- CD105
endoglin
- CV
cresyl violet
- DAPI
dihydrochloride
- KO
knockout
- MCAO
middle cerebral artery occlusion
- NeuN
neuronal nuclear antigen
- PAIS
perinatal arterial ischemic stroke
- P10
post-natal day 10
- RVM
Rice–Vanucci model
- TTC
triphenyltetrazolium chloride
- Verge
vascular early response gene
- VWF
Von Willebrand factor
- WT
wild-type
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Bao Y, Qin L, Kim E, Bhosle S, Guo H, Febbraio M, Haskew-Layton RE, Ratan R, Cho S. CD36 is involved in astrocyte activation and astroglial scar formation. J Cereb Blood Flow Metab. 2012;32:1567–1577. doi: 10.1038/jcbfm.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley J, Soltau T, Wimborne H, Kim S, Martin-Studdard A, Hess D, Hill W, Waller J, Carroll J. BrdU-positive cells in the neonatal mouse hippocampus following hypoxic-ischemic brain injury. BMC Neurosci. 2005;6:15. doi: 10.1186/1471-2202-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Semisch M, Culmsee C, Plesnila N, Hatzopoulos AK. Egr-1 regulates expression of the glial scar component phosphacan in astrocytes after experimental stroke. Am J Pathol. 2008;173:77–92. doi: 10.2353/ajpath.2008.070648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek A, Moricz K, Juranyi Z, Gigler G, Levay G, Harsing LG, Jr, Matyus P, Szenasi G, Albert M. Use of TTC staining for the evaluation of tissue injury in the early phases of reperfusion after focal cerebral ischemia in rats. Brain Res. 2006;1116:159–165. doi: 10.1016/j.brainres.2006.07.123. [DOI] [PubMed] [Google Scholar]
- Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease. PLoS ONE. 2011;6:e23789. doi: 10.1371/journal.pone.0023789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JL, Cowan FM. Neonatal arterial ischaemic stroke: obstetric issues. Semin Fetal Neonatal Med. 2009;14:267–271. doi: 10.1016/j.siny.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17:984–992. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- Harrison DE. Life span as a biomarker. The Jackson Laboratory; 2011. sebsite: http://research.jax.org/faculty/harrison/ger1vLifespan1.html. [Google Scholar]
- Jiang X, Mu D, Biran V, Faustino J, Chang S, Rincon CM, Sheldon RA, Ferriero DM. Activated Src kinases interact with the N-methyl-D-aspartate receptor after neonatal brain ischemia. Ann Neurol. 2008;63:632–641. doi: 10.1002/ana.21365. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle MP, Bix GJ. Neuronal restoration following ischemic stroke: influences, barriers, and therapeutic potential. Neurorehabil Neural Repair. 2013;27:469–478. doi: 10.1177/1545968312474119. [DOI] [PubMed] [Google Scholar]
- Kleman N, Sun D, Cengiz P. Mechanisms underlying neonatal hypoxia ischemia. Open Drug Discov J. 2010;2:129–137. [Google Scholar]
- Knox R, Zhao C, Miguel-Perez D, Wang S, Yuan J, Ferriero D, Jiang X. Enhanced NMDA receptor tyrosine phosphorylation and increased brain injury following neonatal hypoxia-ischemia in mice with neuronal Fyn overexpression. Neurobiol Dis. 2013;51:113–119. doi: 10.1016/j.nbd.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Yang SN. Perinatal hypoxic-ischemic encephalopathy. J Biomed Biotechnol. 2011;2011:609813. doi: 10.1155/2011/609813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranconi S, Locatelli F, Corti S, Candelise L, Comi GP, Baron PL, Strazzer S, Bresolin N, Bersano A. Growth factors in ischemic stroke. J Cell Mol Med. 2011;15:1645–1687. doi: 10.1111/j.1582-4934.2009.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leker RR, Toth ZE, Shahar T, Cassiani-Ingoni R, Szalayova I, Key S, Bratincsak A, Mezey E. Transforming growth factor alpha induces angiogenesis and neurogenesis following stroke. Neuroscience. 2009;163:233–243. doi: 10.1016/j.neuroscience.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- Liu F, Benashski SE, Persky R, Xu Y, Li J, McCullough LD. Age-related changes in AMP-activated protein kinase after stroke. Age (Dordr) 2012a;34:157–168. doi: 10.1007/s11357-011-9214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J Neuroendocrinol. 2012b;24:319–330. doi: 10.1111/j.1365-2826.2011.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Turtzo LC, Li J, Regard J, Worley P, Zeevi N, McCullough LD. Loss of vascular early response gene reduces edema formation after experimental stroke. Exp Transl Stroke Med. 2012c;4:12. doi: 10.1186/2040-7378-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH. Experimental models, neurovascular mechanisms and translational issues in stroke research. Br J Pharmacol. 2008a;153 (Suppl 1):S396–S405. doi: 10.1038/sj.bjp.0707626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008b;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Tu S, Shin Y, Cui J, Kurokawa T, Zhang D, Chen HS, Tong G, Lipton SA. Neuroprotection by the NR3A subunit of the NMDA receptor. J Neurosci. 2009;29:5260–5265. doi: 10.1523/JNEUROSCI.1067-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kataoka K, Chou H, Ishikawa N, Matsumoto N, Iwashita Y, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187:266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H, Kocsis JD. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010;1343:226–235. doi: 10.1016/j.brainres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Regard JB, Scheek S, Borbiev T, Lanahan AA, Schneider A, Demetriades AM, Hiemisch H, Barnes CA, Verin AD, Worley PF. Verge: a novel vascular early response gene. J Neurosci. 2004;24:4092–4103. doi: 10.1523/JNEUROSCI.4252-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE, Payne EM, Haskard DO, Hughes AD, Cutler DF, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071–1080. doi: 10.1182/blood-2010-01-264507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G, Xiang X, Guo W, Zhang B, Chen W, Yang J. Study of the impact of uterine artery embolization (UAE) on endometrial microvessel density (MVD) and angiogenesis. Cardiovasc Intervent Radiol. 2013;36 (4):1079–1085. doi: 10.1007/s00270-013-0599-x. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Vemuganti R, Sailor KA, Dempsey RJ. Infarct volume quantification in mouse focal cerebral ischemia: a comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J Neurosci Methods. 2004;139:203–207. doi: 10.1016/j.jneumeth.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics. 1997;100:1004–1014. doi: 10.1542/peds.100.6.1004. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CC, Brooks KJ, Buchan AM, Szele FG. Cellular and molecular determinants of stroke-induced changes in subventricular zone cell migration. Antioxid Redox Signal. 2011;14:1877–1888. doi: 10.1089/ars.2010.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Pepper MS, Kiss JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163:1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]