Abstract
Objective
Patients with suspected acute aortic syndromes (AAS) often undergo CT with negative results. We sought clinical and diagnostic criteria to identify low risk patients, an initial step in developing a clinical decision rule.
Methods
We retrospectively identified all adults presenting to our Emergency Department (ED) from 1/1/2006- 8/1/2010 who underwent CT angiography for suspected AAS without prior trauma or AAS. 1,465 patients met inclusion criteria; a retrospective case-controlled review (ratio 1:4) was conducted. Cases were diagnosed with aortic dissection, intramural hematoma, penetrating atherosclerotic ulcer or ruptured aneurysm.
Results
2.7% (40/1,465) of patients who underwent CT had an AAS, 2 additional cases were diagnosed after admission [ED miss rate 5% (2/42)]. Patients with AAS were significantly older than controls (66 vs 59 yrs; p=.008). Risk factors included abnormal chest radiograph [sensitivity 79% (26/33), specificity 82% (113/137)] and acute chest pain [sensitivity 83% (29/35), specificity 71% (111/157)]. None of the 19 patients with resolved pain upon ED presentation had AAS. These data support a two-step rule: first screen for ongoing pain; if present, screen for acute chest pain or an abnormal chest radiograph. This approach achieves a 54% (84/155) reduction in CT usage with a sensitivity for AAS of 96% (95% CI: 89%-100%), negative predictive value of 99.8% (99.4%-100%) and a false negative rate of 1.7% (1/84).
Conclusions
Our results demonstrate a need to safely identify patients at low risk for AAS who can forgo CT. We developed a preliminary two-step clinical decision rule, which requires validation.
Keywords: Aortic Dissection, Acute Aortic Syndrome, CT, Diagnosis, Emergency Department
Introduction
Acute aortic dissection is a catastrophic event associated with a pre- and in-hospital mortality rates of 20% and 30% respectively [1], with an estimated annual incidence of 3/100,000 [1-3]. The more general term “acute aortic syndrome” (AAS) has evolved to include aortic dissection, rupture, intramural hematoma and penetrating atherosclerotic ulcer, entities with similar clinical presentations.
To improve survival, rapid diagnosis in the Emergency Department (ED) is essential. Unfortunately, aortic dissection is notoriously difficult to diagnose with only 15-43% of cases suspected upon initial evaluation [3, 4] and up to 30% undiagnosed antemortem [5]. Clinical exam and simple testing such as electrocardiogram and chest radiograph are almost always non-diagnostic, requiring advanced imaging [6-9]. Several imaging modalities can be used to diagnose AAS, but computed tomography (CT) has emerged as the method of choice given its wide availability, speed and accuracy [10, 11]. However, CT scans are often negative. As CT is increasingly relied upon to “rule out” acute aortic syndromes in the ED, there are rising concerns regarding the associated radiation exposure [12, 13] and costs.
The purpose of this study was to explore whether history, physical examination, and simple diagnostic tests can be used to establish pretest probability for AAS in ED patients. Identification of low risk patients is essential to better target CT utilization in order to reduce radiation exposure and costs for ED patients.
Methods
Study Population
We retrospectively reviewed our institutional database for all adults initially presenting to our urban, academic medical center's ED from 1/1/2006-8/1/2010 who underwent a CT scan for suspected AAS and who did not have a history of trauma, AAS or aortic surgery. The study was approved by the Institutional Review Board and was HIPAA compliant. Study participants were identified using Clinical Looking Glass (CLG)®[14], a software application developed to evaluate health care quality, effectiveness, and efficiency using clinical and administrative datasets.
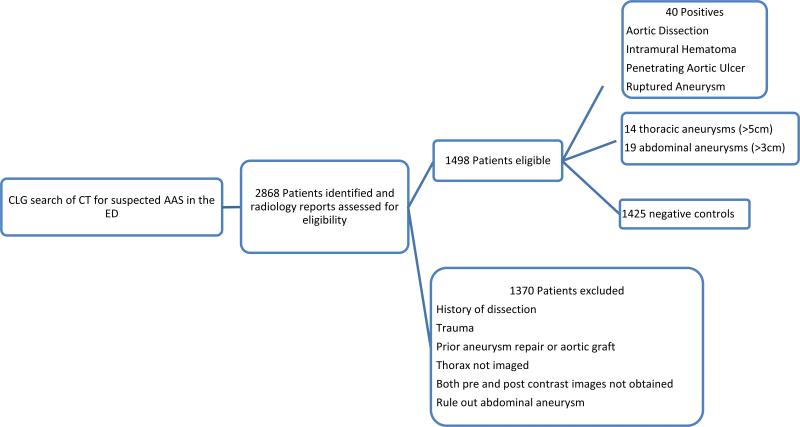
Cases and controls were identified through a 3 step process: CLG database search, radiology report review and ED chart review. Inclusion was limited to patients with a clinical suspicion of AAS in the ED who underwent pre and post contrast CT of the chest and abdomen specifically for suspected AAS. Exclusion criteria included history of trauma, transfer, AAS, or aortic surgery (Fig. 1). Cases were defined as patients with acute aortic dissection, acute aortic intramural hematoma, acute aortic penetrating atherosclerotic ulcer and acute aortic rupture as stated in the CT report and confirmed by image review. A total of 1,425 negative controls and 40 cases were identified. 33 non-ruptured new or enlarging aneurysms (≥5cm for the thoracic aorta, ≥3cm for the abdominal aorta)[1, 11] were identified as an ambiguous group and excluded from the analysis.
Figure 1.
Schematic depicting the identification of cases and controls. Abbreviation: CLG, Clinical Looking Glass; AAS, Acute Aortic Syndrome; ED, Emergency Department
Chart Review
Four controls for each case were randomly selected from the larger pool of 1,425 negatives for detailed chart review. The one to four case: control ratio was chosen to improve the power of the study, since the event rate was low. Review of the ED chart was then performed for case and controls with the reviewer blinded to the ultimate diagnosis. Data on 32 clinical variables including clinical history, physical examination findings, past medical and surgical history and current medications were extracted from the standardized ED physician chart at our institution. Onset of pain was determined to be acute if the ED physician indicated abrupt, acute or immediate onset. Pain was considered resolved if the ED physician documented that the pain had resolved upon initial evaluation. Data on CT and chest radiographic findings were extracted from radiology reports. A chest radiograph was considered abnormal if a widened mediastinum or abnormal aortic contour was reported.
Cost Effectiveness Analysis
Cost effectiveness of the incremental cost of CT to diagnose AAS was compared between current clinical practice and proposed usage according to the algorithm developed from the present study. Cost per quality adjusted life year was also calculated by attributing all years of life of patients whose death data was available to CLG to CT. Patients not known to be dead were assigned a life expectancy estimated using Social Security Administration actuarial tables based on 2007 mortality rates [15]. Recognizing the potential overestimation of life-years gained by assuming “a normal life expectancy”, a sensitivity analysis was conducted for living patients using 10%, 25%, 50%, 75% and 90% discounted life expectancy. Cost per dissection protocol CT was provided by the Department of Radiology billing office ($8,789).
Statistical Analyses
Statistical analysis was conducted using STATA software (Version 11, College Station, TX). Descriptive statistics were performed using χ2 or Fisher exact test for categorical data and Student's T-test or Mann-Whitney U test for continuous data. All variables were assessed for normality and equal variance.
We conducted bivariate analyses to determine the relationship between each risk factor and AAS. Next, we built a predictive multivariate logistic regression model for acute aortic syndrome for patients without missing data. We included all variables with a p<0.25 on bivariate analysis and then performed a stepwise backward elimination using p>0.05 for exclusion. Any variable which perfectly discriminated cases and controls was described separately, as logistic regression fails for perfect. Likelihood ratio tests confirmed that removed variables were not significant predictors (p>0.05), and all variables were tested for confounding and interaction. Regression diagnostics were performed looking for influential observations. The final model was assessed for goodness of fit using the Hosmer-Lemeshow test, and predictive accuracy was assessed using the area under the receiver operating characteristic (ROC) curve and classification tables. Negative and positive predictive values and their 95% confidence intervals were modeled in Revolution R (Palo Alto, CA) by randomly resampling observed prevalence and best model sensitivity and specificity 10,000 times.
Results
Forty of 1,465 patients (2.7%) had CT scans that were positive for AAS. Patients with AAS were older than controls (66 vs 59 years; p=0.008) and were more likely to be white (35.0% vs 17.4%; p=0.01) (Table 1). Among the 40 cases, there were 11 type A dissections, 10 type B dissections, 8 intramural hematomas, 8 aortic ruptures, 2 impending aortic ruptures, and 1 penetrating atherosclerotic ulcer. One day mortality for AAS was 15.4% (6/40) and 15 day mortality was 27.5% (11/40). Of the 1,425 controls, 417 were discharged home from the ED and 990 were admitted to the hospital with an average length of stay of 4.1 ± 5.5 days. The most common admission diagnoses among controls were chest pain (23%), other (20%), other cardiovascular disease (16%), and other gastrointestinal disease (9%) (eTable 1). Among controls, 1 year mortality was 0.79% (113/1,425) and 5 year mortality was 1.1% (164/1,425).
Table 1.
Demographic Characteristics
| Acute Aortic Syndromes n=40 | Negative Controls n=172 | P valuea | Eligible Negatives n=1253 | P valueb | |
|---|---|---|---|---|---|
| Age (yr) | 66 ± 15 | 59 ± 16 | 0.008 | 60 ± 16 | 0.34 |
| Male | 17 (42.5%) | 68 (39.5%) | 0.73 | 541 (43.1%) | 0.48 |
| Race | |||||
| White | 14 (35.0%) | 30 (17.4%) | 0.01 | 218 (17.1%) | 0.9 |
| Black | 10 (25.0%) | 61 (35.5%) | 0.21 | 421 (33.6%) | 0.51 |
| Hispanic | 13 (32.5%) | 69 (40.1%) | 0.55 | 539 (43.0%) | 0.6 |
| Preferred Language | |||||
| English | 30 (75.0%) | 138 (83.6%) | 0.46 | 987 (78.8%) | 0.39 |
| Spanish | 7 (17.5%) | 27 (16.4%) | 0.78 | 244 (19.5%) | 0.28 |
P-value comparing Negative Controls and Acute Aortic Syndromes
P-value comparing Negative Controls to Eligible Negatives
Univariate analysis revealed 11 factors associated with AAS (eTable 2). Chest pain, acute onset of pain, radiation to the back and severe pain were all significant positive predictors of AAS. Past medical history of hyperlipidemia and diabetes were both negative predictors for AAS (p=0.002 and p=0.008, respectively). Among controls, patients with a history of hyperlipidemia were significantly more likely to be admitted with a cardiovascular related diagnosis than patients without a history of hyperlipidemia [OR=2.1, 95% CI 1.04-4.03, p=0.025]. Hypertension was present in a large majority of both cases (82.1%) and controls (74.4%), (p=0.32). Assessment of differential blood pressures was absent in the large majority cases (82.5%) and controls (91.3%) (eTable 2).
Sensitivity and specificity analyses of the five a priori variables hypothesized to be the most relevant predictors of AAS revealed that an abnormal chest radiograph [OR=17.5, p<0.001; sensitivity 78% (26/33), specificity 83% (113/137)] and acute onset chest pain [OR =11.7, p<0.001; sensitivity 83% (29/35), specificity 71% (111/157)] were significant positive predictors (Table 2). Aortic pain, defined as acute onset of chest pain with radiation was also positively associated with AAS [OR=5.2, p<0.001; sensitivity 56% (18/32), specificity 80% (125/156)]. All patients with AAS had unresolved pain upon presentation to ED, a sensitivity of 100% (31/31). Additionally, AAS was ruled out in all 19 subjects with resolved pain upon ED presentation.
Table 2.
Sensitivity, Specificity, PPV and NPV of Acute Aortic Pain Risk Factors
| Sensitivity | 95% CI | Specificity | 95% CI | PPVa | NPVa | |
|---|---|---|---|---|---|---|
| Abnormal Chest Radiograph | 78.8% | 61.1%-91.2% | 82.5% | 75.1%-88.4% | 11% | 99% |
| Aortic Pain | 56.3% | 37.7%-73.6% | 80.1% | 73.0%-86.1% | 0.07% | 99% |
| Acute Chest Pain | 82.9% | 66.4%-93.4% | 70.7% | 62.9%-77.7% | 0.07% | 99% |
| Age > 50 Years | 85.0% | 70.2%-94.3% | 28.5% | 21.9%-35.9% | 0.03% | 99% |
| Unresolved Pain | 100.0% | 89.1%-100% | 12.6% | 7.8%-19.0% | 0.03% | 100% |
PPV and NPV calculated using 2.7% prevalence (40/1465). Abbreviation: PPV, Positive Predictive Value; NPV, Negative Predictive Value
The final logistic model (n=109 controls, n=27 cases) contained acute onset chest pain, abnormal chest radiograph and hyperlipidemia as independent predictors of AAS (Table 3). Patients with resolved pain upon ED presentation were excluded from the second stage logistic model as resolved pain perfectly predicted a negative CT. Acute chest pain was chosen over aortic pain for its higher sensitivity.
Table 3.
Multivariate Regression of Acute Aortic Syndrome Risk Factors
| OR | SE | 95% CI | P Value | |
|---|---|---|---|---|
| Abnormal Chest Radiograph | 66.8 | 58.2 | 12.1 - 368.2 | <0.001 |
| Acute Chest Pain | 17.6 | 14.5 | 3.5 - 88.6 | 0.001 |
| Hyperlipidemia | 0.057 | 0.053 | 0.009 - 0.35 | 0.002 |
Inclusion limited to patients with unresolved pain; n=136; Positive cases n=27; Hosmer-Lemeshow p=0.96; P(Acute Aortic Syndrome)=4.20*(Abnormal Chest Radiograph) + 2.87*(Acute Chest Pain) + −2.86*(Hyperlipidemia) - 4.07
With three dichotomous variables in our logistic model, there were 8 potential probability cutoffs to classify low and high risk patients (eTable 3). A predicted probability of AAS ≤0.0169, corresponding to the absence of both acute chest pain and abnormal chest radiograph with or without hyperlipidemia was chosen as the cutoff for low risk.
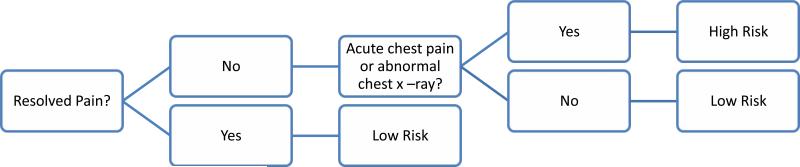
Practically, the approach becomes -first assess for persistent pain. Those with resolved pain are low risk and can forgo CT. For those with persistent pain proceed with the logistic model. If the patient has neither acute chest pain nor an abnormal chest radiograph, the model has a predicted probability of ≤0.019, suggesting that CT can be safely omitted (Fig. 2).
Figure 2.
Diagram of proposed two step screening method to identify patients at high or low risk of acute aortic syndromes.
This two-step rule was applied to the 155 patients who comprised the case-controlled study population. Patients with resolved pain were identified (n=19). Acute chest pain and an abnormal chest radiograph were sought among the remaining patients. For patients with neither acute chest pain nor an abnormal chest radiograph, the preliminary model demonstrated a sensitivity of 96% (26/27) and a specificity of 59% (64/109) for AAS. Adding the 19 patients with resolved pain upon ED presentation to the model resulted in classifying 54% (84/155) of patients at low risk of AAS with 1 false negative among the 84 (1.7%). This two-step method had a sensitivity of 96% (95% CI: 89% - 100%), specificity of 65% (56%-73%), and negative predictive value of 99.8% (99.4% - 100%).
In the process of accruing our study population we identified two patients with AAS who were admitted to the hospital and whose diagnosis was not suspected in the ED, corresponding to an ED miss rate of 5% (2/42). Review of the charts for these misses revealed that, if AAS had been suspected in the ED and the decision rule applied, both patients would have been triaged to undergo CT. Both patients had persistent chest pain and abnormal chest radiographs and one had acute chest pain. Review of case missed in our decision model revealed a type A dissection in a 42 year old female with a history of rheumatic heart disease that presented with acute onset epigastric pain tearing pain that radiated to the back following cocaine use. However the chart did not indicate chest pain and the chest radiograph was normal, thus classifying the patient as negative.
Cost effectiveness analysis for a CT diagnosis of AAS comparing usage based on the proposed algorithm to clinical practice in the study population, revealed a cost savings of $297,896 per AAS diagnosed (eTable 4). Cost per QALY was $21,371 vs $1,548 and sensitivity analysis of 10% to 90% discounted life expectancy revealed a range of $23,735-$206,308 with current clinical practice and $1,719-$14,875 using the described 2-step screening practice (eTable 5).
Discussion
In this study we document the need for a clinical decision rule for patients who present to the ED and are suspected of having an AAS. In our busy urban academic medical center, the positivity rate for patients who underwent CT for suspected AAS was only 2.7%. The ED miss rate was 5%, likely an underestimate given the impossibility of tallying misses that were never detected. These data underscore the need for refining the criteria for appropriately targeting high-risk patients for imaging and safely avoiding imaging in the remaining population. It is desirable to avoid performing CT scans that are not medically necessary because of the risks of intravenous contrast and radiation exposure, as well as the costs of medical imaging which have become a major driver of increasing health care costs in the United States.
Resolved pain perfectly predicted the absence of AAS and is our proposed initial step. Multivariate analysis revealed that acute chest pain, abnormal chest radiograph and absence of hyperlipidemia were independent predictors of AAS. Although atherosclerosis has been postulated to be protective against AAS [3, 16], this result may reflect the generally poorer health status of patients with hyperlipidemia, rather than a protective effect. Patients with hyperlipidemia likely had alternative diagnoses explaining their clinical presentation more often than the general ED population. This is supported by our finding that controls with a history of hyperlipidemia were more likely to be admitted to the hospital with a cardiovascular diagnosis than controls without hyperlipidemia (p=0.025). When reviewing the multivariate model for potential cutoffs, an additional cutoff corresponding to hyperlipidemia in the absence of an abnormal chest x-ray or acute chest pain was considered. This model had an unchanged sensitivity of 96% with an improved specificity of 77%. This cutoff was rejected to facilitate timely risk stratification in the ED and because high sensitivity is the focus of this analysis in order to identify low risk patients who can safely forgo CT scanning.
Acute onset of pain was noted in 89% of cases (31/35) and an abnormal chest radiograph in 79% (26/33), similar to previous literature [6, 9, 17]. Presence or absence of a blood pressure differential, an established risk factor for AAS [6], was not recorded in the charts of 83% (33/40) of cases and 91.3% (157/172) of controls. This is not surprising as the International Registry of Acute Aortic Dissection (IRAD), similarly found that pulse deficit assessment was reported in fewer than 20% of patients [17]. Incomplete documentation has been previously reported, with only 42% of conscious patients asked three basic questions about their pain (quality, radiation, intensity at onset) [18].
In a retrospective review of CT utilization for AAS in the ED, Hayter et al [11] reported a positivity rate of 18% (67/373) without documenting an ED miss rate. Although this number is much higher than the incidence of 2.7% in the present study, there were key differences in the study designs. The present study included only unambiguous incident cases of AAS and excluded patients with prior aortic surgery, aortic dissection and aneurysms. Heyter et al's series was more heterogeneous and included these patients. 27% (18/67) of Hayter el al's positives were patients who had a history of prior dissection or aneurysm repair [11]. These patients should not be considered low risk in a decision rule, the focus of the present study.
In a prospective trial evaluating independent predictors of acute aortic dissection, Von Kodolitsch et al [9] culled a study population of 250 from 41,495 patients seen in the ED for chest or back pain. They eliminated 93.5% (38,819) of patients with an alternative diagnosis which was ascertained through an extensive workup including exercise electrocardiography, Holter monitoring and esophagogastroduodenoscopy [9]. An additional 5.8% (2,426) were culled by the concurrence of two ED physicians [9]. The remaining 250 patients underwent risk stratification criteria based upon abnormal chest radiographs, and pulse/blood pressure differentials. Absence of all three resulted in a 7% probability of dissection. Over half the patients were ultimately diagnosed with acute aortic dissection or intramural hematoma (128/250). The reason for the large discrepancy between Von Kodolitsch et al's 51.2% and our 2.7% incidence of AAS among patients with a clinical suspicion may reflect differences in ED practice patterns between the United States and Europe, study population or study design. The extensive work-up that established an alternative diagnosis in 93.5% of their patients would be difficult to complete during an ED visit in the United States. Additionally, they did not describe an ED miss rate.
The low positivity rate of CT for suspected AAS in the present study population underscores the timeliness of developing a clinical decision rule to effectively identify low risk patients, especially given increasing concerns of radiation exposure from CT angiography [12, 13, 19]. The estimated mean effective dose of a dissection protocol CT is 30 mSv [20], and is associated with a measurable lifetime attributable risk of cancer, even among patients greater than 60 years of age [21].
Comparison of cost for case finding under the current usage rates to proposed usage, revealed a savings of $297,896 per CT diagnosis of AAS. Furthermore, cost per QALY with up to a 90% discounted life expectancy was $21,371-$206,308 based on current usage patterns compared to $1,548-$14,875 with proposed usage. Although no absolute standard exists in the US to determine whether an intervention is “cost effective” with $50,000 as a rough cutoff [22]. Our results compare to $27,540 for single vessel percutaneous coronary intervention [23], and $40,881 for intensive glycemic control in type 2 diabetics [24], and $69,000 for dual air bags vs driver-side air bag only [25]. Even at a 90% discounted life expectancy CT diagnosis is justified in the proposed model, however current clinical practice with greater than a 50% discounted life expectancy is unsupported as cost effective. However, given the wide variation in both inter and intra-state medical imaging costs [26], the generalizability of our cost analysis may be limited.
Major limitations of our study are inherent to its retrospective design including missing data points, small number of events and the fact that it represents a single institution experience. A benefit of our study includes the racially and ethnically diverse population, representing the norm of many urban environments.
The present study documents the need for a clinical decision rule to identify patients at low risk for AAS, who can safely forgo CT scanning. The current practice at our large urban academic ED yields a CT positivity rate of 2.7% and a minimum miss rate of 5% for AAS. We developed a preliminary two-step clinical decision rule based on a retrospective analysis. First assess for chest pain. If the pain has resolved, the patient is at low risk. If the pain has not resolved the patient is low risk if the following two criteria are both met: the pain must be non-acute and the mediastinal and aortic contours must be normal on chest radiography. This preliminary decision rule requires validation in prospective clinical trials in diverse practice settings.
Supplementary Material
Acknowledgements
This publication was made possible by the CTSA Grant UL1 RR025750 and KL2 RR025749 and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessary represent the official view of the NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at Radiological Society of North America (RSNA) Annual Meeting 2011, Chicago, IL, 12/1/11
References
- 1.Olsson C, Thelin S, Stahle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006. 114(24):2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 2.Clouse WD, Hallett JW, Jr., Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176–180. doi: 10.4065/79.2.176. [DOI] [PubMed] [Google Scholar]
- 3.Meszaros I, Morocz J, Szlavi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117(5):1271–1278. doi: 10.1378/chest.117.5.1271. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan PR, Wolfson AB, Leckey RD, et al. Diagnosis of acute thoracic aortic dissection in the emergency department. Am J Emerg Med. 2000;18(1):46–50. doi: 10.1016/s0735-6757(00)90047-0. [DOI] [PubMed] [Google Scholar]
- 5.Bansal RC, Chandrasekaran K, Ayala K, et al. Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography. J Am Coll Cardiol. 1995;25(6):1393–1401. doi: 10.1016/0735-1097(94)00569-C. [DOI] [PubMed] [Google Scholar]
- 6.Klompas M. Does this patient have an acute thoracic aortic dissection? JAMA. 2002;287(17):2262–2272. doi: 10.1001/jama.287.17.2262. [DOI] [PubMed] [Google Scholar]
- 7.Marill KA. Serum D-dimer is a sensitive test for the detection of acute aortic dissection: a pooled meta-analysis. J Emerg Med. 2008;34(4):367–376. doi: 10.1016/j.jemermed.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372(9632):55–66. doi: 10.1016/S0140-6736(08)60994-0. [DOI] [PubMed] [Google Scholar]
- 9.von Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160(19):2977–2982. doi: 10.1001/archinte.160.19.2977. [DOI] [PubMed] [Google Scholar]
- 10.Shiga T, Wajima Z, Apfel CC, et al. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med. 2006;166(13):1350–1356. doi: 10.1001/archinte.166.13.1350. [DOI] [PubMed] [Google Scholar]
- 11.Hayter RG, Rhea JT, Small A, et al. Suspected aortic dissection and other aortic disorders: multi-detector row CT in 373 cases in the emergency setting. Radiology. 2006;238(3):841–852. doi: 10.1148/radiol.2383041528. [DOI] [PubMed] [Google Scholar]
- 12.Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurwitz LM, Reiman RE, Yoshizumi TT, et al. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: implications for cancer induction. Radiology. 2007;245(3):742–750. doi: 10.1148/radiol.2453062046. [DOI] [PubMed] [Google Scholar]
- 14.Bellin E, Fletcher DD, Geberer N, et al. Democratizing information creation from health care data for quality improvement, research, and education-the Montefiore Medical Center Experience. Acad Med. 2010;85(8):1362–1368. doi: 10.1097/ACM.0b013e3181df0f3b. [DOI] [PubMed] [Google Scholar]
- 15.Period life tables. [12/19/2011];Social Security Online. http://www.ssa.gov/oact/STATS/table4c6.html.
- 16.Achneck H, Modi B, Shaw C, et al. Ascending thoracic aneurysms are associated with decreased systemic atherosclerosis. Chest. 2005;128(3):1580–1586. doi: 10.1378/chest.128.3.1580. [DOI] [PubMed] [Google Scholar]
- 17.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283(7):897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 18.Rosman HS, Patel S, Borzak S, et al. Quality of history taking in patients with aortic dissection. Chest. 1998;114(3):793–795. doi: 10.1378/chest.114.3.793. [DOI] [PubMed] [Google Scholar]
- 19.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 20.Mettler FA, Jr., Huda W, Yoshizumi TT, et al. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 21.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold MR. Cost-effectiveness in health and medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 23.Cohen DJ, Bakhai A, Shi C, et al. Cost-effectiveness of sirolimus-eluting stents for treatment of complex coronary stenoses: results from the Sirolimus-Eluting Balloon Expandable Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions (SIRIUS) trial. Circulation. 2004;110(5):508–514. doi: 10.1161/01.CIR.0000136821.99814.43. [DOI] [PubMed] [Google Scholar]
- 24.Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA. 2002;287(19):2542–2551. doi: 10.1001/jama.287.19.2542. [DOI] [PubMed] [Google Scholar]
- 25.Chapman RH, Stone PW, Sandberg EA, et al. A comprehensive league table of cost-utility ratios and a sub-table of “panel-worthy” studies. Med Decis Making. 2000;20(4):451–467. doi: 10.1177/0272989X0002000409. [DOI] [PubMed] [Google Scholar]
- 26.Sinaiko AD, Rosenthal MB. Increased price transparency in health care--challenges and potential effects. N Engl J Med. 2011;364(10):891–894. doi: 10.1056/NEJMp1100041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.