Abstract
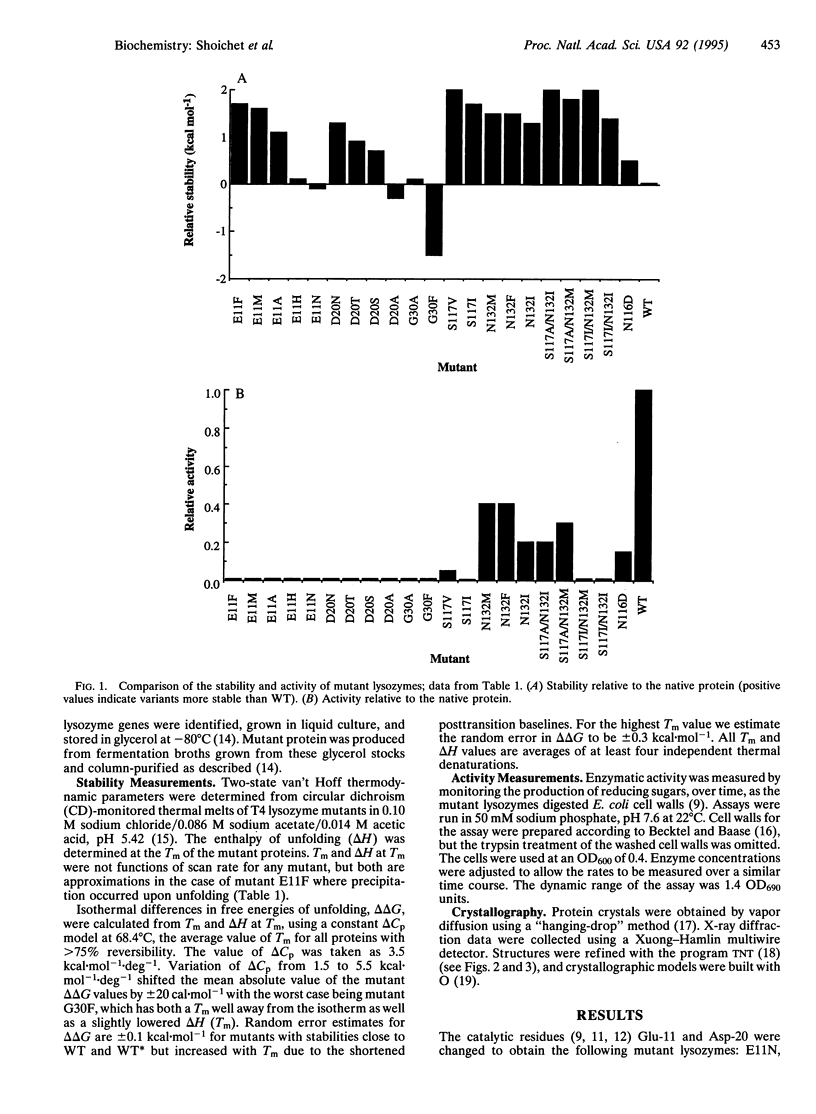
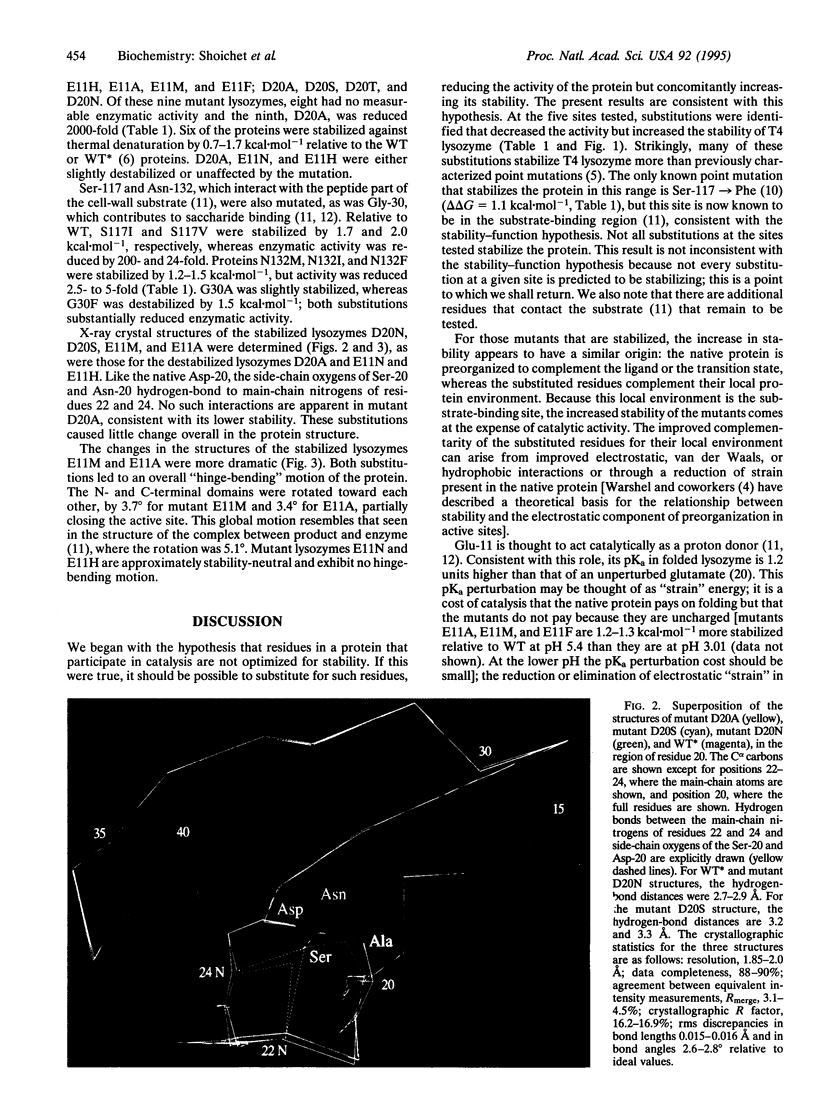
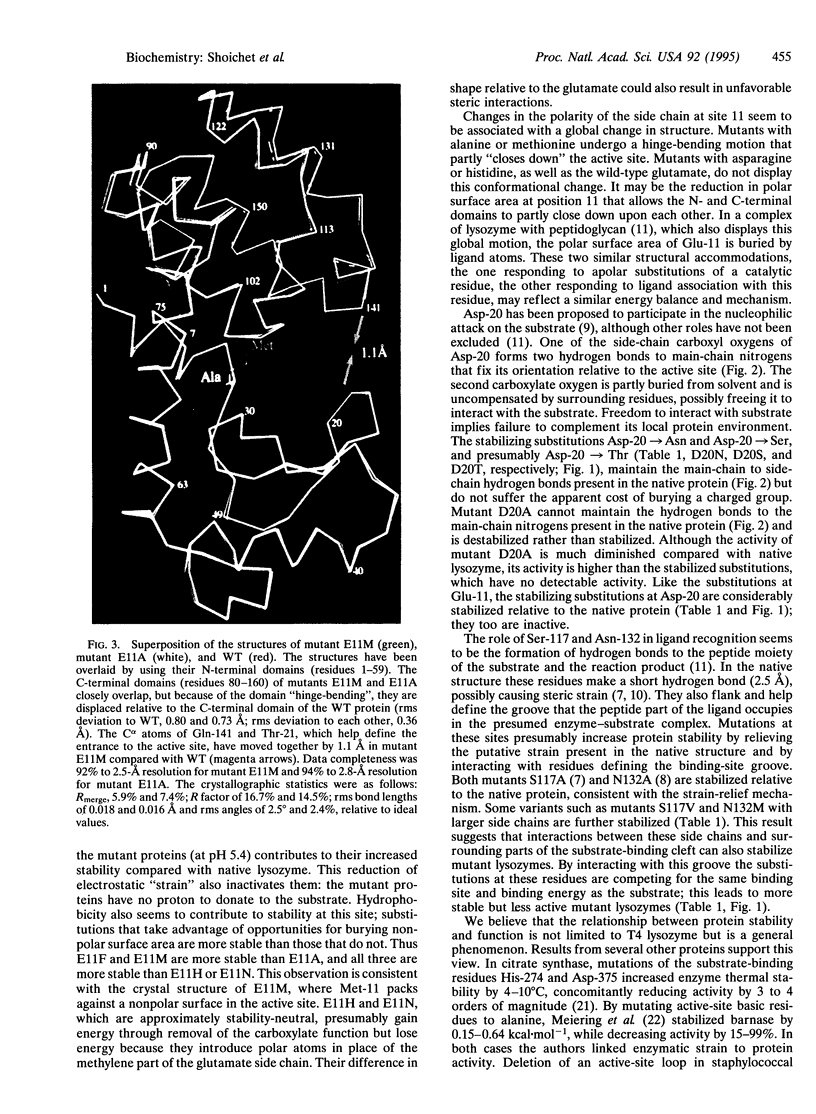
Enzymes are thought to use their ordered structures to facilitate catalysis. A corollary of this theory suggests that enzyme residues involved in function are not optimized for stability. We tested this hypothesis by mutating functionally important residues in the active site of T4 lysozyme. Six mutations at two catalytic residues, Glu-11 and Asp-20, abolished or reduced enzymatic activity but increased thermal stability by 0.7-1.7 kcal.mol-1. Nine mutations at two substrate-binding residues, Ser-117 and Asn-132, increased stability by 1.2-2.0 kcal.mol-1, again at the cost of reduced activity. X-ray crystal structures show that the substituted residues complement regions of the protein surface that are used for substrate recognition in the native enzyme. In two of these structures the enzyme undergoes a general conformational change, similar to that seen in an enzyme-product complex. These results support a relationship between stability and function for T4 lysozyme. Other evidence suggests that the relationship is general.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. E., Hurley J. H., Nicholson H., Baase W. A., Matthews B. W. Hydrophobic core repacking and aromatic-aromatic interaction in the thermostable mutant of T4 lysozyme Ser 117-->Phe. Protein Sci. 1993 Aug;2(8):1285–1290. doi: 10.1002/pro.5560020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. F., Grütter M. G., Remington S. J., Weaver L. H., Matthews B. W. Crystallographic determination of the mode of binding of oligosaccharides to T4 bacteriophage lysozyme: implications for the mechanism of catalysis. J Mol Biol. 1981 Apr 25;147(4):523–543. doi: 10.1016/0022-2836(81)90398-3. [DOI] [PubMed] [Google Scholar]
- Becktel W. J., Baase W. A. A lysoplate assay for Escherichia coli cell wall-active enzymes. Anal Biochem. 1985 Nov 1;150(2):258–263. doi: 10.1016/0003-2697(85)90508-1. [DOI] [PubMed] [Google Scholar]
- Braxton S., Wells J. A. Incorporation of a stabilizing Ca(2+)-binding loop into subtilisin BPN'. Biochemistry. 1992 Sep 1;31(34):7796–7801. doi: 10.1021/bi00149a008. [DOI] [PubMed] [Google Scholar]
- Dao-Pin S., Baase W. A., Matthews B. W. A mutant T4 lysozyme (Val 131----Ala) designed to increase thermostability by the reduction of strain within an alpha-helix. Proteins. 1990;7(2):198–204. doi: 10.1002/prot.340070208. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Baase W. A., Matthews B. W. Similar hydrophobic replacements of Leu99 and Phe153 within the core of T4 lysozyme have different structural and thermodynamic consequences. J Mol Biol. 1993 Feb 5;229(3):747–769. doi: 10.1006/jmbi.1993.1077. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Baase W. A., Wozniak J. A., Matthews B. W. A cavity-containing mutant of T4 lysozyme is stabilized by buried benzene. Nature. 1992 Jan 23;355(6358):371–373. doi: 10.1038/355371a0. [DOI] [PubMed] [Google Scholar]
- Gleason F. K. Mutation of conserved residues in Escherichia coli thioredoxin: effects on stability and function. Protein Sci. 1992 May;1(5):609–616. doi: 10.1002/pro.5560010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy L. W., Poteete A. R. Reexamination of the role of Asp20 in catalysis by bacteriophage T4 lysozyme. Biochemistry. 1991 Oct 1;30(39):9457–9463. doi: 10.1021/bi00103a010. [DOI] [PubMed] [Google Scholar]
- Herzberg O., Moult J. Analysis of the steric strain in the polypeptide backbone of protein molecules. Proteins. 1991;11(3):223–229. doi: 10.1002/prot.340110307. [DOI] [PubMed] [Google Scholar]
- Hibler D. W., Stolowich N. J., Reynolds M. A., Gerlt J. A., Wilde J. A., Bolton P. H. Site-directed mutants of staphylococcal nuclease. Detection and localization by 1H NMR spectroscopy of conformational changes accompanying substitutions for glutamic acid-43. Biochemistry. 1987 Sep 22;26(19):6278–6286. doi: 10.1021/bi00393a048. [DOI] [PubMed] [Google Scholar]
- Inoue M., Yamada H., Hashimoto Y., Yasukochi T., Hamaguchi K., Miki T., Horiuchi T., Imoto T. Stabilization of a protein by removal of unfavorable abnormal pKa: substitution of undissociable residue for glutamic acid-35 in chicken lysozyme. Biochemistry. 1992 Sep 22;31(37):8816–8821. doi: 10.1021/bi00152a018. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kanzaki H., McPhie P., Miles E. W. Effect of single amino acid substitutions at positions 49 and 60 on the thermal unfolding of the tryptophan synthase alpha subunit from Salmonella typhimurium. Arch Biochem Biophys. 1991 Jan;284(1):174–180. doi: 10.1016/0003-9861(91)90280-v. [DOI] [PubMed] [Google Scholar]
- Kelley R. F., DeVos A. M., Cleary S. Thermodynamics of ligand binding and denaturation for His64 mutants of tissue plasminogen activator kringle-2 domain. Proteins. 1991;11(1):35–44. doi: 10.1002/prot.340110105. [DOI] [PubMed] [Google Scholar]
- Kimura S., Nakamura H., Hashimoto T., Oobatake M., Kanaya S. Stabilization of Escherichia coli ribonuclease HI by strategic replacement of amino acid residues with those from the thermophilic counterpart. J Biol Chem. 1992 Oct 25;267(30):21535–21542. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuroki R., Weaver L. H., Matthews B. W. A covalent enzyme-substrate intermediate with saccharide distortion in a mutant T4 lysozyme. Science. 1993 Dec 24;262(5142):2030–2033. doi: 10.1126/science.8266098. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Matthews B. W. Control of enzyme activity by an engineered disulfide bond. Science. 1989 Feb 10;243(4892):792–794. doi: 10.1126/science.2916125. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Structural and genetic analysis of protein stability. Annu Rev Biochem. 1993;62:139–160. doi: 10.1146/annurev.bi.62.070193.001035. [DOI] [PubMed] [Google Scholar]
- Meiering E. M., Serrano L., Fersht A. R. Effect of active site residues in barnase on activity and stability. J Mol Biol. 1992 Jun 5;225(3):585–589. doi: 10.1016/0022-2836(92)90387-y. [DOI] [PubMed] [Google Scholar]
- Mozhaev V. V., Berezin I. V., Martinek K. Structure-stability relationship in proteins: fundamental tasks and strategy for the development of stabilized enzyme catalysts for biotechnology. CRC Crit Rev Biochem. 1988;23(3):235–281. doi: 10.3109/10409238809088225. [DOI] [PubMed] [Google Scholar]
- Pakula A. A., Sauer R. T. Amino acid substitutions that increase the thermal stability of the lambda Cro protein. Proteins. 1989;5(3):202–210. doi: 10.1002/prot.340050303. [DOI] [PubMed] [Google Scholar]
- Poole L. B., Loveys D. A., Hale S. P., Gerlt J. A., Stanczyk S. M., Bolton P. H. Deletion of the omega-loop in the active site of staphylococcal nuclease. 1. Effect on catalysis and stability. Biochemistry. 1991 Apr 16;30(15):3621–3627. doi: 10.1021/bi00229a005. [DOI] [PubMed] [Google Scholar]
- Poteete A. R., Sun D. P., Nicholson H., Matthews B. W. Second-site revertants of an inactive T4 lysozyme mutant restore activity by restructuring the active site cleft. Biochemistry. 1991 Feb 5;30(5):1425–1432. doi: 10.1021/bi00219a037. [DOI] [PubMed] [Google Scholar]
- Scrutton N. S., Deonarain M. P., Berry A., Perham R. N. Cooperativity induced by a single mutation at the subunit interface of a dimeric enzyme: glutathione reductase. Science. 1992 Nov 13;258(5085):1140–1143. doi: 10.1126/science.1439821. [DOI] [PubMed] [Google Scholar]
- Tronrud D. E. Conjugate-direction minimization: an improved method for the refinement of macromolecules. Acta Crystallogr A. 1992 Nov 1;48(Pt 6):912–916. doi: 10.1107/s0108767392005415. [DOI] [PubMed] [Google Scholar]
- Varley P. G., Pain R. H. Relation between stability, dynamics and enzyme activity in 3-phosphoglycerate kinases from yeast and Thermus thermophilus. J Mol Biol. 1991 Jul 20;220(2):531–538. doi: 10.1016/0022-2836(91)90028-5. [DOI] [PubMed] [Google Scholar]
- Warshel A. Energetics of enzyme catalysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5250–5254. doi: 10.1073/pnas.75.11.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A., Sussman F., Hwang J. K. Evaluation of catalytic free energies in genetically modified proteins. J Mol Biol. 1988 May 5;201(1):139–159. doi: 10.1016/0022-2836(88)90445-7. [DOI] [PubMed] [Google Scholar]
- Williams R. J. The entatic state. Cold Spring Harb Symp Quant Biol. 1972;36:53–62. doi: 10.1101/sqb.1972.036.01.010. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu Z. P., Jones T. A., Gierasch L. M., Sambrook J. F. Mutating the charged residues in the binding pocket of cellular retinoic acid-binding protein simultaneously reduces its binding affinity to retinoic acid and increases its thermostability. Proteins. 1992 Apr;13(2):87–99. doi: 10.1002/prot.340130202. [DOI] [PubMed] [Google Scholar]
- Zhang X. J., Baase W. A., Matthews B. W. Multiple alanine replacements within alpha-helix 126-134 of T4 lysozyme have independent, additive effects on both structure and stability. Protein Sci. 1992 Jun;1(6):761–776. doi: 10.1002/pro.5560010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi W., Srere P. A., Evans C. T. Conformational stability of pig citrate synthase and some active-site mutants. Biochemistry. 1991 Sep 24;30(38):9281–9286. doi: 10.1021/bi00102a021. [DOI] [PubMed] [Google Scholar]





