Abstract
Background
Uncertainty exists whether hearts from infants who have died of sudden infant death syndrome (SIDS) are acceptable for transplantation because the mechanism of death in SIDS remains unclear. We analyzed post-transplant outcomes in infants who received a heart from a donor where SIDS was the primary cause of brain death.
Methods
This retrospective multicenter cohort study used data from the Organ Procurement and Transplant Network (OPTN). All infants aged <12 months undergoing heart transplant between 1994 and 2008 were included. A Cox proportional hazards model was used to determine whether donor SIDS was independently associated with post-transplant graft loss (death or retransplant).
Results
During the study period, 66 of 1033 infants (6.4%) who underwent heart transplant received an allograft from a SIDS donor. These infants were similar to the remaining infants with respect to age, diagnosis, blood type, and invasive support. In multivariable analysis, graft loss was associated with congenital heart disease (hazard ratio [HR], 1.6; 95% confidence interval [CI], 1.2–2.1), ventilator (HR, 1.4; 95% CI, 1.1–1.9), and extracorporeal membrane oxygenation support (HR, 3.0; 95% CI, 2.2–4.3), but not donor SIDS (HR, 1.0; 95% CI, 0.6–1.5), suggesting graft survival in SIDS-donor heart recipients was similar to the remaining infants. Primary causes of post-transplant death in infants receiving SIDS-donor hearts and the remaining infants were similar.
Conclusions
Graft survival was similar in infants who received SIDS-donor hearts compared with those who received hearts from donors who died of other causes. There was no increase in incidence of non-rejection-related cardiac deaths after transplant in these children.
Keywords: infant heart transplantation, sudden infant death syndrome, outcome
Sudden infant death syndrome, or SIDS, is a leading cause of death for infants aged younger than 1 year, with an estimated annual incidence of 0.57 per 1,000 live births.1 Most infants with SIDS are declared dead shortly after presentation after cardiopulmonary resuscitation is unsuccessful. However, infants who can be resuscitated may progress to brain death secondary to the hypoxic-ischemic event, thus becoming potential organ donors. Because the precise mechanism in SIDS remains unclear,2–5 controversy exists within the pediatric heart transplant (HT) community about whether it is safe to accept donor hearts from infant donors who have died of SIDS. Yet because of the critical organ shortage for infants awaiting HT, a better understanding of post-HT outcomes of infants who receive a donor heart from an infant who died of SIDS would be helpful in determining whether organ recovery from SIDS patients can be performed without undue risk to the recipient. Therefore, the specific aims of this study were:
to determine the prevalence and recent trends in the use of hearts from infants who became organ donors after SIDS,
to determine whether graft survival for infants receiving a SIDS-donor heart is similar to that of infants who receive donor hearts from children who died of other causes, and
to determine whether the causes of death after HT are similar for infants receiving SIDS-donor hearts vs non-SIDS-donor hearts.
The primary study hypothesis was that graft survival in infants who received a donor heart from an infant who died of SIDS is similar to graft survival in infants who received a donor heart from a child with a cause of death other than SIDS, after adjusting for patient factors.
Methods
Study population
All infants aged <12 months and weighing <10 kg who underwent their first orthotopic HT in the United States between January 1994 and May 2008 were identified retrospectively using Organ Procurement Transplant Network (OPTN) data provided by the United Network for Organ Sharing (UNOS). The OPTN database is internally audited, mandatory, government-sponsored, and collects information on all solid-organ transplants performed in the United States. Demographic and clinical information is reported by the transplant centers to the OPTN. The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN contractor. Infants who underwent re-HT or multivisceral HT were excluded. All patients were followed-up from the time of HT until death or the last day of observation on May 20, 2008.
Study definitions and outcome measures
The primary outcome variable was time to graft loss (death or retransplant). Patients were monitored from the time of their HT to the time of death, retransplant, or last follow-up on May 20, 2008. All clinical and demographic variables were defined at the time of transplant, unless otherwise specified. Race/ethnicity data (categories included black, white, Hispanic, Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, multiracial, and other) and cause of death were analyzed as reported by the transplant center. Non-rejection graft loss, defined as graft loss due to causes other than rejection (primary graft dysfunction, non-specific graft dysfunction, cardiac arrest, ventricular failure, and multi-organ failure), was used as an end point because many patients who experience sudden/arrhythmic cardiac death may be miscategorized for cause of death. Creatinine clearance was estimated using the Schwartz formula.6
Statistical analysis
Summary statistics are presented as median with interquartile range (IQR) or as number and percentage. Patient characteristics were compared using Fisher’s exact test for categoric variables and the Wilcoxon rank sum test for continuous variables. Post-transplant graft survival was estimated by the Kaplan-Meier method. Univariate relationships between patient characteristics and post-transplant graft loss were evaluated using the log-rank test. A multivariable Cox proportional hazards model using a stepwise selection technique was developed. Only risk factors that were statistically significant at the 0.05 level were retained in the final model. The proportion of children who died of non-rejection graft loss at 1 year in each group was compared using a chi-square test. Data were analyzed using statistical software SAS 9.1 (SAS Institute Inc, Cary, NC) and STATA 10.0 software (StataCorp LP, College Station, TX).
Results
Patients
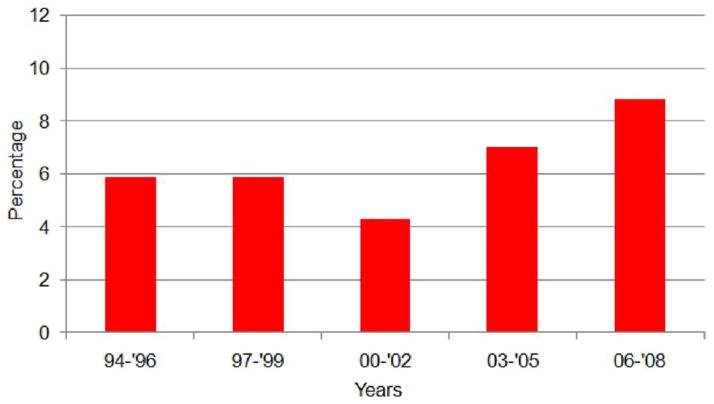
Between 1994 and 2008, 1,033 infants aged < 12 months underwent HT in the United States, of which 66 (6.4%) received a heart from a SIDS donor. Figure 1 shows the time trend in the percentage of hearts that had a donor cause of death listed as SIDS. Although not statistically significant, there has been a gradual trend toward greater use of SIDS-donor hearts (p = 0.21 test for trend) during the past 15 years, with the number of SIDS donors used from 2006 to 2008 reaching nearly 9%. There were no significant regional differences in the use of these donor hearts.
Figure 1.
Proportion of transplanted infant donor hearts where the cause of death was sudden infant death syndrome.
A comparison of baseline characteristics between infants in the 2 groups (infants who received a SIDS-donor hearts and the remaining infants or controls) in the study population is summarized in Table 1. There was no significant difference in the use of invasive support (ie, extracorporeal membrane oxygenation, ventilator, or dialysis), blood type, or percentage hospitalized at the time of transplant between infants who received SIDS-donor hearts and controls. Infants who received a SIDS-donor heart were more likely to be girls (p = 0.02) and require prostaglandin support (p = 0.035). There was a trend toward a higher prevalence of congenital heart disease in the SIDS donor group (p = 0.06). Left ventricular ejection fraction was reported in 48% of infants in the SIDS-donor heart group and in 49% of the controls and was not different between the 2 groups. Ischemic time was available in more than 95% of infants in both groups was also similar in the 2 groups (Table 1).
Table 1.
Baseline Characteristics of the Study Cohort
| Characteristicsa | Heart recipient | p-value | |
|---|---|---|---|
| SIDS donor (N = 66) | Non-SIDS donor (N = 967) | ||
| Weight, kg | 4.1 (3.3, 5.0) | 4.5 (3.5, 5.8) | 0.068 |
| Age, mon | 2.6 (1.6, 4.2) | 3.1 (1.3, 6.0) | 0.19 |
| Female gender, % | 56 | 41 | 0.020 |
| Non-Caucasian race, % | 29 | 32 | 0.59 |
| Support pre-transplant, % | 0.69 | ||
| ECMO | 12 | 9 | |
| Ventilator | 24 | 27 | |
| Other | 64 | 63 | |
| Dialysis peri-transplant, % | 2 | 2 | 1.0 |
| Hospitalized | 85 | 81 | 0.62 |
| Diagnosis | |||
| CHD | 79 | 67 | |
| Other | 21 | 33 | 0.056 |
| Prostaglandin support, % | 35 | 23 | 0.035 |
| Donor LVEF, % | 65 (60, 73) | 65 (60, 72) | 0.58 |
| Ischemic time, hours | 4.1 (3.1, 4.8) | 3.8 (3.0, 4.5) | 0.16 |
CHD, congenital heart disease; ECMO, extracorporeal membrane oxygenation; LVEF, left ventricular ejection fraction.
Values represent percentage of reported values or median (interquartile range).
Outcome
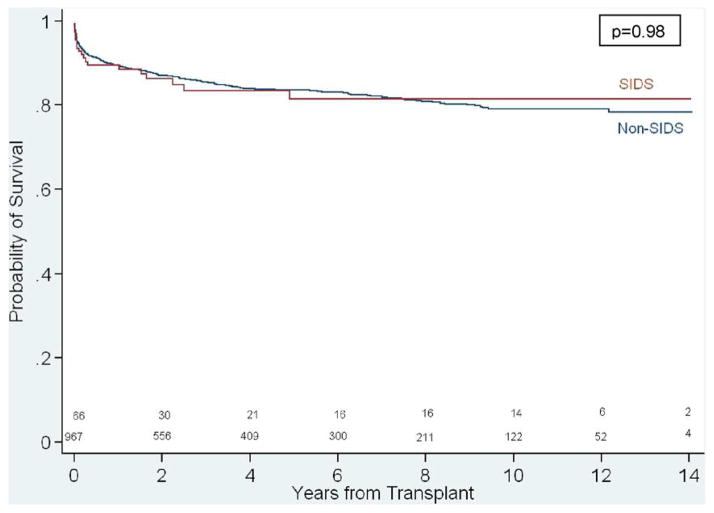
Overall, graft loss occurred in 311 patients (307 deaths, 4 retransplants) with a median graft survival of 14.0 years. Adjusted graft survival in infants who received a SIDS-donor heart and controls was similar (p = 0.98, Figure 2). In multivariable analysis, risk factors of post-transplant graft loss (Table 2)included congenital heart disease, extracorporeal membrane oxygen support, ventilator support, dialysis, and earlier era (1994–1997). After adjustment for these risk factors, receiving a SIDS-donor heart was not associated with post-transplant graft loss (HR, 1.0; 95% CI, 0.6–1.5, p = 0.96). When patient survival was used as the dependent variable in the multivariable analysis, use of a SIDS-donor heart was not associated with patient survival after adjusting for patient factors (HR, 1.0; 95% CI, 0.6–1.6; p = 0.98).
Figure 2.
Unadjusted Kaplan-Meier survival of outcomes comparing sudden infant death syndrome (SIDS) and non-SIDS heart transplant recipients.
Table 2.
Multivariable Factors Associated With Graft Loss After Heart Transplant Using Cox Proportional Hazards Modela
| Variable | HR (95% CI) | p-value |
|---|---|---|
| CHD diagnosis | 1.6 (1.2–2.1) | 0.001 |
| ECMO support | 3.0 (2.2–4.3) | <0.001 |
| Ventilator support | 1.4 (1.1–1.9) | 0.005 |
| Dialysis peri-transplant | 3.4 (2.0–5.9) | <0.001 |
| Year of listing 1994–1997 | 1.3 (1.1–1.7) | 0.019 |
| Donor cause of death: SIDS | 1.0 (0.6–1.5) | 0.96 |
CHD, Congenital heart disease; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; SIDS, sudden infant death syndrome.
The number of graft loss events in each group were as follows: CHD, 235 events/702 patients; ECMO support, 50 events/99 patients; ventilator support, 90 events/280 patients; dialysis, 15 events/20 patients; transplant year 1994–1997, 120 events/303 patients; donor death due to SIDS, 21 events/66 patients.
For infants undergoing HT with a SIDS vs non-SIDS-donor heart, the proportion of patients who died from non-rejection-related cardiac deaths was similar in both groups at 1 year after HT (1 year survival, 92% in SIDS group vs 93% in non-SIDS group, p = 0.98). Also, there was no difference in rejection related deaths between the SIDS and non-SIDS recipients at 1 and 5 years after HT (p = 0.67 and p = 0.72, respectively).
Discussion
Infant (<1 year old) HT currently accounts for approximately 30% of all pediatric HTs in North America.7 The number of infant HTs is approximately 100 per year and has remained relatively constant during the past 15 years.7 Indeed, infants are both the most common group listed for HT and face the highest waiting list mortality of any age group listed for HT.8 Of the 3098 infants listed in the past 6 years, 533 (17%) have died while awaiting a suitable donor. 8 The possibility of expanding the donor pool to include SIDS donors may be a potential strategy for safely increasing the number of infant donors. Although only a small proportion of these SIDS infants can be expected to regain a spontaneous circulation with cardiopulmonary resuscitation to become a candidate for heart-beating organ donation, unpublished data from the Pediatric Health Information System (PHIS) database from 2004 suggests that more than 100 infants with a final diagnosis of SIDS had sufficient circulatory function to be admitted to an inpatient pediatric bed before their eventual death.
There has long been concern about the safety of using donor hearts from infants who died of SIDS, largely because the precise mechanism of death is poorly understood. Potential underlying conditions that may be undiagnosed but result in SIDS include cardiac channelopathies,2–5, 9–15 metabolic syndromes,16 and infectious etiologies.17 Nevertheless, the lack of a clear mechanism of death leaves open the possibility that the donor’s death may have been caused by an unrecognized cardiac event to which a HT recipient could also be susceptible.
Despite these concerns, our study found (1) the use of SIDS-donor hearts for infant HT has increased slightly since the mid-1990s, with SIDS-donor hearts accounting for approximately 10% of all infant heart transplants, (2) graft survival in infants receiving a SIDS-donor heart was similar to controls after adjusting for known risk factors for post-transplant mortality, and (3) the proportion of infants receiving a SIDS-donor heart who experienced a non-rejection-related cardiac death was not different compared with children who had received a non-SIDS-donor heart.
Our findings are consistent with work by Boucek et al that analyzed donor and recipient data for 25 pediatric HT patients and found that the use of SIDS-donor hearts was not associated with post-transplant mortality.18 However, this study was limited by small sample size, with only 4 of the 25 donors (16%) having SIDS listed as the cause of death. The present study is the first, to our knowledge, to systematically analyze outcomes in pediatric HT recipients from SIDS donors in a relatively large cohort of patients with extended follow-up available.
Our findings also suggest that the use of brain-dead SIDS donors with normal left ventricular function may not necessarily be as high risk as one may assume. Although approximately 10% of SIDS victims may have cardiac channelopathies5 that predispose to sudden cardiac death, we did not find increased mortality amongst SIDS-donor heart recipients compared with non-SIDS-donor heart recipients. Although it is difficult to exclude entirely some risk associated with this group due to the small numbers of patients, our finding suggest that the overall risk for patients receiving a SIDS-donor heart is not significantly different to the risk conferred by other donors. A potential etiology for this may be that autonomic denervation occurs in the process of performing a heart transplant, which may by a therapeutic strategy for channelopathies and may potentially reduce the adverse effect of transplanting donor hearts from SIDS victims with these channelopathy diagnoses.19
Our findings should not be interpreted to mean that all SIDS-donor hearts can be used safely for HT. Other findings on routine pre-transplant evaluation, including 12-lead electrocardiogram, echocardiogram, and detailed history (including family history) should all be considered in decision making when such a heart is offered. Our findings suggest that when this evaluation is within reasonable limits for an acceptable donor, a donor cause of death of SIDS does not lead to increased post-transplant mortality or increased risk of sudden cardiac death.
There are several potential limitations related to the study design. First, this was a retrospective cohort study and has the study limitations associated with this type of study design. Second, despite this analysis containing the largest collection of SIDS-heart infant transplant recipients, the sample size is relatively small and may be underpowered to detect a small difference. Third, the possibility of misclassification for donor cause of death cannot be excluded. We were unable to review the surface 12-lead electrocardiograms (ECGs) from donor patients to measure corrected QT intervals or to analyze T wave morphology. Similarly, we do not have genetic testing for cardiac channelopathies from donors. The calculated corrected QT interval from donors was not collected as part of the database. It is likely, however, that patients selected as suitable donors were likely to have normal-appearing 12-lead ECGs and echocardiograms, whereas those with very abnormal ECGs or echocardiograms were likely rejected as potential heart donors. We believe this possibility, instead of being looked at as an inherent selection bias in this analysis, should be viewed as a real-world evidence for our conclusions that carefully selected SIDS-donor hearts may not necessarily represent high-risk donors.
In summary, infant heart transplant recipients who receive a SIDS-donor heart appear to have post-transplant graft survival similar to that of infants who receive non-SIDS-donor hearts. Use of donor hearts from infants who died of SIDS but are otherwise judged suitable for transplant may be a reasonable strategy to expand the donor pool.
Footnotes
Disclosure statement
This study was supported in part by the Alexa Clinton Family, the Boston Children’s Heart Foundation and the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
References
- 1.Malloy MH, Freeman DH. Age at death season and day of death as indicators of the effect of the back to sleep program on sudden infant death syndrome in the United States 1992–1999. Arch Pediatr Adolesc Med. 2004;158:359–365. doi: 10.1001/archpedi.158.4.359. [DOI] [PubMed] [Google Scholar]
- 2.Southall DP, Arrowsmith WA, Oakley JR, McEnery G, Anderson RH, Shinebourne EA. Prolonged QT interval and cardiac arrhythmias in two neonates: sudden infant death syndrome in one case. Arch Dis Child. 1979;54:776–779. doi: 10.1136/adc.54.10.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maron BJ, Clark CE, Goldstein RE, Epstein SE. Potential role of QT prolongation in sudden infant death syndrome. Circulation. 1976;54:423–430. doi: 10.1161/01.cir.54.3.423. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman MJ, Siu BL, Sturner WQ, et al. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. JAMA. 2001;286:2264–2269. doi: 10.1001/jama.286.18.2264. [DOI] [PubMed] [Google Scholar]
- 5.Tester DJ, Ackerman MJ. Cardiomyopathic and channelopathic causes of sudden unexplained death in infants and children. Annu Rev Med. 2009;60:69–84. doi: 10.1146/annurev.med.60.052907.103838. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 7.Boucek MM, Aurora P, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: tenth official pediatric heart transplantation report—2007. J Heart Lung Transplant. 2006;26:796–807. doi: 10.1016/j.healun.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Almond CS, Thiagarajan RR, Piercey GE, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation. 2009;119:717–727. doi: 10.1161/CIRCULATIONAHA.108.815712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz PJ, Stramba-Badiale M, Segantini A, et al. Prolongation of the QT interval and the suddent infant death syndrome. N Engl J Med. 1998;338:1709–1714. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 10.Arnestad M, Crotti L, Rognum TO, et al. Prevalence of Long-QT syndrome gene variants in sudden infant death syndrome. Circ. 2007;115:361–367. doi: 10.1161/CIRCULATIONAHA.106.658021. [DOI] [PubMed] [Google Scholar]
- 11.Otagiri T, Kijima K, Osawa M, et al. Cardiac ion channel gene mutations in sudden infant death syndrome. Pediatr Res. 2008;64:482–487. doi: 10.1203/PDR.0b013e3181841eca. [DOI] [PubMed] [Google Scholar]
- 12.Behr E, Wood DA, Wright M, et al. Cardiological assessment of first-degree relatives in sudden arrhythmic death syndrome. Lancet. 2003;362:1457–1459. doi: 10.1016/s0140-6736(03)14692-2. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz PJ. Cardiac sympathetic innervations and the sudden infant death syndrome: a possible pathogenic link. Am J Med. 1976;10:167–172. doi: 10.1016/0002-9343(76)90425-3. [DOI] [PubMed] [Google Scholar]
- 14.Maron BJ, Clark CE, Goldstein RE, Epstein SE. Potential role of QT interval prolongation in sudden infant death syndrome. Circulation. 1976;54:423–430. doi: 10.1161/01.cir.54.3.423. [DOI] [PubMed] [Google Scholar]
- 15.Tester DJ, Ackerman MJ. Sudden infant death syndrome: how significant are the cardiac channelopathies? Cardiovasc Res. 2005;67:388–396. doi: 10.1016/j.cardiores.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Lantz PE, Ibdah JA. Post-mortem analysis for two prevalent beta-oxidation mutations in sudden infant death. Pediatr Int. 2007;49:883–887. doi: 10.1111/j.1442-200X.2007.02478.x. [DOI] [PubMed] [Google Scholar]
- 17.Stray-Pedersen A, Vege A, Rognum TO. Helicobacter pylori antigen in stool is associated with SIDS and sudden infant deaths due to infectious disease. Pediatr Res. 2008;65:405–410. doi: 10.1203/PDR.0b013e31818095f7. [DOI] [PubMed] [Google Scholar]
- 18.Boucek MM, Kanakriyeh MS, Mathis CM, Trimm RF, Bailey LL. Cardiac transplantation in infancy: donors and recipients. J Pediatr. 1990;116:171–176. doi: 10.1016/s0022-3476(05)82870-7. [DOI] [PubMed] [Google Scholar]
- 19.Atallah J, Fynn-Thompson F, Cecchin F, et al. Video-assisted thoracoscopic cardiac denervation: a potential novel therapeutic option for children with intractable ventricular arrhythmias. Ann Thorac Surg. 2008;86:1620–1625. doi: 10.1016/j.athoracsur.2008.07.006. [DOI] [PubMed] [Google Scholar]