Abstract
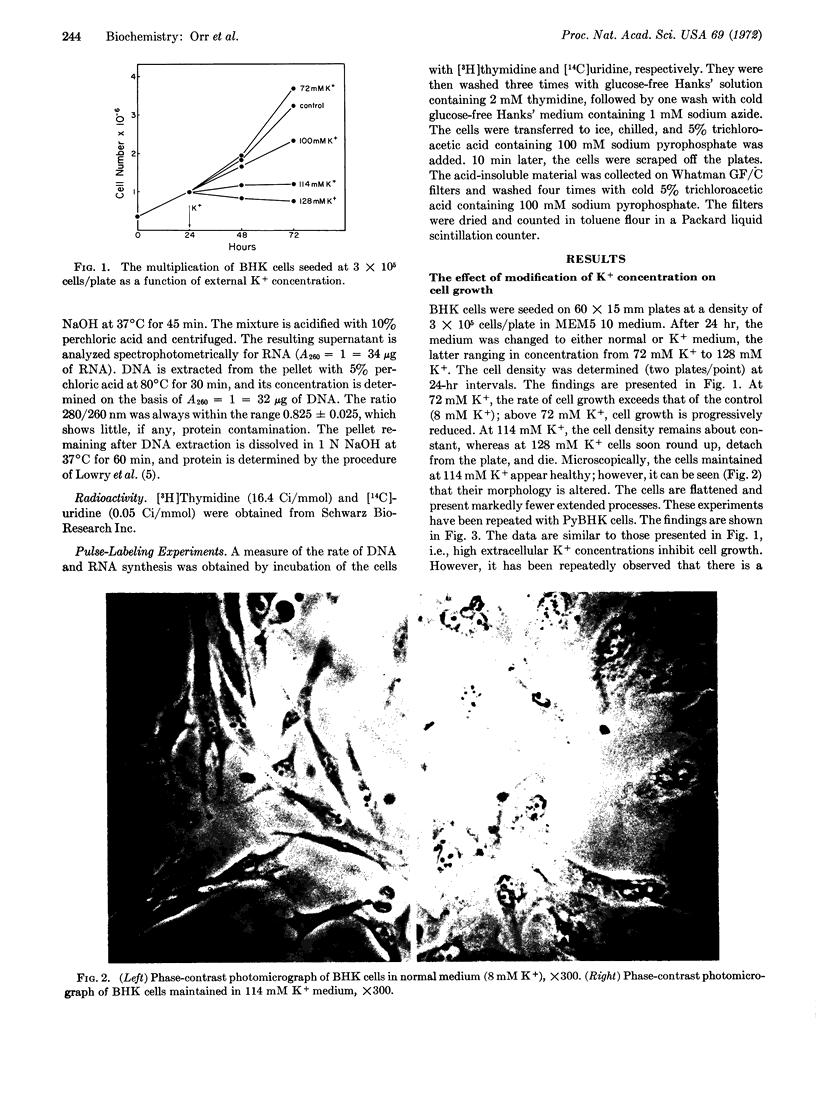
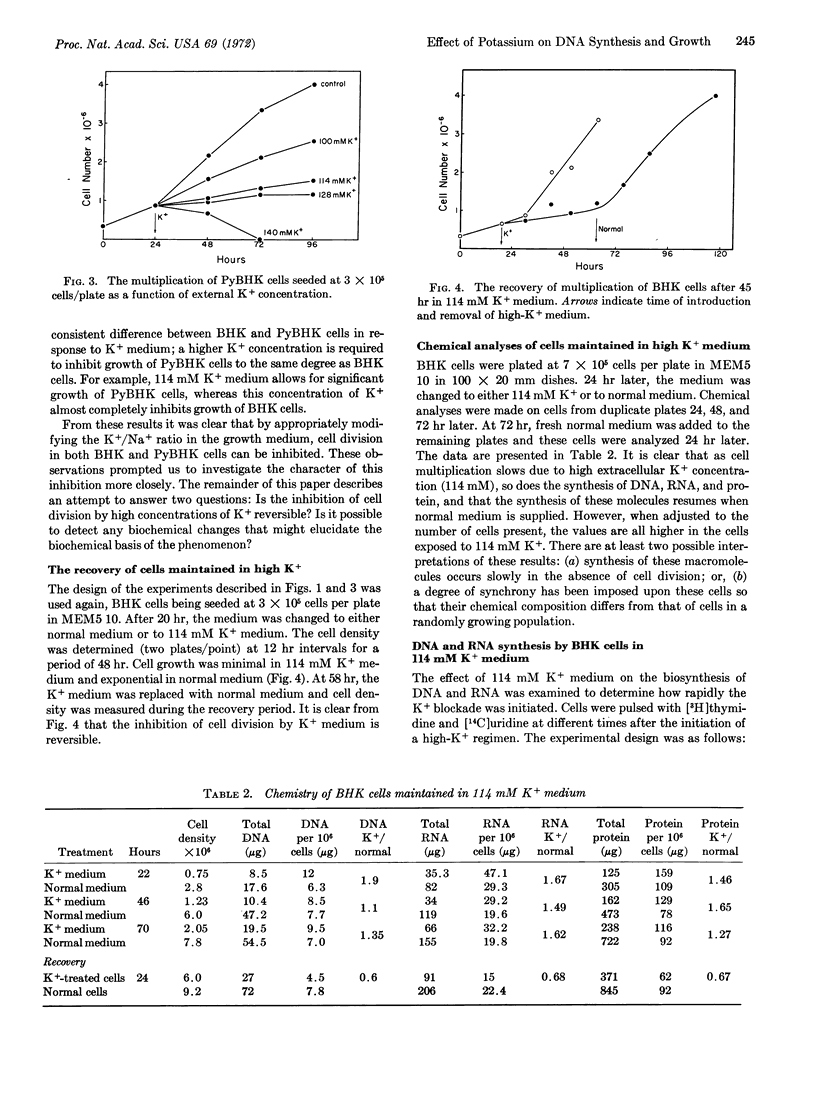
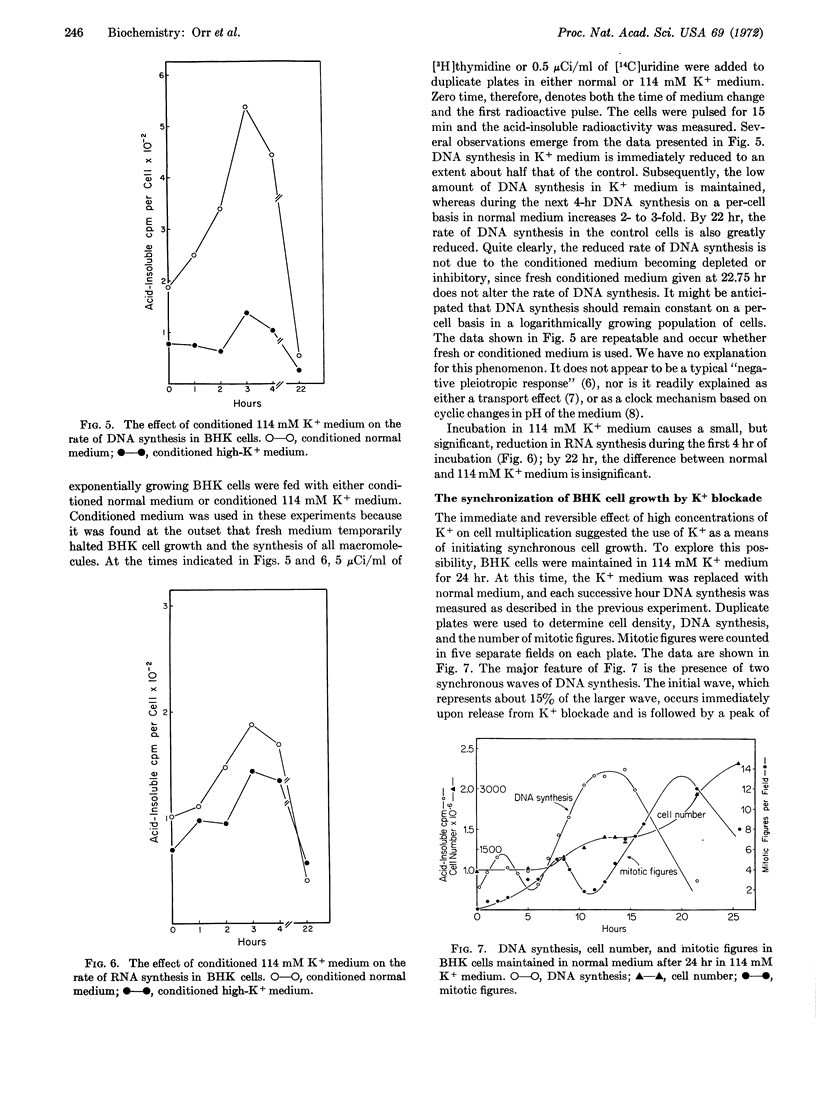
The relations between DNA synthesis, cell multiplication, and external potassium concentration have been investigated in cultured baby-hamster kidney cells. When the potassium concentration was raised from 8 mM to 114 mM by equimolar replacement of sodium, DNA synthesis and cell multiplication were almost completely inhibited. This inhibition was reversible even after 72 hr of incubation in medium with a high concentration of potassium. There is a consistent difference between the cultured cells and polyoma virus-transformed cells in response to high-potassium medium, a higher-potassium concentration being required to inhibit multiplication of polyoma virus-transformed cells to the same extent as that of the nontransformed cells.
Keywords: cell cycle, membrane potential, synchronization, transformation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceccarini C., Eagle H. pH as a determinant of cellular growth and contact inhibition. Proc Natl Acad Sci U S A. 1971 Jan;68(1):229–233. doi: 10.1073/pnas.68.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill J. R., Studzinski G. P. Thymidine as synchronizing agent. 3. Persistence of cell cycle patterns of phosphatase activities and elevation of nuclease activity during inhibition of DNA synthesis. J Cell Physiol. 1970 Jun;75(3):297–303. doi: 10.1002/jcp.1040750306. [DOI] [PubMed] [Google Scholar]
- Cone C. D., Jr Maintenance of mitotic homeostasis in somatic cell populations. J Theor Biol. 1971 Jan;30(1):183–194. doi: 10.1016/0022-5193(71)90043-9. [DOI] [PubMed] [Google Scholar]
- Cunningham D. D., Pardee A. B. Transport changes rapidly initiated by serum addition to "contact inhibited" 3T3 cells. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1049–1056. doi: 10.1073/pnas.64.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Kuchler R. J. The role of sodium and potassium in regulating amino acid accumulation and protein synthesis in LM-strain mouse fibroblasts. Biochim Biophys Acta. 1967 Apr 25;136(3):473–483. doi: 10.1016/0304-4165(67)90006-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]



