Highlights
-
•
Definition of a focus for a pregnancy of unknown location should use all clinical, laboratory data available.
-
•
Choriocarcinoma should be considered among the possibilities in the diagnosis of pregnancy of unknown location.
-
•
Radiological whole body imaging for this purpose should especially include FDG-PET.
-
•
Differentiating a nongestational choriocarcinoma may change the whole therapeutical approach.
Keywords: Choriocarcinoma, Primary renal artery, Secondary renovascular hypertension, Pregnancy of unknown location
Abstract
INTRODUCTION
Choriocarcinoma is a rare primary germ cell tumour of the ovary composed of cyto- and syncytotrophoblast cells. Most of the choriocarcinomas are normally arising in the gestational trophoblast, gonads and, less frequently, mediastinum, pineal gland and retroperitoneum.
PRESENTATION OF CASE
We report a case of primary choriocarcinoma of renal artery causing secondary renovascular hypertension in a 28 years old woman of reproductive age, presenting with abdominal pain, minimal vaginal bleeding and a delayed menstrual period.
DISCUSSION
Non-gestational choriocarcinomas, are histologically related to the pregnancy related gestational choriocarcinomas. These two subtypes may have to be differentiated according the clinical and radiological findings and DNA analysis may be used for this purpose as well. In many studies, authors have stated that nongestational choriocarcinoma diagnosis could be implemented in situations where the presence of a pregnancy could not be considered like the prepubertal period.
CONCLUSION
Choriocarcinoma should as well be considered among the possibilities in the differential diagnosis of the causes for secondary hypertension, especially within a picture of pregnancy of unknown location, albeit being one of the rarest.
1. Introduction
Choriocarcinoma is a rare primary germ cell tumour of the ovary composed of cyto- and syncytotrophoblast cells.1 Most of the gestational choriocarcinomas stem from the uterine cavity. These may show up as consequences of normal or abnormal gestations. The incidence in the United States is within a rate of 110–120 per 100,000 pregnancies.2 Non-gestational choriocarcinomas may be presented as a component of a mixed germ cell tumours of the ovary or as pure choriocarcinomas. Non-gestational choriocarcinomas of the ovary comprise a very small percentage of all the ovarian carcinomas (0.6%); and extremely rarely observed as ‘pure choriocarcinoma of the ovary’.3 Extragonadal nongestational choriocarcinomas may be misdiagnosed as ‘gestational choriocarcinoma metastasis’; and in many patients, the prognosis is worsened due to delayed diagnosis. In performing the differential diagnosis for postmenarcheal cases gestational choriocarcinoma metastasis must be ruled out.1 Due to their rarity, information about their clinical behaviour, diagnosis and treatment is limited.3
2. Case report
A 28 years old woman applied to the emergency section of our hospital with lower pelvic pain, flank pain, minimal vaginal bleeding and a delayed menstrual period. She had nothing remarkable in her obstetrical and medical histories. She had 2 previous vaginal deliveries with the latest being 4 years prior to the time being. In the initial evaluation, her general condition was stable, blood pressure (100/60 mmHg) and pulse values (90 beats per minute) normal, body temperature: 37.2 °C. and had a minimal abdominal guarding. Speculum examination revealed no more than a minimal extra uterine oozing. Transvaginal sonography revealed normal pelvic organs and endometrial thickness as 13 mm. Initial lab findings were within normal limits. Blood electrolyte levels, hepatic and renal function tests were normal. (AST: 11 mIU/dl; ALT: 6 mIU/dl; ALP: 109 mIU/dl; LDH: 141 mIU/dl; total bilirubin: 0.27 mg/dl; total protein: 6.86gm/dl; urea: 17 mg/dl; creatinine: 0.72 mg/dl). Blood CRP (C-reactive protein) level was 278 mg/l. The β-human chorionic gonadotropin (β-hCG) was 344.4 mIU/dl. The patient was initially hospitalized with a preliminary diagnosis of pregnancy of unknown location (ectopic pregnancy?). In the differential diagnosis, complete abortion and abortus imminens of an early gestation were considered. Two days later, following a blood β-hCG level of 445 mIU/ml, an endometrial sampling was performed being reported as proliferative phase endometrium. A single cure of 50 mg methotrexate (MTX) applied. On the 4th and the 7th days, the β-hCG levels were 460 mIU/ml and 412 mIU/ml, respectively. The response was suboptimal. The condition was discussed with the patient and a laparoscopy was performed revealing completely normal pelvic findings. Due to the persisting high blood β-hCG levels, 3 cycles of double dose 75 mg MTX (on day 0 and day 4) cures were carried out. β-hCG levels were measured on the 4th and 7th day of each treatment cycle; with results: 434, 547, 701, 770, 1131, 1275 mIU/ml, respectively. Cranial and lower abdominal magnetic resonance imagings (MRI) were reported as normal. Despite weekly MTX therapies, the blood β-hCG levels were as high as 47915 mIU/ml; hence the patient, accepted as ‘MTX therapy resistant’ and the decision made to institute the multiagent EMA/CO protocol as 12 cures, every 14 days (etoposide 170 mg/m2/d, MTX 500 mg/m2/d, actinomycine 0.5 mg/d, calcium folinate 15 mg twice daily, cyclophosphamide 1000 mg/m2/d, vincristine sulfate 1.4 mg/m2/d). Due to the C-reactive protein blood level being 278 mg/dl, Ceftriaxon (Rocephine®) 500 mg 1 × 2 and moxifloxacine (Avelox®) 400 mg 1 × 1 were started. A whole abdominal computed tomography (CT) imaging revealed a mass totally occluding the left renal artery and reaching the abdominal aorta (As shown in Fig. 1). Static and dynamic scintigraphies of the kidneys showed that the left kidney was totally nonfunctional. The mass obstructing the renal artery was interpreted as a thrombus and enoxaparin sodium (0.6cc × 2) was started. In this period, the patient had a left flank pain not responding to narcotic analgesics. Doppler ultrasonographic examination could not view any blood flow in the left renal artery or vein. Gastroscopic and colonoscopic images of the gastrointestinal system were also normal. Pheochromocytoma was ruled out by checking the urinary metanephrine and catecholamine levels. Occasional hypertensive episodes were initially treated with perindopril 5 mg 1 × 1 and amlodipin 5 mg 1 × 1, afterwards. With fluorine-18-fluorodeoxyglucose positron emission tomography (FDF-PET) hypermetabolic areas were observed at the junction of the left renal artery and the aorta at the left lateral of the abdominal aorta. Following a final β-hCG blood level of 70,373 mIU/ml a diagnostic laparotomy was planned for extraction of the mass obstructing the left renal artery. During the operation, the left renal artery and the abdominal aorta-within the segment starting from where the left renal artery connection, as low down to the iliac bifurcation were filled with thrombus (As shown in Fig. 2). Left nephrectomy and abdominal aorta thromboendarterectomy were performed. No intra- or postoperative complications were observed. In the gross morphological evaluation of the nephrectomy specimen, the pelvicalyceal system looked normal, and no mass or mass effect was observed in the renal parenchyma. The corticomedullary junction was apparent. Histopathological examination revealed widespread atrophy of the renal parenchyma, patchy sclerosis of the glomeruli and rare fibrotic foci were observed. Within the left proximal renal artery lumen, choriocarcinoma infiltration of the endothelium and an overlying thrombus was observed. Pathological examination concluded that the tumour was not originating from the kidney.
Fig. 1.
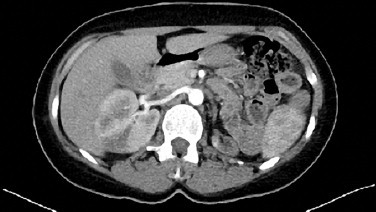
A whole abdominal CT imaging revealed a mass totally occluding the left renal artery and reaching the abdominal aorta.
Fig. 2.
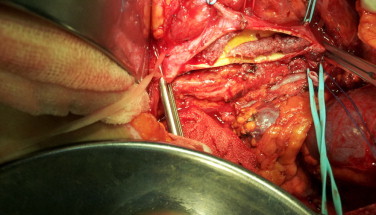
Choriocarcinoma is seen along the left side of the abdominal aorta.
When the whole specimen was examined, there was no tumour observed within the renal parenchyma. Yet, accounting for the current clinical and radiological data, the case was diagnosed as ‘primary choriocarcinoma originating from the renal artery’. The patient completed 12 cures of the EMA/CO multiagent chemotherapy protocol. The β-hCG blood levels regressed and within 1 year following the operation, no hypertensive episodes or choriocarcinoma recurrences were observed.
3. Discussion
Nongestational choriocarcinomas are a histological subtype of germ cell tumours and in fact originate from primordial germ cells of the ovary. These tumours display the same histomorphology of their analogue in men, the testicular germ cell choriocarcinoma; but its incidence is about one tenth.4 In both sexes, the tumour may originate the midline structures especially the anterior mediastinum, the pineal gland, the suprasellar region, the sacrococcyx; and rarely the vagina, the orbitas, the thyroid gland, the supramandibular region, the anterior abdominal wall, the prostate, the stomach, the liver, the brain, the bladder and the kidneys.5–7 Still a matter of debate, the origins of the extragonadal germ cell tumours have been hypothesized by 3 theories: displacement of primitive germ cells during the migration stage from the yolk sac ectoderm to the gonadal region; the persistence of pluripotential stem cells sequestered within primitive residual tissues during somatic development; or their malpositioning during the blastula or morula stages of embryogenesis.4 More recent studies have revealed that germ cells do migrate to extragonadal organs like the liver, the thymus, the bone marrow or the brain and have the potential to be the origin of primary tumours. Non-gestational choriocarcinomas, are histologically related to the pregnancy related gestational choriocarcinomas. These two subtypes may have to be differentiated according the clinical and radiological findings and DNA analysis may be used for this purpose as well.1,8 The non-gestational type, may be differentiated according to the absence of the paternal component or human leucocyte antigen (HLA) subtyping in DNA polymorphism analysis; however, their clinical applicability is limited. In many studies, authors have stated that nongestational choriocarcinoma diagnosis could be implemented in situations where the presence of a pregnancy could not be considered like the prepubertal period.9 In the current literature, cases which have been stated as extragonadal nongestational choriocarcinomas must actually have been gestational choriocarcinomas with unidentified primaries. Also in clinical practise, most of the extragonadal nongestational cases stated as primary pathologies have been shown to be nongestational choriocarcinoma metastasis with unknown primaries.5,8 Because the therapeutical approaches are different, it is essential that gestational and nongestational choriocarcinomas are differentiated. While the gestational choriocarcinomas respond favourably to MTX based chemotherapies, nongestational choriocarcinomas may be resistant to these modalities and may more favourably be responsive to multiagent chemotherapies.3 The case found to be resistant to MTX therapy was reevaluated, taking into consideration the radiological, clinical and surgical findings; and decided to be treated with the EMA/CO multiagent chemotherapy protocol as a nongestational or gestational choriocarcinoma with unknown primary. Despite this, the serum β-hCG levels continued to rise. Because most of developments in the treatment modalities of primary nongestational choriocarcinomas are stemming from the data related to testicular tumours, there is no standard treatment modality for the treatment of these tumours. Generally, there are 2 accepted treatment options. The first is to accept this tumour as a gestational germ cell tumour and to start a single agent MTX or a multiagent protocol like EMA/CO; the second option is to administer a nongestational germ-cell tumour protocol like VAC (vincristine, actinomycin D, cyclophosphamide) or BEP (bleomycine, etoposide, cisplatin).10 In addition to these, there are studies which report that treatment regimens like MAC (methotrexate, actinomycin D, cyclophosphamide) or VBP (vincristine, bleomycine, cisplatin) can provide complete cure.12 Because of the rarity of these nongestational choriocarcinomas, the literature regarding the chemotherapy of these tumours is still too scant and there is no consensus. We initially preferred the EMA/CO protocol. β-hCG monitorization may help in the assessment of the response of choriocarcinoma to medical treatment; however, it has no use in the differentiation of the gestational subtype from the nongestational one.10 The mass originating from the renal artery which was initially defined as a thrombus was, interestingly later when rescreened with the FDG-PET was defined as a possible metabolically active neoplasia. A preoperative FDG-PET was performed: viewing hypermetabolic areas in the left renal arterial lumen and along the left side of the abdominal aorta suggesting the locations of neoplastic tissues. Against all odds, including the risk of a systemic thromboembolism, a nephrectomy and a thromboendareterectomy was performed subserving both therapeutic and definitive objectives. Eventually, as described in the previous section, pathological examination concluded that the tumour was not originating from the kidney.
An intracranial pathology had been ruled out with a cranial CT; a primary or a secondary thoracic pathology was as well ruled out with a thorax spiral CT. The FDG-PET had not shown any other remarkable hypermetabolic foci in any other locations suggesting that there was no other primary. Regarding all the radiological and clinical findings we could not define a different primary location for the tumour. To our knowledge, one case of choriocarcinoma of vascular origin, presenting with nonspecific pulmonary symptoms, causing secondary pulmonary hypertension has been reported.11 As of the current time a choriocarcinoma originating from the renal artery causing secondary hypertension has never been reported in the literature. In conclusion, choriocarcinoma should as well be considered among the possibilities in the differential diagnosis of the primary causes for secondary hypertension especially within a clinical picture of pregnancy of unknown location, albeit being one of the rarest.
Conflict of interest
The authors have no conflicts of interest to declare regarding this manuscript.
Funding
No funding source was used.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Study design: Taner A. Usta. Data analysis: Eser Ozturk, Taner A. Usta, Tolga Karacan and M. Murat Naki. Data collection: Suat Nail Omeroglu, Taner A. Usta, and Tolga Karacan. Writing paper: Fuat Demirkiran, Taner A. Usta, Tolga Karacan, Eser Ozturk, M. Murat Nak and Suat Nail Omeroglu.
References
- 1.Altaner S., Tastekin E., Aksu B., Puyan F.O., Kutlu K. Pure nongestational choriocarcinoma of the ovary in a child: case report. JPS. 2009;23:81–84. [Google Scholar]
- 2.Altieri A., Franceschi S., Ferlay J. Epidemiology and aetiology of gestational trophoblastic diseases. Lancet Oncol. 2003;4:670–678. doi: 10.1016/s1470-2045(03)01245-2. [DOI] [PubMed] [Google Scholar]
- 3.Lv L., Yang K., Wu H., Lou J., Peng Z. Pure choriocarcinoma of the ovary: a case report. J Gynecol Oncol. 2011;22:135–139. doi: 10.3802/jgo.2011.22.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslan D., Temiz Y., Turkeri L. Extragonadal germ cell tumors: scientific letter. Turkiye Klinikleri J Med Sci. 2006;26:589–593. [Google Scholar]
- 5.Schmoll H.J. Extragonadal germ cell tumors. Ann Oncol. 2002;13:265–272. doi: 10.1093/annonc/mdf669. [DOI] [PubMed] [Google Scholar]
- 6.Chawla Grover S., Bansal R., Sangeeta, Agarwal N., Khare A., Jain V. Non gestational cervical choriocarcinoma with urinary bladder metastases: an unusual case report. JK Sci. 2010;12:147–148. [Google Scholar]
- 7.Campbell P.A., McKendrick J. Choriocarcinoma of the urinary bladder. Aust N Z J Surg. 1999;69:533–537. doi: 10.1046/j.1440-1622.1999.01607.x. [DOI] [PubMed] [Google Scholar]
- 8.Fisher R.A., Newlands E.S., Jeffreys A.J., Boxer G.M., Begent R.H., Rustin G.J. Gestational and nongestational trophoblastic tumors distinguished by DNA analysis. Cancer. 1992;69:839–845. doi: 10.1002/1097-0142(19920201)69:3<839::aid-cncr2820690336>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Vance R.P., Geisinger K.R. Pure nongestational choriocarcinoma of the ovary: report of a case. Cancer. 1985;56:2321–2325. doi: 10.1002/1097-0142(19851101)56:9<2321::aid-cncr2820560931>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Park S.H., Park A., Kim J.Y., Kwon J.H., Koh S.B. A case of non gestational choriocarcinoma arising in the ovary of a postmenopausal woman. J Gynecol Oncol. 2009;20:192–194. doi: 10.3802/jgo.2009.20.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trübenbach J., Pereira P.L., Huppert P.E., Farnsworth C., Mayer R., Feine U. Primary choriocarcinoma of the pulmonary artery mimicking pulmonary embolism. Br J Radiol. 1997;70:843–845. doi: 10.1259/bjr.70.836.9486052. [DOI] [PubMed] [Google Scholar]
- 12.Gerbie M.V., Brewer J.I., Tamimi H. Primary choriocarcinoma of the ovary. Obstet Gynecol. 1975;46:720–723. [PubMed] [Google Scholar]


