Abstract
The morphology of the toe epithelium of the rock frog, Staurois parvus (Family Ranidae), was investigated using a variety of microscopical techniques. The toe pad epithelium is stratified (four to five cell layers), the apical parts of the cells of the outermost layer being separated by fluid-filled channels. The surface of these cells is covered by a dense array of nanopillars, which also cover the surface of subarticular tubercles and unspecialized ventral epithelium of the toes, but not the dorsal epithelium. The apical portions of the outer two layers contain fibrils that originate from the nanopillars and are oriented approximately normal to the surface. This structure is similar to the pad structure of tree frogs of the families Hylidae and Rhacophoridae, indicating evolutionary convergence and a common evolutionary design for reversible attachment in climbing frogs. The main adaptation to the torrent habitat seems to be the straightness of the channels crossing the toe pad, which will assist in drainage of excess water. The presence of nanopillar arrays on all ventral surfaces of the toes resembles that on clingfish suckers and may be a specific adaptation for underwater adhesion and friction. The relevance of these findings to the development of new biomimetically inspired reversible adhesives is discussed.
Keywords: rock frog, adhesive toe pad, functional morphology, cell shape, electron microscopy, biomimetics
1. Introduction
In addition to tree frogs that are largely arboreal (though smaller species may be found in the ground flora), there are also frogs that live around freshwater streams (stream frogs) and waterfalls (rock and torrent frogs) that also possess adhesive toe pads [1]. The purpose of this study is to investigate the morphology of the toe pads of one such rock frog, the Lesser rock frog, Staurois parvus Inger and Haile, found in and around waterfalls within the rainforests of Borneo. Is the pad morphology very similar to those of tree frogs, or can we find differences that might relate to its rather different habitat?
The foundations of our understanding of the structure and function of tree frog toe pads dates back 40 years, but has received a significant boost in recent years by the realization of the biomimetic significance of mechanisms of animal adhesion (e.g. [2,3]). Many of these biomimetic studies have been aimed at replicating the dry adhesive mechanisms of geckos (e.g. [4–7]), but tree frog adhesion has also been highlighted as having potential for the development of new smart adhesives able to adhere under wet conditions [8–11].
The morphology of the adhesive toe pads of tree frogs has been extensively investigated by both transmission and electron microscopy [12–21]. There is also a recent study by Barnes et al. [22] that combined atomic force microscopy (AFM) and cryo-scanning electron microscopy (including freeze-fracture). These investigations show that the cells that make up the stratified columnar epithelium of the toe pads differ from those of the skin on the dorsal surface of the toes or indeed any other epidermal cell type [1], being specialized for their adhesive function. Indeed, circumferal and, in many species, proximal margin grooves separate the toe pad epithelium from neighbouring, less specialized skin. Accessory adhesive tubercles, found more proximally on the digits as well as on the main body of the feet, may be similarly specialized, but their precise role in adhesion and/or friction remains to be confirmed. The outer layer of cells, largely hexagonal in surface view, appears flat-topped at the microscale, the apical portions of individual cells being separated from each other by deep, fluid-filled channels, giving an aspect ratio of ca 0.5 for the outer parts of the epithelial cells. The fluid within the channels is secreted by mucous glands that have ducts ending in these channels, the channels serving to spread the mucus over the surface of the pad. Additionally, on surfaces wetted by rain, the channels may act to disperse excess fluid to the pad edge. Finally, the epithelial cells are covered by a dense array of nanopillars [23] that originate from desmosomes [12]. Within the epithelial cells, there are large numbers of keratin fibrils (tonofilaments), arising from the nanopillars, that are oriented approximately normal to the surface [12,20]. They are the main cytoskeletal elements of the toe pad epithelium and will confer a degree of rigidity to the cells [15]. Additionally, by analogy with comparable research on insects [24–26], it is probable that they are important for both toe pad adhesion and detachment.
Arboreal frogs (tree frogs) are found in at least seven different families including the Hylidae and Rhacophoridae, whose members are almost exclusively arboreal in the New and Old Worlds, respectively. The morphological features described above show little variation between the different families of tree frog and, as common ancestors with toe pads are lacking, clearly demonstrate convergent evolution [1].
Frog pads employ wet adhesion [27,28] that functions optimally when the fluid layer under the pad is very thin ( ) and there are no air pockets [29,30]. Given the presence of this fluid joint, the fact that toe pads also generate significant friction forces is surprising. Federle et al. [29] have proposed that this is achieved through direct contact between the tops of the nanopillars and the substrate in adhering frogs, and their experimental data demonstrate static as well as dynamic friction. Toe pads detach by peeling from the rear (i.e. in a proximal to distal direction) [28]. This is a natural consequence of the forward and upward movement of the distal part of the limb at the beginning of the locomotor swing phase, but does mean that, on slopes, downward-facing pads would have a tendency to peel spontaneously. As a consequence, tree frogs on slopes usually place their pads so that they face uphill [30]. Frictional forces prevent sliding on sloping surfaces, produce the close contact upon which good adhesion depends and, on overhanging surfaces where the limbs are spread out sideways [31], prevent peeling of the pads from the surface [32].
) and there are no air pockets [29,30]. Given the presence of this fluid joint, the fact that toe pads also generate significant friction forces is surprising. Federle et al. [29] have proposed that this is achieved through direct contact between the tops of the nanopillars and the substrate in adhering frogs, and their experimental data demonstrate static as well as dynamic friction. Toe pads detach by peeling from the rear (i.e. in a proximal to distal direction) [28]. This is a natural consequence of the forward and upward movement of the distal part of the limb at the beginning of the locomotor swing phase, but does mean that, on slopes, downward-facing pads would have a tendency to peel spontaneously. As a consequence, tree frogs on slopes usually place their pads so that they face uphill [30]. Frictional forces prevent sliding on sloping surfaces, produce the close contact upon which good adhesion depends and, on overhanging surfaces where the limbs are spread out sideways [31], prevent peeling of the pads from the surface [32].
Much less is known about adhesion and toe pad morphology in rock or torrent frogs. Ohler [33] carried out a preliminary study of toe pad morphology of torrent-living ranid frogs, demonstrating that the toe pads of Amolops spp. had distinct anatomical differences from the typical pattern seen in tree frogs. The toe pad epithelial cells were elongated, providing much straighter channels between the centre of the pad and the periphery. Experimental evidence is lacking, but an obvious function for these shorter pathways from centre to edge of pad would be to promote rapid drainage of excess fluid from underneath the pad. Such elongated channels were also found in the torrent frog, Odorana hosii, but only around the edges of the toe pads in the rock frog, Staurois guttatus [34]. A biomechanical study has been carried out on the Trinidadian stream frog, Mannophryne trinitatis, by Barnes et al. [35], while Endlein et al. [34] made a detailed comparison between the adhesive abilities of the rock frog S. guttatus and a rhacophorid tree frog of comparable size. In both studies, the stream/rock frog was good at adhering to rough wet surfaces, but very poor at sticking to dry rough ones. They also slipped on smooth wet surfaces. These data suggest that torrent/stream frogs can cope with running water so long as the surfaces are rough. This study searched for morphological features that could underlie these abilities and discusses the implications of these findings for the development of new smart adhesives.
2. Material and methods
2.1. Animals
The frog species of our study was the Lesser rock frog, S. parvus (family Ranidae), a native of Borneo. The frogs (N = 5) were sub-adult with a snout–vent length of 30 mm. They were donated by Schönbrunn Zoo (Vienna, Austria).
2.2. Sample preparation for scanning electron microscopy
Frog toes were cut off with a scalpel and fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer for 2 h at room temperature. They were then rinsed three times (5 min per rinse) in 0.1 M phosphate buffer containing 2% sucrose. Toes were post-fixed with 1% osmium tetroxide in 0.1 M phosphate buffer for 1 h, then washed three times in distilled water (15 min per wash), before en bloc staining with 0.5% aqueous uranyl acetate for 1 h in the dark (as this is a light-sensitive stain). Following two further washes in distilled water, samples were dehydrated through an acetone series (30, 50, 70 and 90%, 15 min in each), then four changes of absolute acetone (15 min each) and four changes of dried absolute acetone (3A molecular sieve). Toe samples were placed in critical point drying (CPD) specimen processing capsules (type C211, TAAB Laboratories Equipment Ltd, Aldermaston, Berkshire, UK) under dried absolute acetone and then critical point dried using a Polaron Critical Point Drying Apparatus E3000 (Quorum Technologies, Lewes, East Sussex, UK). Dried frog toes were orientated on aluminium pin scanning electron microscopy (SEM) stubs using double sided copper conductive tape and quick drying silver DAG paint, all from Agar Scientific (Stansted, Essex, UK). Mounted toes were gold/palladium coated (approx. 20 nm thickness) using a Polaron SC515 SEM coating system (Quorum Technologies) and then viewed on a JEOL6400 SEM (JEOL, Tokyo, Japan) running at 6–10 kV. The above describes procedures used in Glasgow. Procedures carried out in Kiel were similar, with specimens being viewed on a Hitachi S-4800 SEM (Hitachi High-Technologies Corp., Tokyo, Japan).
2.3. Sample preparation for transmission electron microscopy
Frog toes were cut off as described earlier, with the initial sample preparation procedures following those described above for SEM. Following fixation in glutaraldehyde, post-fixation in osmium tetroxide, en bloc staining with uranyl acetate and washing in distilled water, the toes were processed through a dehydration series of ethanol starting with 30%, then 50, 70 and 90% (15 min in each solution), before four changes in absolute ethanol (for 15 min each) and drying in absolute ethanol (3A molecular sieve; four changes of 15 min each). The absolute ethanol was then replaced with 100% propylene oxide for three 15 min changes, and then into a 50 : 50 propylene oxide/Araldite 502/812 resin mix (TAAB Laboratories Equipment Ltd) minus accelerator overnight. The toes were given several changes of pure resin minus accelerator over the next day; then placed in pure resin plus accelerator overnight and fresh resin embedded in flat-bed moulds the next morning. They were then left to polymerize at 60°C for 48 h. Semi-thin 0.35 µm light microscope sections were cut using a Leica Ultracut UTC Ultratome (Leica Microsystems, Milton Keynes, UK) and a Drukker histomicrotome knife with trough (Drukker International, Cuijk, The Netherlands). Sections were dried on a glass slide on a hotplate (80°C) and then stained with 1% toluidine blue/1% borax in distilled water. Ultrathin sections (60–70 nm) were also cut using the Leica Ultracut UTC Ultratome and a Drukker Ultramicrotome knife. Sections were picked up on slot or 100 mesh formvar-coated copper grids and contrast-stained with 2% methanolic uranyl acetate for 5 min and Reynolds lead citrate for 5 min. Toe samples were viewed on a LEO912AB transmission electron microscope (Carl Zeiss Ltd, Cambridge, UK), running at 120 kV. Images were captured using an Olympus iTEM soft imaging system.
2.4. Sample preparation for cryo-SEM
Frog toes were tied with a synthetic fibre to prevent blood loss, cut off with a scalpel and plunge-frozen in liquid propane cooled by liquid nitrogen. This procedure avoided formation of ice crystals and prevented damage of pad structure. The specimens were stored in liquid nitrogen. Cryo-SEM analysis was performed with a Hitachi S-4800 SEM (Hitachi High-Technologies Corp.) using a cryo-preparation chamber Gatan ALTO-2500 (Gatan Inc., Abingdon, UK). The frozen frog toes were fixed with Tissue-Tek O.C.T (Sakura Finetek Europe B.V., Zoeterwoude, The Netherlands) to the specimen holder and immediately plunged in liquid nitrogen to avoid thawing. They were then placed in the cooled (−140°C) cryo-preparation chamber (using liquid nitrogen). The frozen specimens were sputter-coated with a gold–palladium layer (1 : 9, layer thickness 10–20 nm) and transferred to the microscope. The samples were examined at −120°C at an accelerating voltage of 2–5 kV.
2.5. Sample preparation for atomic force microscopy
The AFM studies were performed with a NanoWizard I AFM (JPK Instruments AG, Berlin, Germany). The frog toe was tied with a synthetic fibre and cut off with a scalpel. The toe was fixed with superglue to a glass slide in an AFM BioCell chamber (JPK Instruments AG, Berlin, Germany) and submerged in frog Ringer solution. The measurements were conducted with a pyrex-nitride-probe cantilever (silicon nitride SPM-Sensor, NanoWorld AG, Neuchâtel, Switzerland) in intermittent (tapping) mode. The resolution of the scans was 1024 × 1024 pixels. The images were processed with the SPIP software (Scanning probe image processing software, v. 5.1.2, Image Metrology A/S, Hørsholm, Denmark).
3. Results
3.1. Toe morphology (SEM and cryo-SEM)
3.1.1. Toe pads
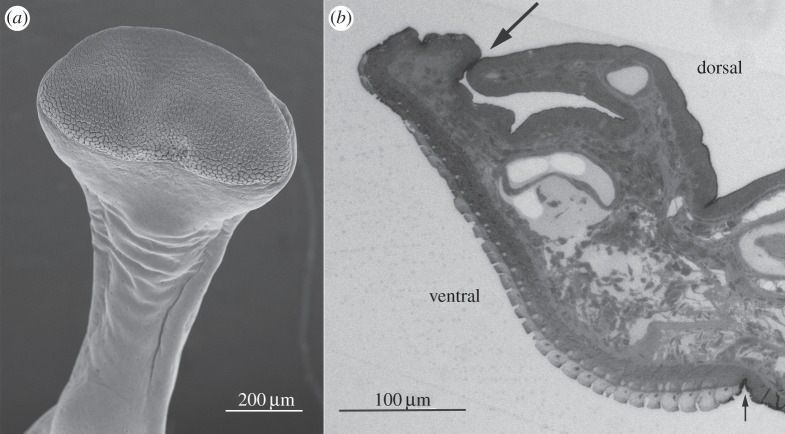
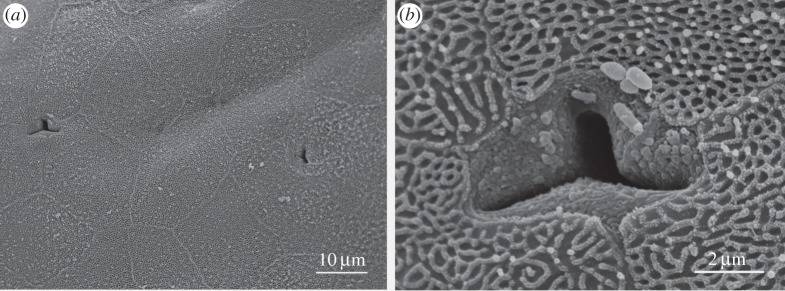
The toe pads are located on the expanded tips of each digit, of which there are four in each forelimb (figure 1) and five in each hindlimb. Additionally, there are accessory adhesive structures called subarticular tubercles located more proximally on the digits (figure 1, arrows). The toe pads are surrounded by circumferal and proximal grooves (figures 1 and 2). The proximal groove, which delineates the proximal margin of the pad, is shallow as seen in the longitudinal section of the pad (figure 2b, small arrow), while the circumferal groove that delineates the distal and lateral pad margins is a large deep channel, especially at the distal tip of the pad (figure 2b, large arrow). Interestingly, as this is not a common feature of tree frogs, the larger pads extend distally around the front of the toe (figure 2a,b). The following features were visible.
Figure 1.
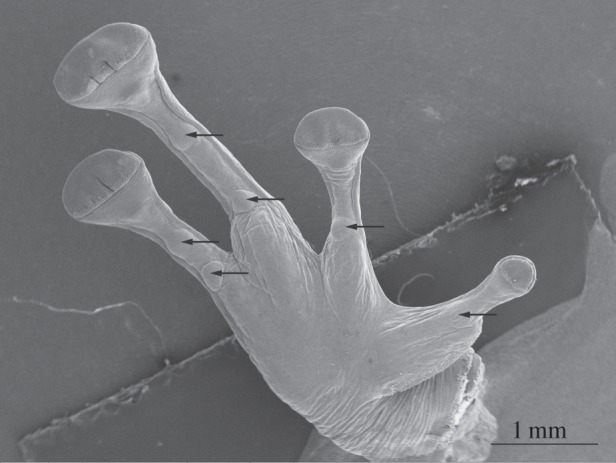
SEM image of forelimb of S. parvus; ventral view showing toe pads and subarticular tubercles (arrows). The ‘thumb’ (digit 1) is the small pad on the right hand side.
Figure 2.
(a) SEM image of digit 2 of forelimb of S. parvus, showing toe pad epithelium, circumferal and proximal grooves. (b) Longitudinal section of toe pad (light microscope image) showing the same features. Note relatively large size of circumferal groove (large arrow) compared with proximal groove (small arrow).
3.1.2. Toe pad surface micropattern—epithelial cells
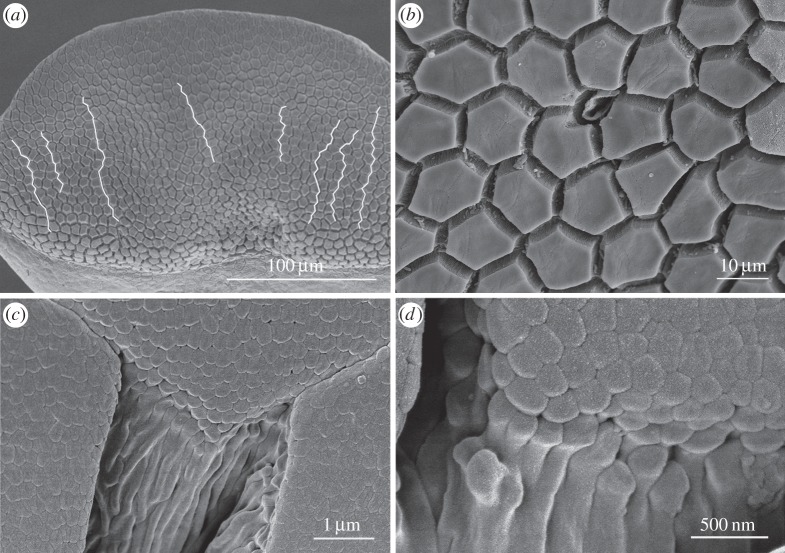
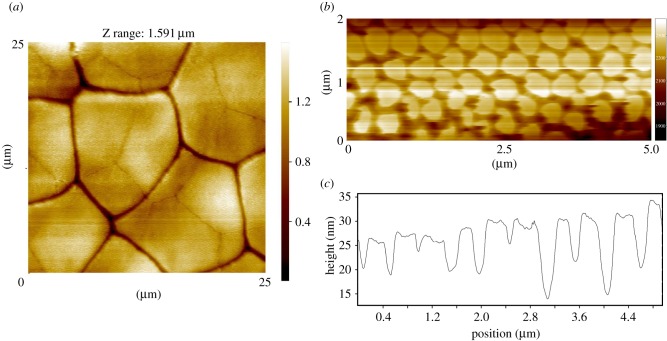
As shown in figure 3a,b, the toe pad surface is made up of flat-topped epithelial cells of ca 13 μm diameter separated by channels with a width of ca 2 μm in fixed material, but probably narrower in living cells where channel width varies with the amount of fluid under the pad. Mucus glands are rare in toe pads (less than five per pad), less common than in other skin areas. The duct of one such mucus gland is shown in figure 3b, opening into an intercellular channel. The majority of epithelial cells are irregular hexagons, but other shapes are also seen. In a total number of 122 epithelial cells, the hexagonal shape was predominant (56%), followed by pentagonal (33%) and heptagonal (11%). A single octagonal epithelial cell was also recorded. The white lines on figure 3a show the existence of relatively straight channels crossing the pad in an approximately anterior–posterior direction. We have quantified the lengths of such channels in two pads (10 channels in each pad), including those in figure 3a. Relative to a straight line (length = 1), such channels have a length of 1.13 ± 0.04 (mean ± s.d., N = 20). This is significantly better than a pattern of regular hexagons, which have a relative channel length of 1.33. Interestingly, channels going across the pad also have a relative channel length of less than 1.33, but are not as straight as the anterior–posterior channels (p < 0.001, Wilcoxan signed-rank test). Such channels should promote good drainage of excess water in the rock frogs' stream habitat, those running in an anterior–posterior direction being of greater significance as pads normally face uphill. For full details of this analysis, see electronic supplementary material, table S1 and figure S1. Figure 3c shows such a channel at a point where three epithelial cells meet. The channel walls can be seen to be ridged, the ridges probably reflecting the presence of bundles of keratin tonofilaments beneath the surface.
Figure 3.
SEM images of toe pad epithelium of S. parvus. (a) White lines show relatively straight channels crossing the pad. (b) Polygonal epithelial cells (mainly hexagonal in shape) surrounded by deep channels with a single mucous pore (centre). (c) Edge of a pad epithelial cell showing dense array of nanopillars covering the pad surface. (d) High-power view of nanopillars.
3.1.3. Toe pad surface nanopattern—nanopillars
As in tree frogs, the surface of the epithelial cells is covered in a dense array of nanopillars that form a surface nanopattern consisting of regular polygons 200–300 nm in diameter (figure 3d). Their height is not measurable from this image, partly because they are so densely packed and partly because they merge with the ridges that characterize the channel walls. However, transmission electron microscopy (TEM) images show their height to be 400–500 nm, giving them an aspect ratio of 1.4–2.0 (figure 10). Bundles of filaments, presumed to be keratin tonofilaments from immunohistochemical studies on other frog species (e.g. [36]), are visible through the walls of the nanopillars in some of our cryo-SEM images (figure 4b). Such images (figure 4a,b) also revealed that the nanopillars have a slightly concave terminal and in, unspecialized ventral toe epithelium at least, can have aspect ratios of up to 2.5. One of the advantages of using cryo-SEM is that it allows one to view the mucus covering of the pad. In figure 4a, the frozen mucus can be seen surrounding but not covering the nanopillars and also filling their dimpled tops.
Figure 4.
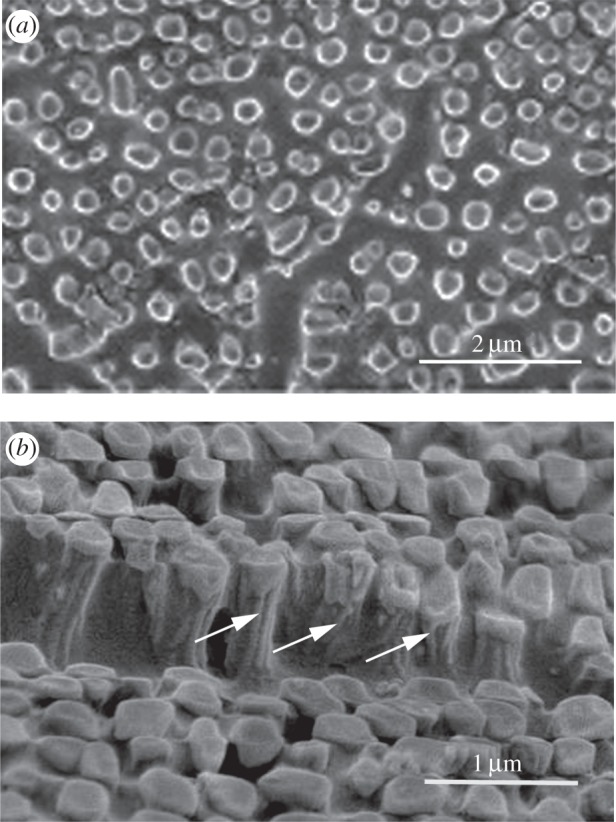
High-power images of nanopillars in S. parvus. Cryo-SEM images of unspecialized ventral epithelium showing (a) the tops of the nanopillars surrounded by frozen mucus and (b) fibrils (bundles of tonofilaments) visible through the walls of the nanopillars (arrows).
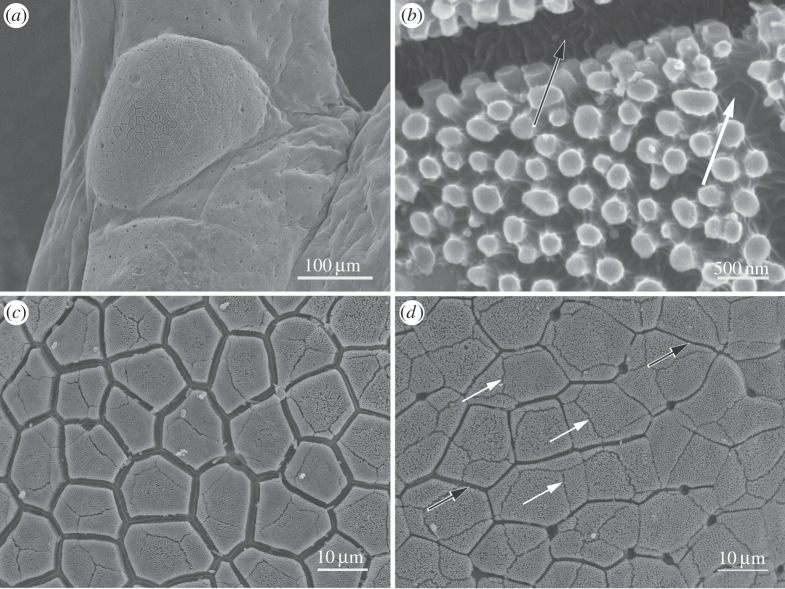
3.1.4. Epithelium of toe tubercles
Proximal to each toe pad are located subarticular tubercles (figures 1 and 5a). Their epithelial surface possesses similar features to those of the toe pads (figure 5c,d). Polygonal epithelial cells (mostly 5 or 6-sided) are separated by channels (figure 5c,d), but the channel depth in tubercular epithelium is significantly shallower (1–3 µm, figure 8b) than in the pad epithelium (10 µm, figure 8a), and significantly shallower and narrower in the peripheral region of the tubercles (figure 5d) than in the centre (figure 5c). Epithelial cells in the tubercle are also covered by an array of nanopillars with similar dimensions and arrangement as in toe pads, but at a significantly lower density (figure 5b). By contrast, the density of mucous pores (several of which are visible in both figure 5c,d) appears higher than in the toe pad epithelium. These images also show marks on their surface (actually gaps in the nanopillar array as figure 5b makes clear) that clearly indicate the position of the layer of cells that previously had lain above them (figure 5d, arrows). Such marks are also present on the toe pad epithelium (obvious in figure 11a, less so in figure 3b). The different cell layers are thus displaced with respect to each other in both x and y axes, as previously noted for tree frogs [22].
Figure 5.
SEM images of subarticular tubercle epithelium of S. parvus. (a) Basal tubercle of digit 4 of forelimb. (b) High-power view of nanopillars, intercellular channel (black arrow) and gap in the nanopillar array at the position of a cell boundary of the previous outer cell layer (white arrow). (c) Epithelium of centre of tubercle with larger intercellular channels. (d) Epithelium near edge of tubercle with smaller intercellular channels (black arrows indicate channels; white arrows, gaps in the nanopillar array as described in b).
3.1.5. Ventral unspecialized toe epithelium
Examination of the ventral epithelium of the toes, excluding toe pads and subarticular tubercles, shows a degree of adaptation for adhesion and friction. As figure 6 shows, the epithelial cell surface is covered by nanopillars, but at lower density than in the more specialized epithelia of the toe pads and subarticular tubercles. Such nanopillars are absent at the cell boundaries, giving rise to narrow, shallow, intercellular channels. Mucous pores are common. The cryo-SEM images of nanopillars discussed earlier (figure 4a,b) were taken from this area, their lower density making structural features easier to see and measure.
Figure 6.
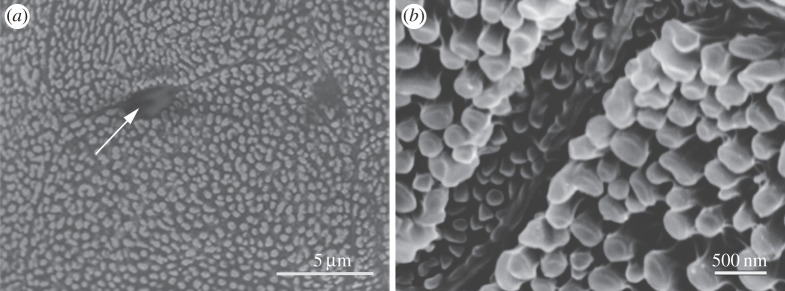
SEM images of nanopillar array and intercellular channels of unspecialized ventral epithelium of S. parvus digits. (a) Low power; arrow indicates opening of mucous gland. (b) High power.
3.1.6. Dorsal toe epithelium
In contrast to the ventral epithelium, the epithelium of the dorsal surface of the toes has neither nanopillars nor intercellular channels; i.e. it shows no adaptations for either adhesion or friction. At low power (figure 7a), the surface appears relatively smooth, though large Y-shaped pores, possibly of mucous glands (though the pore structure is very different than that of the ventrally located mucous pores), are common. At higher power (figure 7b), the dorsal epithelium is seen to be covered by nanostructures (150 nm across) that are joined end to end to form a network that covers the cell surface. Their appearance is similar to that of vermicular (worm-like) structures seen on lizard scales [37].
Figure 7.
SEM images of dorsal epithelium of S. parvus digits. (a) Low-power image of several epithelial cells. (b) High-power image of cell surface and the pore of a (presumed) mucous gland.
3.2. Toe pad anatomy (light microscopy and TEM)
3.2.1. Epithelial cell layers
Examination of longitudinal sections of the toes under the light microscope reveals that the epithelium of the toes of S. parvus is stratified. In the toe pads, it has a thickness of ca 50 µm, consisting of four to five cell layers, with deep channels separating the outer portions of the outer cell layer (figure 8a). This layer has dark pyknotic nuclei and consists of dying or dead cells. Peripheral portions of the cytoplasm of the two outmost layers are stained darkly, owing to the presence of keratin. The large vesicles in the second layer will give rise to the intercellular channels when the second layer becomes the outer layer, through the shedding of the outer layer. By contrast, unspecialized ventral epithelium and the dorsal epithelium of the toe are only 15–20 µm thick (figures 2b and 8b). The dorsal epithelium has no channels, while the channels separating the outer portions of the outer layer of cells in unspecialized ventral toe epithelium are only about 1 µm deep. The extent of the keratinization is much reduced. The epithelium of the subarticular tubercles is broadly similar to that of the toe pads, though its thickness is reduced (data not shown).
Figure 8.
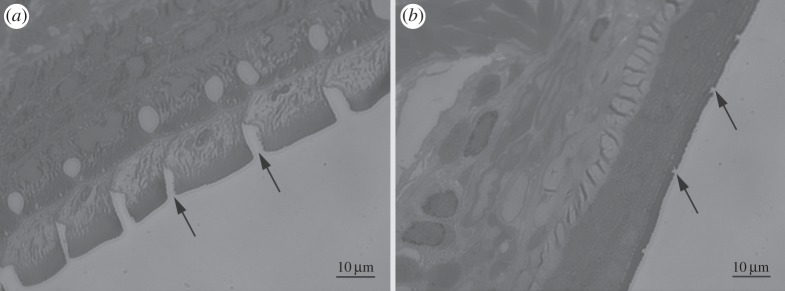
Light microscope sections of ventral epithelium of toes of S. parvus (×100 objective). (a) Toe pad epithelium showing four cell layers with deep channels between the outer portions of the outer cell layer (arrows). (b) Unspecialized ventral epithelium (near proximal groove) showing small shallow grooves (arrows) between the outer portions of the outer cell layer.
3.2.2. Transmission electron microscopy
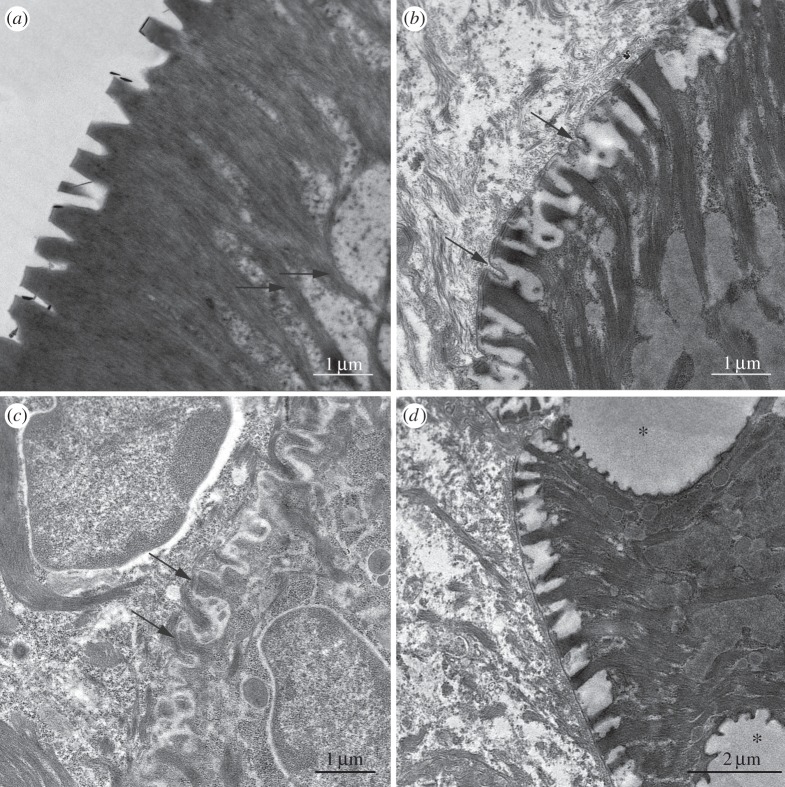
The electron microscope reveals a lot more detail of the ultrastructure of the different layers. As, with each sloughing of the outer layer, layer IV will progressively become layer III, then II and finally I (the outer layer), examination of the different layers also provides insights into how the specialized outer layer with its deep channels, nanopillar arrays and keratin fibrils develops from unspecialized beginnings. The outer layer, seen in figure 9a, shows the cells to be filled with dark-staining keratin tonofilaments that have their origin in the nanopillars, three of which are shown in enlarged view in figure 10. Completely filling the nanopillars (figure 10), they initially run normal to the cell surface (figure 9a), but gradually bend proximally before stopping about halfway across the cell. Even the peripheral cytoplasm appears dark, owing to keratin deposition. Figure 9b shows the cell border between layers I and II. Here, the outer cell layer is devoid of both tonofilaments and keratin, though both are present in the peripheral part of layer II. The outermost part of layer II also shows how the nanopillars arise, as bundles of tonofilaments are becoming separated from each other by clear cytoplasm and there are some invaginations of the cell boundary into these clear areas (figure 9b, arrows). Figure 9d shown the same layer I/layer II boundary, but now including one of the large vesicles (seen in figure 8a) that will give rise to the deep channels between the outer parts of the epithelial cells in layer I. Figure 9c, which illustrates the boundary between layers II and III, shows an even earlier stage in the development of the structure of the outer layer. Both cell layers are clearly living tissue, with typical nuclei visible in the cells shown. The cell boundary is invaginated, but to a lesser extent than the layer I/II boundary. In some cases, short tonofilaments can be seen to be going both inwards and outwards from desmosomes joining the two surfaces together (figure 9c, arrows). Electron-dense particles, probably corneous (horny) material containing keratin [38], are visible in both layers.
Figure 9.
TEM images of toe pad epithelium of S. parvus digits. (a) Outer cell layer showing nanopillars and dense bundles of keratin tonofilaments (arrows). (b) Border between outer cell layer on left and the second layer of cells; arrows show invaginations of cell membrane. (c) Border between cell layers II (left) and III; arrows indicate desmosomes. (d) Lower-power image of border between layers I and II showing large vacuoles (stars) that will form the channels between the epithelial cells when the outer layer is shed.
Figure 10.

High-power TEM image of nanopillars in S. parvus. Section of toe pad epithelium showing nanopillars filled with tonofilaments lying at right angles to the pad surface.
3.3. Atomic force microscopic studies of the surface of the toe pads
3.3.1. Cell surface probed by the atomic force microscopy
The AFM studies provide further insight into the structure and components of toe pad epithelium. Unlike SEM, TEM and light microscopy, the tissue does not require to be fixed, so problems of shrinkage and distortion are avoided. However, as an AFM cantilever is not able to penetrate narrow clefts, it cannot provide data on the depths of the channels between either epithelial cells or nanopillars. AFM scans showed the polygonal epithelial cells separated by channels that did not exceed 1 µm in width (figure 11a), considerably narrower than they appear in SEM images (figure 3b). They also show the gaps in the nanopillar array that reflect the position of the previous cell layer, a feature not obvious in figure 3b. The narrowness of the channels did not allow the cantilever tip to penetrate them and, therefore, the depth of the channels could not be estimated. Figure 11b shows the nanostructures on the epithelial cell surfaces. The height profile (figure 11c) quantifies the extent to which the tops of the nanopillars are not flat-terminated but slightly concave. Precise quantification is difficult because it is necessary for the profile to run precisely through the centre of the nanopillar. Our best data give a figure for the depth of the dimple in the range 6–8 nm.
Figure 11.
AFM images of toe pad epithelium cells of S. parvus. (a) Topographical image at the microscale. (b) Topographical image of the toe pad surface nanostructures. The horizontal line indicates the position of the height profile shown in (c). (Online version in colour.)
4. Discussion
The surface of toe pads of tree, rock and torrent frogs shows a particular topography with distinct features at the micro- and nanoscales [20–23,29,33,34,39–41]. The role that this surface structure plays in reversible attachment and locomotion of the animals in wet and even flooded environments is a current focus of research for zoologists and material scientists eager to transfer new ‘wet’ adhesive principles from natural to artificial systems.
The outermost cell layer of the toe pad consists of epithelial cells forming polygonal pillars of 10–15 μm width. As in hylid and rhacophorid tree frogs [22,39], the majority are hexagonal but pentagonal and heptagonal geometries also occur. The occurrence of such geometries in a predominantly hexagon pattern has recently been studied in the corneal nipple arrays that cover the ommatidia (facets) of the compound eyes of the butterfly, Nymphalis antiopa [42]. Nipples are occasionally surrounded by either seven or five neighbours and five/seven pairs are relatively common, a feature shared by the toe pads of the tree frog, Rhacophorus prominanus, where pentagonal epithelial cells are surrounded by more heptagons than would be expected by chance and vice versa [22]. Space filling due to the domed surface of each ommatidium is considered by Lee and Erb to be one reason for the existence of such irregularities. Indeed, this mechanism of introducing curvature into a close-packed structure may be quite common in nature. For example, the honeycombs of bees and social wasps use similar interruptions of the hexagonal pattern to account for curvature, confined boundary conditions and transition zones from smaller to larger cells [43,44]. Tree and rock frog epithelial cells are much less regular in shape than either corneal nipple arrays or honeycombs and the presence of relatively straight channels crossing the pad (see below) also necessarily affects cell shape, changing regular hexagons into rather squarer cells. However, as in butterfly corneal nipple arrays, the coexistence of heptagonal and pentagonal shapes with the predominantly hexagonal one is probably required to fill the pad space and accommodate irregularities caused by the pad curvature and the mucus pores.
The polygonal epithelial cells are separated at their tips by narrow (ca 1 μm width) channels of around 10 μm depth. This is a characteristic feature of the toe pad epithelia of tree frogs, suggesting an important functional role for the channels. In ranid frogs, this feature has been observed in torrent frogs such as Amolops spp. [33], in rock frogs of the genus Staurois ([34] and this study) and also, somewhat surprisingly, in the unexpanded toe pads of the amphibious leopard frog, Rana pipiens [45]. In S. parvus, the channels are restricted to the ventral epithelium of the toes; i.e. pads (deep channels), subarticular tubercles (shallower channels) and unspecialized ventral epithelium (channels are hardly more than gaps in the nanopillar array) as illustrated in figures 3b, 5, 6 and 8. They are absent from dorsal toe epithelium, where nanopillars are also absent (figure 7). The role of the channels in reversible ‘wet’ adhesion has been recently investigated in artificial tree frog toe pad replicas [11]. The channel structure allows draining of a wetting fluid out of the contact surface and establishment of direct (dry) contact in the presence of shear forces (i.e. friction). This conclusion is supported by data from live frogs studied using interference reflection microscopy, which allows direct measurement of the fluid layer thickness under the pad [29]. This draining role would be expected to be more important in frogs that live in wet environments, such as rock and torrent frogs. Indeed, Ohler [33] observed elongated epithelial cells in the toe pads of torrent frogs (Amolops spp.) which, she hypothesized, provided shorter and straighter channels for the removal of excess fluid from under the pad. Elongated epithelial cells do not appear to be a common feature of rock frog pads in spite of their habitat in the vicinity of waterfalls. However, as shown by Endlein et al. [34] in S. guttatus and here in S. parvus, there are channels crossing the pad (particularly in a distal/proximal direction as would be appropriate for water drainage) that, while not straight, are significantly shorter than would be found in an array of regular hexagons (figure 3a). A role in drainage of excess fluid thus seems probable. Although the presence of a deep circumferal groove (figure 2b) is not unique to rock frogs (WJP Barnes 2012, unpublished data on rhacophorid tree frogs), this groove, which surrounds the distal and lateral parts of the pads, is ideally placed to channel fluid around rather than under the pad.
Studies on toe pad mimics have also investigated the importance of aspect ratio [11]. Microstructures with low aspect ratio features are more resistant to bend and collapse during shear and allow more effective establishment of direct contact; i.e. higher friction forces are achieved. It is thus possible that the lower aspect ratio subarticular tubercles (epithelial cells are surrounded by shallow channels as described above) have evolved to generate high friction forces, but this remains to be tested experimentally.
Ventral surfaces are also characterized by the presence of nanopillar arrays that cover the surface. These arrays are particularly dense on the toe pads, but less so on the subarticular tubercles and unspecialized ventral toe epithelium (figures 3c,d; 4; 5b and 6b). They are unlikely to play a role in capillarity as such forces are generated by the meniscus (air–water interface) around the edge of the pad [9], but a role in friction and/or viscous flow between adhering surfaces seems likely. Indeed, the thickness of the fluid layer under the pad is such that the tops of the nanopillars are likely to make direct dry contact with the substrate. This is shown both by cryo-SEM images such as figure 4a and by calculations of the thickness of the fluid layer thickness using interference reflection microscopy [29]. Also, as noted previously in a tree frog [23], the tops of the nanopillars are clearly concave (figure 4b, a cryo-SEM image and figure 11c, the AFM height profile), with a dimple depth in the range 6–8 nm, similar to those measured in tree frogs [22,23]. The reason for this concavity is unclear, but it is noteworthy that the shape (flat, T-shaped, concave) of the tops of fibrillar microstructures can significantly affect both adhesion and friction [11,46]. Varenberg and Gorb propose that, under appropriate circumstances, each element can act as a passive suction device, but this is disputed by Drotlef et al., who favour a combination of capillary and direct contact forces.
Interestingly, nanopillar arrays have also been reported in clingfish, where they occur around the margin of the ventrally placed suction disc. Clingfish use their adhesive disc to stick under water to many different kinds of surfaces and, surprisingly as they adhere by suction, adhesion is not hindered by surface roughness [47]. The array of nanopillars (called microvilli by the authors), aided by secreted mucus, is able to maintain a good seal and resist frictional forces, even on the roughest of surfaces. As S. parvus lives in and around fast-flowing water, it is unsurprising that the nanopillar arrays in these frogs are present not only on the toe pads and subarticular tubercles but also on unspecialized ventral epithelium in the toe region. Indeed, as Endlein et al. [34] have shown for S. guttatus, most of the ventral body surface is used when they are clinging onto rough surfaces under high rates of water flow.
Material properties are also critical for adhesion and friction. This paper does not address this matter directly, but the TEM results indicate that the distal regions of the pads are keratinized and that keratin tonofilaments fill the nanopillars and the distal parts of the two outer cell layers (figures 9 and 10). Their dense packing resembles the structure of many composite materials and would confer higher mechanical strength and flexibility to the epithelial cells. As frog toe pads have a low elastic modulus [23,41], such keratinization probably serves to increase the wear resistance of the outermost part of the toe pads. Whether the keratin tonofilaments have a particular role in adhesion, as they do in insects [26], remains to be investigated. However, their orientation (initially normal to the surface they become angled to the surface in a proximal direction as shown in figure 9a) could provide the pad with directional adhesion, so that pads pulled towards the body increase their contact with the substrate, while movements in the other direction cause detachment.
The TEM images of the different cell layers within the toe pad epithelium (figure 9a–d) are broadly similar to those of equivalent studies on hylid and rhacophorid tree frogs [12,14,20,21]. For example, in all groups, it appears that the outer cell layer consists of dying or dead cells and is highly keratinized, that nanopillars originate from desmosomes, and that the keratin filaments are linked to desmosomes. Our images also indicate that the specialized outer layer develops in a similar way to the tree frogs. The degree of evolutionary convergence is thus remarkable, though it is probable that most of the underlying mechanisms (e.g. mechanisms of keratinization) will have been present in common ancestors of these groups. Thus, in our view, it is the detailed structure of the toe pad that arises by convergence in the different frog families and that it does so predominantly using pre-existing cellular mechanisms.
The adhesive mechanisms of climbing animals such as geckos, anoles and skinks among reptiles, tree, torrent and rock frogs among amphibians, and a wide variety of insects have many features that are of interest to materials scientists. These include the combination of good adhesion with easy detachment, the fact that the adhesive can be used repeatedly without deteriorating, the ability to self-clean (e.g. [48]) and thus quickly and efficiently remove attached dirt particles, and the property of only sticking when required [2,4]. The amphibians additionally have the ability to adhere under wet conditions. Thus, understanding the adhesion principles behind the structural features described in this paper will enhance the development of novel adhesion strategies for reversible attachment in artificial systems. The initial work on the tree/rock/torrent frog system has already begun with theoretical studies of capillary adhesion of soft, deformable surfaces [49,50] and nanofabrication and testing of hexagonal patterns of cells surrounded by channels (Varenberg & Gorb [46,51], whose work was insect-inspired, and Drotlef et al. [11], who systematically examined the adhesive and frictional properties of tree frog toe pad analogues under a variety of conditions). More generally, studies on bioinspired adhesive materials are increasingly combining materials with very different properties in the development of new hybrid adhesive surfaces (e.g. [52,53]). In respect of the frog adhesion research, possible applications include improved design in wet weather tyres [35,54,55], non-slip footwear, plasters for surgery able to adhere to tissue, holding devices for surgery and the assembly of functional elements in miniature electronic devices (microelectromechanical systems). As both Barnes et al. [22] and this paper make clear, both nanopillars and fibrils will also need to be considered if the full biomimetic potential of the rock frog adhesive system is to be realized.
Acknowledgements
We thank Margaret Mullin of Glasgow University's Life Sciences Electron Microscopy Facility for her expert assistance with both the scanning and transmission electron microscopy.
Ethics statement
The frogs were sacrificed for these studies by submersion in water containing a lethal dose of benzocaine, in accordance with current laws on animal experimentation in the UK.
Funding statement
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SPP1420 ‘Biomimetic materials research: functionality by hierarchical structuring of materials') to A.d.C. and W.J.P.B. and by an STMS grant from COST Action TD0906 on ‘Biological adhesives: from biology to biomimetics' to D.M.D.
References
- 1.Duellman W, Trueb L. 1997. Biology of amphibians, 2nd edn Baltimore, MA: Johns Hopkins University Press. [Google Scholar]
- 2.Barnes WJP. 2007. Biomimetic solutions to sticky problems. Science 318, 203–204. ( 10.1126/science.1149994) [DOI] [PubMed] [Google Scholar]
- 3.Creton C, Gorb S. 1997. Sticky feet: from animals to materials. MRS Bull. 32, 466–468. ( 10.1557/mrs2007.79) [DOI] [Google Scholar]
- 4.Autumn K. 2007. Gecko adhesion: structure, function and applications. MRS Bull. 32, 473–478. ( 10.1557/mrs2007.80) [DOI] [Google Scholar]
- 5.Bhushan B. 2007. Adhesion of multi-level hierarchical attachment systems in gecko feet. J. Adhes. Sci. Technol. 21, 1213–1258. ( 10.1163/156856107782328353) [DOI] [Google Scholar]
- 6.del Campo A, Greiner C, Arzt E. 2007. Contact shape controls adhesion of bioinspired fibrillar surfaces. Langmuir 23, 10235–10243. ( 10.1021/la7010502) [DOI] [PubMed] [Google Scholar]
- 7.Zhao B, Pesika N, Zeng H, Wei Z, Chen Y, Autumn K, Turner K, Israelachvili J. 2009. Role of tilted adhesion fibrils (setae) in the adhesion and locomotion of gecko-like systems. J. Phys. Chem. B 113, 3615–3621. ( 10.1021/jp806079d) [DOI] [PubMed] [Google Scholar]
- 8.Barnes WJP. 2007. Functional morphology and design constraints of smooth adhesive pads. MRS Bull. 32, 479–485. ( 10.1557/mrs2007.81) [DOI] [Google Scholar]
- 9.Barnes WJP. 2012. Adhesion in wet environments—frogs. In Encyclopedia of nanotechnology, part 2 (ed. Bhushan B.), pp. 70–83. Berlin, Germany: Springer. [Google Scholar]
- 10.Gupta R, Fréchette J. 2012. Measurement and scaling of hydrodynamic interactions in the presence of draining channels. Langmuir 28, 14 703–14 712. ( 10.1021/la303508x) [DOI] [PubMed] [Google Scholar]
- 11.Drotlef D-M, Stepien L, Kappl M, Barnes WJP, Butt H-J, del Campo A. 2013. Insights into the adhesive mechanisms of tree-frogs using artificial mimics. Adv. Funct. Mater. 23, 1137–1146. ( 10.1002/adfm.201202024) [DOI] [Google Scholar]
- 12.Ernst V. 1973. The digital pads of the tree frog, Hyla cinerea. I. The epidermis. Tissue Cell 5, 83–96. ( 10.1016/S0040-8166(73)80007-2) [DOI] [PubMed] [Google Scholar]
- 13.Ernst V. 1973b. The digital pads of the tree frog, Hyla cinerea. II. The mucous glands. Tissue Cell 5, 97–104. ( 10.1016/S0040-8166(73)80008-4) [DOI] [PubMed] [Google Scholar]
- 14.Welsch U, Storch V, Fuchs W. 1974. The fine structure of the digital pads of rhacophorid tree frogs. Cell Tiss. Res. 148, 407–416. ( 10.1007/BF00224267) [DOI] [PubMed] [Google Scholar]
- 15.Green DM. 1979. Treefrog toe pads: comparative surface morphology using scanning electron microscopy. Can. J. Zool. 57, 2033–2046. ( 10.1139/z79-268) [DOI] [Google Scholar]
- 16.McAllister A, Channing C. 1983. Comparisons of toepads of some Southern African climbing frogs. S. Afr. Zool. 18, 110–114. [Google Scholar]
- 17.Green DM, Simon P. 1986. Digital microstructure in ecologically diverse microhylid frogs genera Cophixalus and Sphenophryne (Amphibia: Anura) from Papua New Guinea. Aust. J. Zool. 34, 135–145. ( 10.1071/ZO9860135) [DOI] [Google Scholar]
- 18.Hertwig I, Sinsch U. 1995. Comparative toe pad morphology in marsupial frogs (genus Gastrotheca): arboreal versus ground-dwelling species. Copeia 1, 38–47. ( 10.2307/1446798) [DOI] [Google Scholar]
- 19.Ba-Omar TA, Downie JR, Barnes WJP. 2000. Development of adhesive toe-pads in the tree frog (Phyllomedusa trinitatis). J. Zool. Lond. 250, 267–282. ( 10.1111/j.1469-7998.2000.tb01077.x) [DOI] [Google Scholar]
- 20.Mizuhira V. 2004. The digital pads of rhacophorid tree-frogs. J. Electron Microsc. 53, 63–78. ( 10.1093/jmicro/53.1.63) [DOI] [PubMed] [Google Scholar]
- 21.Chakraborti S, Das D, De SK, Nag TC. 2014. Structural organization of the toe pads in the amphibian Philautus annadalii (Boulenger, 1906). Acta Zool. (Stockholm) 95, 63–72. ( 10.1111/azo.12008) [DOI] [Google Scholar]
- 22.Barnes WJP, Baum M, Peisker H, Gorb SN. 2013. Comparative cryo-SEM and AFM studies of hylid and rhacophorid tree frog toe pads . J. Morphol. 274, 1384–1396. ( 10.1002/jmor.20186) [DOI] [PubMed] [Google Scholar]
- 23.Scholz I, Barnes WJP, Smith JM, Baumgartner W. 2009. Ultrastructure and physical properties of an adhesive surface, the toe pad epithelium of the tree frog, Litoria caerulea White. J. Exp. Biol. 212, 155–162. ( 10.1242/jeb.019232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorb SN, Jiao Y, Scherge M. 2000. Ultrastructural architecture and mechanical properties of attachment pads in Tettigonia viridissima (Orthoptera Tettigoniidae). J. Comp. Physiol. A 186, 821–831. ( 10.1007/s003590000135) [DOI] [PubMed] [Google Scholar]
- 25.Perez-Goodwyn PJ, Peressadko A, Schwarz H, Kastner V, Gorb SN. 2006. Material structure, stiffness, and adhesion: why attachment pads of the grasshopper (Tettigonia viridissima) adhere more strongly than those of the locust (Locusta migratoria) (Insecta: Orthoptera). J. Comp. Physiol. A 192, 1233–1243. ( 10.1007/s00359-006-0156-z) [DOI] [PubMed] [Google Scholar]
- 26.Dirks J-H, Li M, Kabla A, Federle W. 2012. In vivo dynamics of the internal fibrous structure in smooth adhesive pads of insects. Acta Biomater. 8, 2730–2736. ( 10.1016/j.actbio.2012.04.008) [DOI] [PubMed] [Google Scholar]
- 27.Emerson SB, Diehl D. 1980. Toe pad morphology and mechanisms of sticking in frogs. Biol. J. Linn. Soc. 13, 199–216. ( 10.1111/j.1095-8312.1980.tb00082.x) [DOI] [Google Scholar]
- 28.Hanna G, Barnes WJP. 1991. Adhesion and detachment of the toe pads of tree frogs. J. Exp. Biol. 155, 103–125. [Google Scholar]
- 29.Federle W, Barnes WJP, Baumgartner W, Dreschler P, Smith JM. 2006. Wet but not slippery: boundary friction in tree frog adhesive toe pads. J. R. Soc. Interface 3, 589–601. ( 10.1098/rsif.2006.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes WJP, Oines C, Smith JM. 2006. Whole animal measurements of shear and adhesive forces in adult tree frogs: insights into underlying mechanisms of adhesion obtained from studying the effects of size and scale. J. Comp. Physiol. A 192, 1179–1191. ( 10.1007/s00359-006-0146-1) [DOI] [PubMed] [Google Scholar]
- 31.Barnes WJP, Pearman J, Platter J. 2008. Application of peeling theory to tree frog adhesion, a biological system with biomimetic implications. Eur. Acad. Sci. E-Newsletter Sci. Technol. 1, 1–2. [Google Scholar]
- 32.Endlein T, Ji A, Samuel D, Yao N, Wang Z, Barnes WJP, Federle W, Kappl M, Dai Z. 2013. Sticking like sticky tape: tree frogs utilise friction forces to enhance attachment to overhanging surfaces. J. R. Soc. Interface 10, 20120838 ( 10.1098/rsif.2012.0838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohler A. 1995. Digital pad morphology in torrent-living Ranid frogs. Asiatic Herpetol. Res. 6, 85–96. [Google Scholar]
- 34.Endlein T, Barnes WJP, Samuel D, Crawford N, Biaw AE, Grafe U. 2013. Sticking under wet conditions: the remarkable attachment abilities of the torrent frog, Staurois guttatus. PLoS ONE 8, e73810 ( 10.1371/journal.pone.0073810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes WJP, Smith J, Oines C, Mundl R. 2002. Bionics and wet grip. In Tire Technol. Int. December ‘02, pp. 56–60. Dorking, UK: UK & International Press. [Google Scholar]
- 36.Alibardi L. 2001. Keratinization in the epidermis of amphibians and the lungfish: comparison with amniote keratinization. Tissue Cell 33, 439–449. ( 10.1054/tice.2001.0198) [DOI] [PubMed] [Google Scholar]
- 37.Spinner M, Westhoff G, Gorb SN. 2013. Subdigital and subcaudal microornamentation in chamaeleonidae—a comparative study. J. Morphol. 274, 713–723. ( 10.1002/jmor.20137) [DOI] [PubMed] [Google Scholar]
- 38.Alibardi L. 2010. Cornification of the beak of Rana dalmatina tadpoles suggests the presence of basic keratin-associated proteins. Zool. Stud. 49, 51–63. [Google Scholar]
- 39.Smith JM. 2003. Effects of allometric growth and toe pad morphology on adhesion in hylid tree frogs. PhD thesis, University of Glasgow, Glasgow, UK. [Google Scholar]
- 40.Smith JM, Barnes WJP, Downie JR, Ruxton GD. 2006. Structural correlates of increased adhesive efficiency with adult size in the toe pads of hylid tree frogs . J. Comp. Physiol. A 192, 1193–1204. ( 10.1007/s00359-006-0151-4) [DOI] [PubMed] [Google Scholar]
- 41.Barnes WJP, Perez Goodwyn PJ, Nokhbatolfoghahai M, Gorb SN. 2011. Elastic modulus of tree frog adhesive pads. J. Comp. Physiol. A 197, 969–978. ( 10.1007/s00359-011-0658-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee KC, Erb U. 2013. Grain boundaries and coincidence site lattices in the corneal nanonipple structure of the mourning cloak butterfly. Beilstein J. Nanotechnol. 4, 292–299. ( 10.3762/bjnano.4.32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Frisch E. 1974. Animal architecture. New York, NY: Harcourt Brace Jovanovitch. [Google Scholar]
- 44.Capinera JL. 2008. Encyclopedia of entomology, 2nd edn Heidelberg, Germany: Springer. [Google Scholar]
- 45.Fahrenbach WH, Knutson DD. 1975. Surface adaptations of the vertebrate epidermis to friction. J. Invest. Dermatol. 65, 39–44. ( 10.1111/1523-1747.ep12598036) [DOI] [PubMed] [Google Scholar]
- 46.Varenberg M, Gorb SN. 2008. A beetle-inspired solution for underwater adhesion. J. R. Soc. Interface 5, 383–385. ( 10.1098/rsif.2007.1171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wainwright DK, Kleinteich T, Kleinteich A, Gorb SN, Summers AP. 2013. Stick tight: suction adhesion on irregular surfaces in the northern clingfish. Biol. Lett. 9, 20130234 ( 10.1098/rsbl.2013.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crawford N, Endlein T, Barnes WJP. 2012. Self-cleaning in tree frog toe pads; a mechanism for recovering from contamination without the need for grooming. J. Exp. Biol. 215, 3965–3972. ( 10.1242/jeb.073809) [DOI] [PubMed] [Google Scholar]
- 49.Butt H-J, Barnes WJP, del Campo A, Kappl M, Schoenfeld F. 2010. Capillary forces between soft, elastic spheres. Soft Matter 6, 5930–5936. ( 10.1039/c0sm00455c) [DOI] [Google Scholar]
- 50.Zakerin M, Kappl M, Backus EHG, Butt H-J, Schönfeld F. 2013. Capillary forces between rigid spheres and elastic supports: the role of Young's modulus and equilibrium vapour adsorption. Soft Matter 9, 4534–4543. ( 10.1039/c3sm27952a) [DOI] [Google Scholar]
- 51.Varenberg M, Gorb SN. 2009. Hexagonal surface micropattern for dry and wet friction. Adv. Mater. 21, 483–486. ( 10.1002/adma.200802734) [DOI] [Google Scholar]
- 52.Ruffato D, III, Parness A, Spenko M. 2014. Improving controllable adhesion on both rough and smooth surfaces with a hybrid electrostatic/gecko-like adhesive. J. R. Soc. Interface 11, 20131089 ( 10.1098/rsif.2013.1089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shahsavan H, Zhao B. 2014. Bioinspired functionally graded adhesive materials: synergetic interplay of top-viscous-elastic layers with base micropillars. Macromolecules 47, 353–364. ( 10.1021/ma4018718) [DOI] [Google Scholar]
- 54.Barnes WJP 1999. Tree frogs and tire technology. In Tire Technol. Int. March ‘99, pp. 42–47. Dorking, UK: UK & International Press. [Google Scholar]
- 55.Persson BNJ. 2007. Wet adhesion with application to tree frog adhesive toe pads and tires . J. Phys. Cond. Matter 19, 376110 ( 10.1088/0953-8984/19/37/376110) [DOI] [Google Scholar]