Abstract
Gregarious settlement in barnacle larvae (cyprids) is induced by a contact pheromone, the settlement-inducing protein complex (SIPC). The SIPC has been identified both in the cuticle of adult barnacles and in the temporary adhesive secretion (footprint) of cyprids. Besides acting as a settlement inducer, the presence of the SIPC in footprints points to its additional involvement in the adhesion process. SIPC adsorption behaviour was therefore investigated on a series of self-assembled monolayers (SAMs) by surface plasmon resonance at the pH of seawater (8.3). Fibrinogen and α2-macroglobulin (A2M) (blood complement protease inhibitors with which the SIPC shares 29% sequence homology) were used in the adsorption experiments as positive and negative standards, respectively. The mass uptake of the SIPC was comparable to that of fibrinogen, with adsorption observed even on the protein-resistant oligo(ethylene glycol) surface. Notably, on the positively charged SAM the SIPC showed a kinetic overshoot, indicating a metastable configuration causing the amount of adsorbed protein to temporarily exceed its equilibrium value. A2M adsorption was low or negligible on all SAMs tested, except for the positively charged surface, indicating that A2M adsorption is mainly driven by electrostatics. Evaluation of SIPC non-specific adsorption kinetics revealed that it adsorbed irreversibly and non-cooperatively on all surfaces tested.
Keywords: barnacle, self-assembled monolayer, surface plasmon resonance, adhesion, settlement-inducing protein complex, kinetic overshoot
1. Introduction
The settlement-inducing protein complex (SIPC) is a large (approx. 260 kDa native, 169 kDa deglycosylated) glycoprotein, and the principal modulator of conspecific recognition in barnacles [1]. It functions as a contact pheromone, inducing the settlement of cypris larvae as they explore the cuticle-covered exterior of adult barnacles. The SIPC is heavily glycosylated, with M2–9 mannose glycans predominating [2]. The exact role of the glycan moiety is unclear; however, it is likely to be involved in the primary signalling function of the SIPC molecule. Free mannose in solution significantly induces the settlement of cyprids, and yet the de-glycosylated SIPC molecule also remains active in that regard. Therefore, both protein and glycan moieties are necessary for the full functionality to be realized.
The SIPC is also present in the ‘footprints’ of temporary adhesive deposited onto surfaces during exploration by cyprids, thus increasing the likelihood of future cyprid settlement on previously explored surfaces. Although the influence of surface-bound temporary adhesive on subsequently exploring larvae had been recognized decades earlier, it was only recently [3] that the mechanism of induction was attributed with confidence to the SIPC. Immunological experiments performed using antibodies specific to the N- and C-terminal amino acid sequences of the SIPC molecule successfully identified trails of footprints left by larvae exploring a nitrocellulose membrane. For a full discussion of the SIPC and its ecological role in barnacle gregarious settlement, the reader is directed to the review by Clare [1].
Of greater relevance to this study is the fact that, with the identification of SIPC in cyprid temporary adhesive, the possibility arose that SIPC may serve a dual role, both as a conspecific signalling molecule and as an adhesive. The exact contribution to adhesion made by the temporary adhesive has never been completely clear; however, recent work by Aldred et al. [4] strongly supports the original hypothesis that this viscous material is indeed the main functional element in the tenacious and rapidly reversible attachment of cyprids to surfaces. The implied role of the SIPC in temporary adhesion is not as far-fetched as it may at first seem, when it is considered in the light of an unpublished report of the persistence and stability of this molecule in the marine environment [5].
This study was, therefore, designed to investigate the adhesive properties of the SIPC in order to examine its putative role in temporary adhesion. This required investigation of the adsorption characteristics of the intact molecule to a range of well-defined surfaces and also comparison with other relevant proteins in the form of positive and negative standards. The identification of SIPC in the temporary adhesive stemmed from biochemical characterization of the molecule, via amino acid sequencing, and subsequent identification of the full SIPC open reading frame in the barnacle nuclear DNA. In so doing, it became apparent that the SIPC shares significant sequence homology with the thioester-containing family of proteins that includes the α2-macroglobulins (A2Ms), including human A2M [3]. A2M was therefore selected as a negative standard—similar in structure to the SIPC, but with no putative adhesive function.
A2Ms are blood complement protease inhibitors, found throughout the animal kingdom. Their function is to inactivate proteolytic enzymes; an action that occurs via trapping of the enzymes in a so-called ‘bait region’. The whole complex is then identified by macrophages and removed from the system. The relative similarity of the SIPC to commercially available human A2M is, however, difficult to state precisely. Certainly A2M is inert as a settlement cue to barnacles [3] and, unsurprisingly, the SIPC also lacks the highly conserved beta-cysteinyl gamma-glutamyl thioester sequences usually found in A2M-like complement proteins. It is presumed, therefore, that the SIPC plays no role in the immune system of barnacles; a hypothesis supported by the dissimilar sequence of the SIPC and barnacle A2M [3], thus suggesting different roles. It does, on the other hand, have a short sequence likely to be a remnant of a ‘bait region’ and the similarity in amino acid sequence between SIPC and A2M is nonetheless significant, with 29% similarity to an A2M from Limulus sp. and a tertiary structure consistent with TEP1r from the anti-parasite immune system of the mosquito Anopheles gambiae (with which it shares 26% sequence homology). It is possible, if not likely, that the SIPC evolved from an ancestral barnacle A2M gene and, as such, it is not unreasonable to expect structural as well as sequence similarity between the molecules. Indeed, this theory is supported by the work of Pagett et al. [2], who superimposed TEM class averages of the SIPC molecule onto the A2M crystal structure, finding a good visual match.
Fibrinogen (clotting factor I), on the other hand, is totally unrelated to the SIPC in terms of structure and sequence. It is, however, a model protein for molecular adhesion studies having a tendency to adsorb non-specifically to a wide range of surfaces, including the so-called non-fouling chemistries like poly(ethylene glycol) (PEG) [6–9]. The PEG surface functionality is strongly hydrophilic, both in its interior and on its surface, retaining a hydration layer of electrostatically bound water molecules at the surface interface and along the chain that makes adsorption impossible for molecules not specifically adapted to the task. Seemingly, (i) steric stabilization, (ii) lateral packing density, (iii) charge neutrality, (iv) chain mobility and conformation, and (v) the unusually low refractive index of PEG (among water-soluble polymers) that makes van der Waals attractions with proteins very low are also parameters contributing to the protein-repellent nature of PEG and oligo(EG) surfaces [10–14]. Any molecule that even approaches the degree of non-specific surface adsorption demonstrated by fibrinogen, including adsorption to PEG, may therefore be considered to be specifically adapted to the role. For this reason, fibrinogen was included here as a positive standard for adhesion.
To enable comparative investigation of surface adsorption by the SIPC, A2M and fibrinogen, we employed surface plasmon resonance (SPR), a highly surface-sensitive optical method frequently used for monitoring biomolecular binding to surfaces based on changes in refractive index. As the specific contribution to refractive index change is assumed to be linearly related to the molecular weight of the biomolecule, SPR is commonly interpreted as a mass sensor. SPR has been used to demonstrate the protein resistance of OEG (EG3OH) and the adhesion of bacterial and human cells to surfaces [15]. Recently, the non-specific adsorption of lipoproteins from human blood plasma to antifouling polymer brushes comprising PEG and zwitterionic functionalities was investigated by SPR [16]. Imaging SPR has been used previously to investigate the deposition of cyprid footprints to surfaces and preliminary data relating to SIPC binding on a limited range of surfaces were also obtained [17]. The previous study concluded that footprint deposition was directly correlated to SIPC adsorption, with the exception of a positively charged amine-terminated SAM, where SIPC adsorption was unexpectedly high relative to footprint deposition data. Since barnacle cyprids are generally reluctant to settle on positively charged surfaces [18–20], this result came as a surprise and required confirmation.
Based upon this rationale, the present study addresses three main hypotheses: (1) that SIPC adsorption to surfaces is chemistry specific, perhaps based upon surface free energy or charge; (2) that due to their structural relatedness, the specificity of the SIPC and A2M would be similar, and (3) that the SIPC is, in qualitative terms, a ‘sticky’ (promiscuously adsorbing) molecule when compared with fibrinogen. If non-specific adsorption by the SIPC is of the same order as fibrinogen and this characteristic is not also possessed by the related A2M molecule, then it would suggest that the SIPC is adapted to maximize surface adsorption and is likely to be involved directly in temporary adhesion by cypris larvae. Improved understanding of cyprid temporary adhesion would benefit not only the development of biomimetic adhesives for rapidly reversible, yet tenacious, underwater adhesion, but also the marine antifouling coatings industry where development of materials to prevent adhesion of marine invertebrate larvae is a priority.
2. Material and methods
2.1. Purification of the settlement-inducing protein complex
SIPC purification followed the protocol of Matsumura et al. [21]. Briefly adult barnacles, Balanus amphitrite (i.e. Amphibalanus amphitrite) [22] from Beaufort, NC, were snap-frozen, quickly homogenized and the crude protein extract, buffered throughout at pH 7.5, was subjected to salt precipitation and dialysis. Following dialysis, the crude extract was purified further using ion exchange and size exclusion chromatography. After each step, relevant fractions were identified by western blotting of a representative sample using an antibody specific to a 76 kDa subunit of the SIPC. The protein used for experiments, prepared using this process, was therefore intact and non-denatured.
2.2. Determination of settlement-inducing protein complex pI
Protein fractions containing the SIPC were collected from a BioLogic LP system (Biorad) following completion of the chromatography stages referred to above. The active fractions, identified by western blotting, were further separated by SDS-PAGE to confirm the presence of SIPC sub-units. Those containing the SIPC were subjected to isoelectric focusing (IEF) in a 24-well off-gel fractionation system (3100 OFFGEL Fractionator; Agilent Technologies). In this method, a liquid sample is applied in wells along the length of a 24 cm immobilized pH gradient strip (ReadyStrips IPG Strips pH 3–10; Biorad). β-Galactosidase from Aspergillus oryzae (Sigma), cytochrome C from equine heart (Sigma) and commercial IEF standards (IEF standards pI 4.45–9.6; Biorad) were subjected to simultaneous separation procedures on different strips for reference. Sample preparation and the off-gel fractionator protocol were followed exactly as per the manufacturer's recommendations (Agilent Technologies OFFGEL Fractionator User Guide). Following the IEF separation procedure, proteins were eluted back into the wells situated above their final location on the IEF strip. The contents of all wells were then removed and once again run on an SDS-PAGE gel followed by western blotting using an antibody specific to the N-terminal sequence of the B. amphitrite SIPC [23]. The location of the stained fraction (well number), relative to the standards mentioned above, provided a measure of the pI of the SIPC.
2.3. Preparation of self-assembled monolayers
Five types of self-assembled monolayers (SAMs) were used in SPR experiments. The surfaces were prepared as follows: 12 × 12 mm2 glass SPR chips coated with a 45 nm thick layer of gold (acquired from GE Healthcare, Uppsala, Sweden) were washed in a 5 : 1 : 1 mixture of Milli-Q water, 30% hydrogen peroxide and 25% ammonia (TL-1) at 85°C for 5 min. The slides were then rinsed with water and ethanol and placed in thiol solutions overnight prior to testing. The following thiols were used: HS(CH2)10CH3 (Fluka, Germany), HS(CH2)11OH (Prochimia, Poland), HS(CH2)10COOH (Sigma-Aldrich, Germany), HS(CH2)11N(CH3)3+ Cl− (Prochimia, Poland) and HS(CH2)10CONH(C2H4O)11CH3 (Polypure AS, Norway). These treatments produced surfaces with homogeneous methyl (CH3), hydroxyl (OH), carboxyl (COO−), trimethylamine (N(CH3)3+) and oligo(ethylene glycol) (OEG) chemistry, respectively. Prior to the analyses, SPR chips were incubated for 24 h in 100 µM thiol solutions in 99.5% ethanol. The chips were subsequently sonicated, washed with ethanol and blow dried under a stream of nitrogen. Advancing water contact angles were 107 ± 1° for CH3, 60 ± 2° for N(CH3)3+, 45 ± 3° for COO−, 39 ± 2° for OH and 33 ± 2° for OEG. Additional information on ellipsometric thicknesses, surface free energies and infrared reflection absorption spectra can be found in [17–20].
2.4. Surface plasmon resonance
SPR experiments were conducted using a Biacore 3000 unit (GE Healthcare) operated at 25°C and at a flow rate of 10 µl min−1. Tris-base (0.05 M, pH 8.3; Sigma) was used as a running buffer. Solutions of SIPC, fibrinogen (Sigma) and α2-macroglobulin (A2M; Sigma) were made up in running buffer, which had been selected to approximate the native conditions for SIPC. After docking the SPR chip into the instrument, running buffer was allowed to equilibrate the surface for 3 min, followed by a 3 min period of protein injection (30 µl). After this injection, running buffer was allowed to flow over the chip for 5 min to ensure a stable plateau was reached after desorption of loosely bound molecules. Data are presented as absolute differences in resonance units (RUs) immediately before protein injection and 300 s after the end of injection. RUs were converted into mass uptake of proteins (Δm) via the following relation:
where CSPR corresponds to 6.5 × 10−2 ng · cm−2 for the adsorption of proteins to flat surfaces [24,25]. Three replicate measurements were collected for each protein on each surface chemistry (different surface per replicate) allowing later comparison by general linear model analysis of variance (GLM ANOVA) and Tukey post hoc comparisons in Minitab 15. Since SPR can determine the mass of the adsorbed analyte, protein concentrations were in weight per volume (mg/ml) rather than moles per volume (molar concentration). This approach allowed direct comparison between the proteins in terms of their adsorption to the chosen SAMs.
Accurate rate constants for the non-specific adsorption process could not be calculated, since the protein density on the SAM at saturation depends on the concentration of protein in the buffer. It was, nevertheless, possible to draw certain conclusions regarding the kinetics of adsorption. In such circumstances, Mrksich et al. [6] suggested a kinetics analysis to determine the initial rate of protein adsorption by plotting the initial change in resonance units (ΔRU during a chosen time interval) as a function of the bulk protein concentration. Here, the initial rates of adsorption of the SIPC at various bulk concentrations (0.1, 0.05, 0.025, 0.0125 and 0.005 mg ml−1) were evaluated on the five SAM surfaces. The slope of the fitted linear trends (ΔRU/s versus protein bulk concentration) relates directly to the initial rate of adsorption of the SIPC to the test surfaces.
The first second of data, immediately following injection of the protein, was disregarded in the ensuing calculations to circumvent aberrations related to bulk refractive index differences between running buffer and sample. Determination of the exact increase in refractive index within approximately 200 nm from the surface (the SPR probe depth) is daunting since the instant response shift may also, in part, be due to a contribution from rapid adsorption of protein molecules onto the SAM. The RU value 1 s after injection of the SIPC solution (t1) was therefore subtracted from that at 2 (t2) and 5 s (t5), and the resulting values (with the value in the t5 − t1 interval divided by 4) were considered to be indicative of the SIPC initial rate of adsorption.
3. Results
3.1. Isoelectric point of the settlement-inducing protein complex
Western blots identified proteins with SIPC-specific immunological activity in wells 6 and 7 of the 24-well off-gel fractionation system. β-Galactosidase and phycocyanin, with pIs of 4.5 and 4.6, respectively, were also found in similar positions in the IEF system. On the basis of these observations, the pI of the SIPC was determined to be 4.6–4.7, which is in the region predicted by the amino acid sequence.
3.2. Protein adsorption on self-assembled monolayers
Adsorption of the SIPC, fibrinogen and A2M onto a range of SAMs was measured by SPR at a concentration of 0.1 mg ml−1 at pH 8.3, and the results are presented in figure 1. Taken across all surfaces, the three proteins gave different responses (F = 138, p ≤ 0.001) and Tukey pairwise comparisons, reported in the electronic supplementary material, table S1, suggested that all three proteins differed significantly from each other in terms of total adsorption to surfaces. There was also a significant effect of surface, when considering all proteins together (F = 295.44, p ≤ 0.001). Surfaces clustered into three groups, where N(CH3)3+ had the highest adsorption of proteins overall, followed by COO− and CH3 (which did not differ) and then OH and OEG with the lowest adsorption overall. The SPR data presented a clear ranking of adsorption for the three proteins under investigation on all surfaces, except for the N(CH3)3+ SAM (figure 1), where A2M responded differently. In fact, on all but the positively charged surface, fibrinogen had the highest adsorbed mass, followed by SIPC and then A2M. In the case of N(CH3)3+ SAM, the largest adsorbed mass was that of A2M, which was lowest on all other surfaces. Interestingly, the SIPC adsorbed equally well to the two oppositely charged surfaces.
Figure 1.
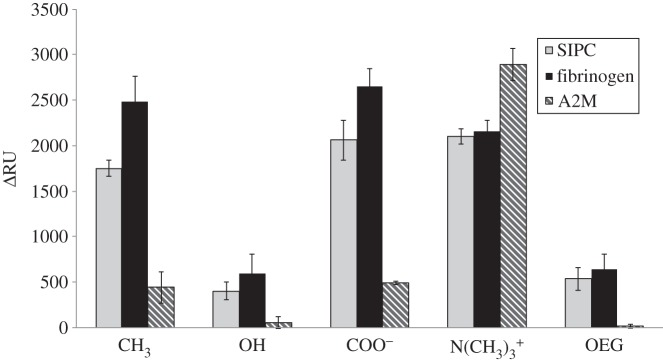
SPR data for the adsorption of fibrinogen, SIPC and α2-macroglobulin (A2M) on a series of SAMs. Data are absolute changes in resonance units (ΔRU) after a 3 min injection of 0.1 mg ml–1 protein solutions at pH 8.3, at 10 µl min–1 flow rate and 25°C, followed by 5 min 0.05 M Tris-base buffer wash. Standard errors were from three replicate SPR experiments.
In statistical terms, there was also a significant GLM ANOVA interaction term (F = 49.87, p ≤ 0.001) between proteins and surfaces, as would be expected from looking at the data in figure 1. That is, different proteins adsorbed in different amounts, depending on the specific surface chemistry of the substrate. It is not necessary to consider here all possible combinations in the analysis (electronic supplementary material, table S1), so specific examples are used for illustrative purposes: the differences in adsorbed mass of SIPC, fibrinogen and A2M were significant on the CH3 and COO− SAMs, but there was no effect of surface on adsorption of the different proteins—i.e. each protein responded similarly to these two surfaces, although there were differences between proteins in terms of absolute adsorption. Further, there were no differences in the response of proteins to OH and OEG, although for both of these surfaces adsorption of A2M was significantly lower than that of fibrinogen and SIPC, which did not differ. For N(CH3)3+, A2M adsorption was significantly higher than SIPC and fibrinogen. SIPC adsorption did not differ between CH3, COO− and N(CH3)3+; however, adsorption of fibrinogen was significantly lower on N(CH3)3+ than on CH3 and COO−.
In the context of the study, the important findings were that for OH, OEG and N(CH3)3+ SAMs the adsorption values for SIPC and fibrinogen were similar. Adsorption of fibrinogen was also significantly reduced by the presence of a positive surface charge, whereas adsorption of SIPC was not. Adsorption of SIPC was only significantly lower than fibrinogen on the CH3 and COO− surfaces and, even so, was still considerably higher than A2M. Adsorption of A2M was very low across the range of surfaces tested, with the exception of N(CH3)3+. SIPC showed much higher adsorption that more closely resembled that of fibrinogen on the charged SAMs and the hydrophilic neutral SAMs (OH and OEG). On the basis of these data, it was immediately apparent that SIPC was markedly different from A2M in terms of its adsorption characteristics, and that its behaviour was broadly similar to that of fibrinogen on the surfaces tested.
3.3. Kinetics of adsorption of the settlement-inducing protein complex
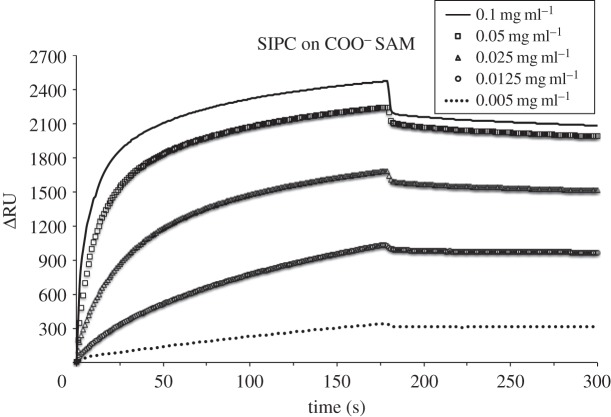
In addition to total adsorbed mass, the kinetics of initial adsorption of SIPC was assessed on the five SAMs. SPR sensorgrams in figures 2–6 show the adsorption of SIPC at five concentrations (0.1, 0.05, 0.025, 0.0125 and 0.005 mg ml−1) on N(CH3)3+, COO−, CH3, OH and OEG SAMs. On all SAMs, the initial rate of SIPC adsorption increased as a function of its bulk concentration, as qualitatively visible from the slopes of the initial part of the SPR curves (see the electronic supplementary material, figures S1–S5). On the contrary, on the positively charged N(CH3)3+ surface, the total amount of adsorbed SIPC was highest for the lowest concentration (0.005 mg ml−1), followed in ascending order by the protein at concentrations of 0.0125, 0.1, 0.025 and 0.05 mg ml−1 (figure 2).
Figure 2.
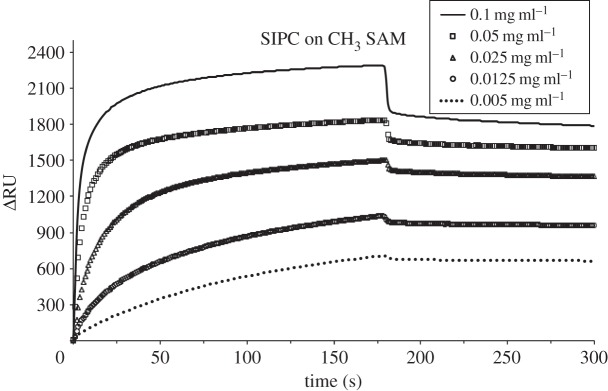
SPR sensorgrams for the adsorption of SIPC on CH3 SAM at 0.1, 0.05, 0.025, 0.0125 and 0.005 mg ml−1 at pH 8.3, 10 µl min−1 flow rate and 25°C. After 3 min protein injection, the desorption phase was followed by 0.05 M Tris-base buffer wash.
Figure 3.
SPR sensorgrams for the adsorption of SIPC on COO− SAM at 0.1, 0.05, 0.025, 0.0125 and 0.005 mg ml−1 at pH 8.3, 10 µl min−1 flow rate and 25°C. After 3 min protein injection, the desorption phase was followed by 0.05 M Tris-base buffer wash.
Figure 4.
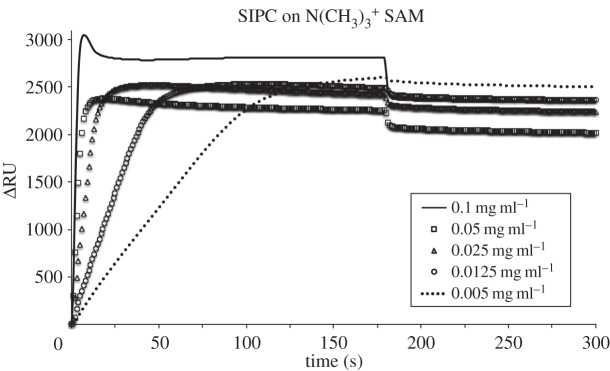
SPR sensorgrams for the adsorption of SIPC on N(CH3)3+ SAM at 0.1, 0.05, 0.025, 0.0125 and 0.005 mg ml−1 at pH 8.3, 10 µl min−1 flow rate and 25°C. After 3 min protein injection, the desorption phase was followed by 0.05 M Tris-base buffer wash.
Figure 5.
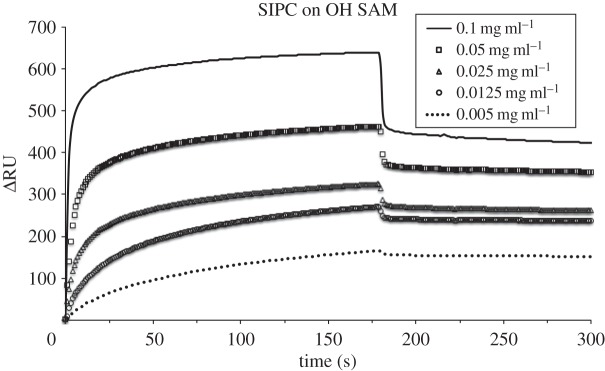
SPR sensorgrams for the adsorption of SIPC on OH SAM at 0.1, 0.05, 0.025, 0.0125 and 0.005 mg ml−1 at pH 8.3, 10 µl min−1 flow rate and 25°C. After 3 min protein injection, the desorption phase was followed by 0.05 M Tris-base buffer wash.
Figure 6.
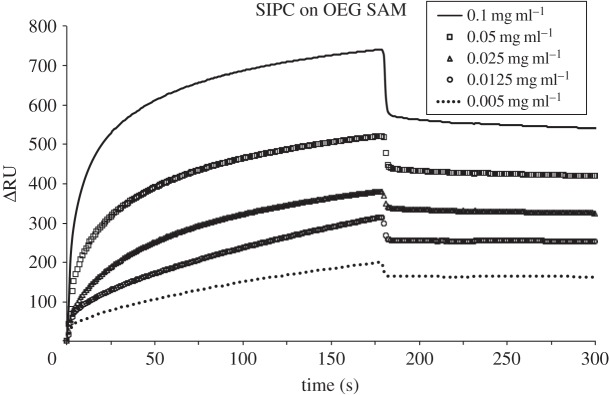
SPR sensorgrams for the adsorption of SIPC on OEG SAM at 0.1, 0.05, 0.025, 0.0125 and 0.005 mg ml−1 at pH 8.3, 10 µl min−1 flow rate and 25°C. After 3 min protein injection, the desorption phase was followed by 0.05 M Tris-base buffer wash.
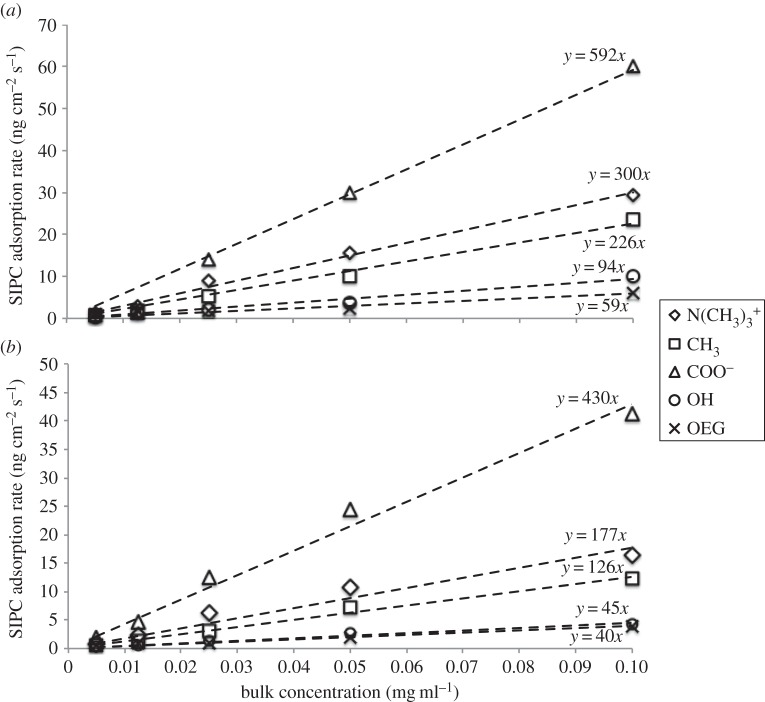
The initial SIPC adsorption rates were calculated via a linear fit to the data in the intervals t1–t2 and t1–t5 for each concentration, for each SAM. These rates are plotted versus the solution concentrations in figure 7. For each SAM chemistry, the rates appear to vary linearly with solution concentration, and linear fits to the rates for a particular SAM yield the slopes indicated in figure 7, and summarized in table 1.
Figure 7.
SIPC adsorption rates during (a) t1–t2 and (b) t1–t5 intervals for five SIPC bulk concentrations (0.1, 0.05, 0.025, 0.0125 and 0.005 mg ml−1) on the SAMs reported in the legend. Linear fits to the rates for each SAM are reported by the dashed lines along with their corresponding equations.
Table 1.
Slopes and corresponding linear correlation coefficients (r) of the linear fitting in figure 7 for the adsorption of SIPC on the investigated SAM surfaces during t1–t2 and t1–t5 intervals.
| SAM surface | t1–t2 slope | t1–t5 slope |
|---|---|---|
| –N(CH3)3+ | 592 (r = 0.99092) | 430 (r = 0.99272) |
| –CH3 | 300 (r = 0.99678) | 177 (r = 0.97288) |
| –COO− | 226 (r = 0.99383) | 126 (r = 0.99526) |
| –OH | 94 (r = 0.9867) | 45 (r = 0.98212) |
| –OEG | 59 (r = 0.91263) | 40 (r = 0.97653) |
Both intervals lead to the same trend for the adsorption rate of the SIPC on the SAMs tested. Kinetics calculations that neglected the initial 2 s of SIPC injection resulted in comparable results (data not shown). The linear increase in adsorption rate with increasing solution concentration for all SAMs is indicative of first-order rate constants, consistent with irreversible and non-cooperative SIPC adsorption on all SAM chemistries [6,26].
3.4. Desorption of the settlement-inducing protein complex
The relative amount of the SIPC desorbed from each SAM upon buffer wash is reported in the electronic supplementary material, figure S6. The data reported as the percentage of desorbed protein (% desorbed) as a function of concentration show that the highest value (33.6%) was recorded for the OH SAM at 0.1 mg ml−1, followed by the OEG SAM (26.9%) at the same concentration. Generally, the desorbed amount percentage increased with the SIPC concentration on each surface. The lowest desorbed percentage was on the positively (3.6%) and negatively (5.9%) charged SAMs, with the CH3 SAM showing intermediate values.
4. Discussion
The adsorption of three proteins, namely SIPC, fibrinogen and A2M, was measured on a range of thiol SAMs using SPR. The functional end-groups were suitably chosen to impart hydrophilic, hydrophobic, neutral and charged characters to the SAMs. Fibrinogen is a large blood plasma protein (340 kDa, pI 5.3–6.1) known to adsorb strongly to a variety of surfaces, and it is thus often used as a ‘sticky’ model protein for comparative purposes [27]. Tetrameric A2M (760 kDa, pI 5.3) [28] was included in this study as it shows a significant homology with SIPC (29%). The pI of the approximately 260 kDa SIPC molecule was found to be 4.6–4.7. At the pH of seawater (8.3), selected for the experiments, all proteins therefore possessed a net negative charge.
The importance of ionic strength and pH should be acknowledged here since these factors certainly affect the range of forces implicated in the molecular adsorption mechanism: electrostatic interactions, hydrogen bonding, molecular conformation, etc. The effect of ionic strength on the adsorption behaviour of the SIPC thus demands further investigation. As explained previously, a pH of 8.3 was chosen to approximate the native conditions in which the SIPC exercises its putative adhesive role and to maintain consistent experimental conditions between treatments. There are, however, some complicating factors. Upon discharging their adhesive material, some organisms have been shown to manipulate the local pH so that it differs from the ambient seawater [29,30], providing conditions conducive to the chemistry of adhesion. In the case of mussels and tubeworms, the secretory granules containing adhesive components have an acidic pH [29,31]. Similarly, an acidic pH has been detected in the cement glands of adult stalked barnacles, Lepas anatifera [32]. Furthermore, two of the test proteins (fibrinogen and A2M) have evolved to perform their respective functions under physiological pH, so the experimental conditions here take them slightly out of context. While imperfect, therefore, this study provided a ‘level playing field’ for all proteins in conditions relevant to the protein of interest, the SIPC, and the findings should be considered with this in mind.
Both the SIPC and fibrinogen adsorbed irreversibly to negatively charged surfaces under conditions where they also carry a net negative charge. Notably, fibrinogen adsorption was reduced on the positively charged SAM, compared with the negatively charged surface, whereas there was no reduction in SIPC adsorption. While A2M adsorbed irreversibly on the N(CH3)3+ SAM, it was significantly reduced on the COO− SAM. The adsorption of all proteins was relatively low on OH and OEG SAMs (protein-resistant surfaces). SIPC and fibrinogen adsorbed in equally small yet quantifiable amounts, while A2M adsorption was negligible on OH SAM and undetected on OEG SAM. Protein net charge alone is therefore insufficient to explain protein adsorption on charged surfaces. This may be due to the role of non-charge interactions, or the patchiness of charged domains where local charge distributions allow proteins to interact with both negatively and positively charged surfaces, depending on the orientation of the molecule [33].
Upon adsorption and binding to a surface, the electron density within the intramolecular bonds of any molecule inevitably undergoes a structural change. Therefore, the structural changes referred to in this work denote major unfolding, denaturating and (re)orientational events. Proteins might unfold to various degrees undergoing conformation and (re)orientational changes. Restructuring may lead to a conformational entropy gain, which can constitute the driving force for adsorption even under conditions of electrostatic repulsion, as observed for the adsorption of SIPC and fibrinogen on a like-charged surface in this SPR study. Additionally, for proteins with a given net charge, adsorption onto like-charged surfaces can be driven by attachment of regions on the protein with opposite charge, and further conformational changes from this state may promote stronger attachment. It is unlikely that the SIPC would have evolved this capability by chance and the most plausible explanation for the similarity in adsorption characteristics between the SIPC and the promiscuously adsorbing fibrinogen, therefore, is that both have similar roles in their respective systems—as ‘sticky’ proteins. The evidence from this preliminary adsorption study thus provides support for the involvement of the SIPC in barnacle cyprid temporary adhesion to the diversity of immersed surfaces that larvae might encounter in nature.
The SIPC and A2M show significant sequence similarity (29% perfect amino acid matches) and, therefore, the prominent discrepancy in adsorption behaviour appears to derive from parameters other than their primary sequences. As displayed in the electronic supplementary material, figure S7, from the pairwise sequence alignment, in addition to the perfect amino acid matches (398 residues), there is a significant number of amino acids (358 residues that score more than 0.5 in the Gonnet PAM 250 matrix) with strongly similar properties and others (229 residues that score greater than or equal to 0.5 in the Gonnet PAM 250 matrix) with weakly similar properties. Besides their homology, the pairwise comparison shows that the SIPC and A2M are highly conserved proteins throughout the entire sequences, and differences in their adsorption behaviour have to be sought in aspects other than their primary sequences.
Furthermore, a comparison of the total amino acid composition (see the electronic supplementary material, table S2) shows that the SIPC contains higher proportions of arginine and aspartic acid, whereas the relative content of histidine is greater in A2M. Arginine and aspartic acid have a hydropathy index of −4.5 and −3.5, respectively, and histidine has an index of −3.2 (the lower the hydropathy index, the greater the hydrophilic nature of an amino acid). This comparison indicates that higher amounts of arginine and aspartic acid in the SIPC confer greater hydrophilicity to the SIPC as compared with A2M. The grand average of hydropathicity (GRAVY) of the SIPC is −0.304, whereas A2M is −0.195, indicating that the SIPC is indeed more hydrophilic than A2M.
Another difference between the SIPC and A2M is revealed by aligning their primary sequences, showing that an insert of 60 amino acids (GQSTPEGTPE SETSGAAHSS LFIPPPTRSQ RFRTDREDAI KPFDEAGFLV LSNLALETRP), located in the SIPC 706–766 region, is missing in A2M. Notably, the GRAVY index for this sequence is −0.697, which is lower than the corresponding values for both proteins. However, it would be highly speculative to provide an explanation for the discrepancy in adsorption behaviour of the SIPC and A2M based solely on this sequence, or on the proteins' GRAVY index. Data relating to the conformations adopted by the SIPC in solution and on surfaces are needed. In addition, glycosylation of the proteins provides further complication since the 60 amino acid insert in the SIPC may well be glycosylated, thus causing the GRAVY index to differ from the predicted values based on its primary sequence.
A kinetic study was carried out to obtain semi-quantitative measurements for the adsorption of SIPC to the range of SAMs tested. SIPC at five concentrations (0.1, 0.05, 0.025, 0.0125 and 0.005 mg ml−1) was injected into the SPR flow cell and the accumulation of mass on the surfaces was quantified. The SPR sensorgrams in figures 2–6 show an increase in the total adsorbed amount of SIPC as a function of its bulk concentration. Interestingly, this trend was not observed on the positively charged SAM, where the lowest concentration (0.005 mg ml−1) resulted in the highest adsorbed amount of SIPC (figure 2). A possible explanation for this unusual pattern of adsorption on the N(CH3)3+ SAM is visible in the ascending portion of the SPR curves at higher concentrations. At 0.1 mg ml−1, the SPR curve shows a maximum after 7 s of SIPC injection peaking at 3048 RU. RU subsequently drops rapidly to 2850 RU at 15 s and then stabilizes to 2800 RU in the following few seconds. It is tempting to disregard such trends as anomalous artefacts, but in reality such a curve can be ascribed to an overshooting effect manifesting a local maximum during the adsorption, exceeding temporarily its equilibrium value [33]. A likely explanation relates to orientational rearrangement that occurs upon adsorption of the SIPC on the positively charged SAM. Wertz & Santore [34] and Daly et al. [35] reported an overshooting effect for the adsorption of fluorescently labelled lysozyme on hydrophilic and hydrophobic surfaces at pH 7.4, caused by the orientational change of adsorbed molecules from an initial end-on to a final side-on orientation with respect to the surface. Assuming similar adsorption behaviour in the case of the SIPC on the N(CH3)3+ SAM, electrostatic interactions could initially drive the rapid adsorption of the net negatively charged SIPC on the positively charged surface in an end-on orientation. Subsequently, the reorientation of the SIPC molecules into a more tightly adhering side-on position would displace more loosely bound SIPC molecules that remained in an end-on orientation.
Besides reorientation, conformational changes during protein adsorption are recurrent, enhancing the affinity for a surface. Plausible mechanisms are presented schematically in figure 8, with both (a) (re)orientational and (b) conformational changes of adsorbed SIPC on N(CH3)3+ SAM. Both rotational and conformational changes cause the adsorbed proteins to increase their surface area in contact with the substrate, thus leading to stronger adsorption and energetically favoured states. These phenomena would explain the decrease in total adsorbed mass and the drop evident in the SPR curves with increasing protein concentration presented to the surface. The overshooting effect was still manifest experimentally at lower SIPC concentrations (0.05, 0.025 and 0.0125 mg ml−1), becoming undetectable at the lowest solution concentration of 0.005 mg ml−1, as the coverage monotonically approached its final value.
Figure 8.
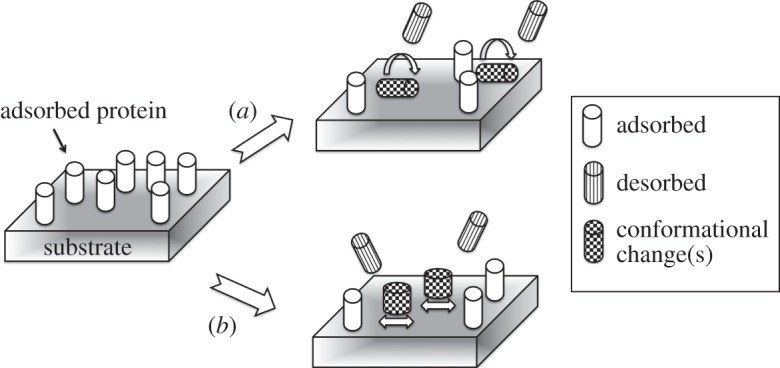
Schematic of the mechanism of overshooting effect observed for the adsorption of SIPC on the N(CH3)3+ SAM. Adsorbed proteins can undergo (a) reorientational and/or (b) conformational changes, displacing neighbouring adsorbed proteins and increasing their surface contact area.
A mathematical model for the overshooting kinetics of solutes at liquid/solid interfaces based on the time delay (τ) was proposed by Ohshima et al. [36]. Solutes with complex structures require some time, denoted as τ, to desorb from a solid surface as a result of their conformation and/or orientational rearrangements. In the period 0 ≤ t ≤ τ only adsorption occurs, and at t = τ desorption begins in addition to adsorption. If τ is sufficiently large, the total adsorbed amount of solute can exceed its equilibrium value, thus causing an overshoot. From figure 4, τ can be deduced from the sensorgrams for each of the four SIPC concentrations as the maximum during adsorption (prior to buffer wash), namely at τ1 = 7 s, τ2 = 19 s, τ3 = 45 s and τ4 = 107 s, corresponding to 0.1, 0.05, 0.025 and 0.0125 mg ml−1, respectively. At τ1, τ2, τ3 and τ4 the surface concentration of the SIPC corresponds to 198, 155, 164 and 165 ng cm–2, respectively. At these concentrations, it can thus be assumed that the rate of conformational change and/or reorientation of the SIPC is lower than its adsorption rate, thus causing the kinetic overshoot.
A marked difference was observed in the relative amount of SIPC desorbed from SAMs upon buffer wash (see the electronic supplementary material, figure S6). A surface that denatures a protein at a faster rate will show a lower tendency towards desorption. In fact, desorption has been shown to correlate strongly to protein stability [37], i.e. the more denaturating a surface, the lower the desorbed protein amount. On the other hand, a higher amount of protein desorption suggests both a slower denaturation rate and a lesser degree of denaturation. The least denaturing of the SAMs during the SIPC adsorption are OH and OEG, on which lower total adsorption occurs. The CH3 SAM is intermediate, while COO− and N(CH3)3+ SAMs show the lowest relative desorption, corresponding to the greatest SIPC denaturation. More specifically, on the positively charged surface, the minimum desorption percentage of the SIPC at 0.005 mg ml−1 was 3.6%, which then increased to 5.1%, 9.8%, 12.8% and 17.3% at, respectively, 0.0125, 0.025, 0.05 and 0.1 mg ml−1. Therefore, the lowest SIPC concentration showed the greatest degree and/or rate of denaturation and, as its concentration increased, the rate of interfacial denaturation decreased. These observations corroborate the assumption that the experimentally observed overshoot kinetic was mainly due to the rate of adsorption overcoming the rate of denaturation at concentrations higher than 0.005 mg ml−1.
The highest total adsorbed mass for the SIPC at the lowest bulk concentrations of 0.005 mg ml−1 can be tentatively explained by the slower rate of adsorption. At low bulk concentrations of SIPC, the surface coverage is maximized, possibly because all SIPC molecules are able to maximize their surface area in contact with the substrate when there are few neighbours. This would increase the fraction of tightly bound molecules and the total adsorbed mass would be augmented.
Regarding the kinetic analysis of SIPC initial adsorption, the initial second of protein injection in the sensorgram adsorption curves was ignored to exclude artefacts presented by the refractive index mismatch between running buffer and the sample [38,39]. The SIPC injection during t1–t2 and t1–t5 intervals was considered to be indicative of the initial phase of protein adsorption on the range of SAMs tested. In fact, regardless of the interval chosen, the trends of SIPC initial adsorption rates were consistent, showing linear dependence as a function of SIPC bulk concentration on each SAM (figure 7). Such linear fitting denotes a first-order rate constant, indicative of irreversible and non-cooperative SIPC adsorption on all SAMs. As reported in table 1, the values of the slopes of the linear relations in figure 7 show the same trend for the two intervals, revealing that the initial SIPC adsorption rate was highest on N(CH3)3+, followed by CH3, COO−, OH and OEG SAMs.
An adsorption mechanism that can create first-order rate constants can be explained as follows: as the protein adsorption starts, the planar, chemically and physically homogeneous SAM surface available for binding can be considered infinite, and the resulting initial adsorption kinetics are of first order (very low sorbate/sorbent ratios). The exclusion of the initial second of adsorption still results in first-order kinetics, thus showing that the surface is not overpopulated by adsorbed proteins and can thus be considered as infinite for the chosen time course. Additionally, the initial protein adsorption takes place on surface sites where no other proteins are present and consequently lateral and cooperative interactions are negligible.
The finding in this work correlates directly with the previous study by Aldred et al. [17] in which the SIPC was shown to have highest adsorption to a positively charged amine-terminated (–NH3+) SAM (N.B. different SAM termination to the present study, different barnacle species and different SIPC purification apparatus yielding a comparable result). However, following surface exploratory behaviour of cyprids, less temporary adhesive material was detected on this surface compared with, for example, a COO− SAM. The relation between the SIPC adsorption and adhesive footprint retention by the other SAMs tested was directly proportional. There are several possible explanations for the dissimilarity of the footprint and the SIPC adsorption data for positively charged SAMs. First, it is possible that cyprids somehow ‘choose’ to deposit fewer footprints on the positively charged surfaces, although no physiological/behavioural mechanism has been described that would allow this and it also seems unlikely in the context of data suggesting a direct correlation between footprint deposition and passive protein adsorption for the other surfaces tested. Second, the cyprid footprints/temporary adhesive could contain other adhesive components, in addition to the SIPC, that could moderate the intrinsic preference of pure SIPC for adsorbing to positively charged surfaces. Last, it is possible that the overshooting effect described above plays a role. If the temporary adhesive footprint is pure SIPC, the immediate saturation of the surface by the SIPC molecules when the cyprid makes contact could lead to extensive reorganization that favours cohesion of the footprint and retention on the adhesive disc, rather than deposition to the surface. After all, positively charged surfaces in the marine environment are presumably rare, and it would be surprising if the temporary adhesive mechanism had adapted to these specifically.
These data present the first mechanistic evidence to support the role of the SIPC in cyprid temporary adhesion to surfaces. In the context of this study, the SIPC performed comparably to fibrinogen and its adsorption characteristics were not shared by the closely related A2M. It is clear, therefore, that the SIPC is a ‘sticky’ protein. It also seems reasonable to conclude, based upon these quantitative findings and the identification of the molecule as a component of cyprid footprints, that the SIPC could also be an adhesive protein. New findings by Gohad et al. [40] revealed that the permanent adhesive of cyprids comprises a bi-phasic system of lipids and phosphoproteins, synergistically acting to maximize underwater adhesion. Future studies should aim towards similar identification of the component(s) of the temporary adhesive of cyprids, for instance by integrating high-throughput RNA-seq with proteomics [41,42] in order to ascertain whether the SIPC is ‘acting alone’ or ‘in concert’ with lipoproteinaceous compounds [40]. In the first case a range of possibilities opens, from use of SIPC adsorption as a rapid assay for approximation of cyprid adhesion, to further investigation of the properties of the SIPC molecule that make it so promiscuously adsorbing. The former has the potential for establishing, for example, a rapid down-selection assay for fouling-resistant chemistries, while the latter may produce findings that can be translated into the development of bioinspired adhesives.
5. Conclusion
— The SIPC appears to be a ‘sticky’ (promiscuously adsorbing) protein with adsorption characteristics more closely resembling those of fibrinogen than the related A2M molecule. This supports a putative role in the temporary adhesion of barnacle larvae to surfaces. Further investigation of this molecule will be required to better understand the mechanism of surface adsorption and potential routes to exploitation.
— The SIPC adsorbed, on all of the SAMs tested, in similar quantities to fibrinogen, exemplifying its ability to interact with diverse surfaces including protein-resistant surfaces such as OEG. Like fibrinogen, adsorption of the SIPC was increased by the presence of a positive or negative surface charge.
— SIPC adsorption was higher than that of A2M for all surfaces, except for the positively charged N(CH3)3+ SAM, indicating a prevalence of ionic interactions during the A2M adsorption process. The kinetics of initial adsorption differed for SIPC between the COO− and N(CH3)3+ SAMs, with faster initial adsorption to the N(CH3)3+ SAM.
— The linearity of the initial rate of adsorption for SIPC indicated first-order rate constants consistent with irreversible and non-cooperative adsorption mechanisms on all SAMs.
— The kinetic overshoot observed on the positively charged SAM can be ascribed largely to conformational changes, as the rate of adsorption overcame the rate of denaturation at concentrations higher than 0.005 mg ml−1.
Funding statement
This study has received funding from the European Community's Seventh Framework Programme FP7/2007–2013 under grant agreement no. (237997) (SEACOAT). N.A. would like to acknowledge Office of Naval Research grant N00014-08-1-1240 to A.S.C. for financial support, as well as COST Action TD0906 for two Short-Term Scientific Missions to Linköping, during which the experimental work was carried out.
References
- 1.Clare AS. 2011. Toward a characterization of the chemical cue to barnacle gregariousness. In Chemical communication in crustaceans (eds Breithaupt T, Thiel M.), pp. 431–450. New York, NY: Springer. [Google Scholar]
- 2.Pagett HE, et al. 2012. Structural characterisation of the N-glycan moiety of the barnacle settlement-inducing protein complex (SIPC). J. Exp. Biol. 215, 1192–1198. ( 10.1242/jeb.063503) [DOI] [PubMed] [Google Scholar]
- 3.Dreanno C, Matsumura K, Dohmae N, Hirota H, Kirby RR, Clare AS. 2006. An α2-macroglobulin-like protein is the cue to gregarious settlement of the barnacle. Proc. Natl Acad. Sci. USA 103, 14 396–14 401. ( 10.1073/pnas.0602763103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldred N, Høeg JT, Maruzzo D, Clare AS. 2013. Analysis of the behaviours mediating barnacle cyprid reversible adhesion. PLoS ONE 8, e68085 ( 10.1371/journal.pone.0068085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yule AB, Walker G. 1987. Adhesion in barnacles. In Barnacle biology, crustacean issues 5 (ed. Southward AJ.), pp. 389–402. Rotterdam, The Netherlands: A.A. Balkema. [Google Scholar]
- 6.Mrksich M, Sigal GB, Withesides GM. 1995. Surface plasmon resonance permits in situ measurement of protein adsorption on self-assembled monolayers of alkanethiolates on gold. Langmuir 11, 4383–4385. ( 10.1021/la00011a034) [DOI] [Google Scholar]
- 7.Harder P, Grunze M, Dahint R, Whitesides GM, Laibinis PE. 1998. Molecular conformation in oligo(ethylene glycol)-terminated self-assembled monolayers on gold and silver surfaces determines their ability to resist protein adsorption. J. Phys. Chem. B 102, 426–436. ( 10.1021/jp972635z) [DOI] [Google Scholar]
- 8.Hu Y, Jin J, Han Y, Yin J, Jiang W, Liang H. 2014. Study of fibrinogen adsorption on poly(ethylene glycol)-modified surfaces using a quartz crystal microbalance with dissipation and a dual polarization interferometry. RSC Adv. 4, 7716–7724. ( 10.1039/C3RA46934D) [DOI] [Google Scholar]
- 9.Jin J, Jiang W, Yin J, Ji X, Stagnaro P. 2013. Plasma proteins adsorption mechanism on polyethylene-grafted poly(ethylene glycol) surface by quartz crystal microbalance with dissipation. Langmuir 29, 6624–6633. ( 10.1021/la4017239) [DOI] [PubMed] [Google Scholar]
- 10.Herrwerth S, Eck W, Reinhardt S, Grunze M. 2003. Factors that determine the protein resistance of oligoether self-assembled monolayers—internal hydrophilicity, terminal hydrophilicity, and lateral packing density. J. Am. Chem. Soc. 125, 9359–9366. ( 10.1021/ja034820y) [DOI] [PubMed] [Google Scholar]
- 11.Jeon S, Andrade J. 1991. Protein–surface interactions in the presence of polyethylene oxide: II. Effect of protein size. J. Colloid Interface Sci. 142, 159–166. ( 10.1016/0021-9797(91)90044-9) [DOI] [Google Scholar]
- 12.Jeon S, Lee J, Andrade J, De Gennes P. 1991. Protein–surface interactions in the presence of polyethylene oxide: I. Simplified theory. J. Colloid Interface Sci. 142, 149–158. ( 10.1016/0021-9797(91)90043-8) [DOI] [Google Scholar]
- 13.Larsson A, Ekblad T, Andersson O, Liedberg B. 2007. Photografted poly(ethylene glycol) matrix for affinity interaction studies. Biomacromolecules 8, 287–295. ( 10.1021/bm060685g) [DOI] [PubMed] [Google Scholar]
- 14.Ekblad T, et al. 2008. Poly(ethylene glycol)-containing hydrogel surfaces for antifouling applications in marine and freshwater environments. Biomacromolecules 9, 2775–2783. ( 10.1021/bm800547m) [DOI] [PubMed] [Google Scholar]
- 15.Ostuni E, Chapman RG, Liang MN, Meluleni G, Pier G, Ingber DE, Whitesides GM. 2001. Self-assembled monolayers that resist the adsorption of proteins and the adhesion of bacterial and mammalian cells. Langmuir 17, 6336–6343. ( 10.1021/la010552a) [DOI] [Google Scholar]
- 16.Gunkel G, Huck WTS. 2013. Cooperative adsorption of lipoprotein phospholipids, triglycerides, and cholesteryl esters are a key factor in nonspecific adsorption from blood plasma to antifouling polymer surfaces. J. Am. Chem. Soc. 135, 7047–7052. ( 10.1021/ja402126t) [DOI] [PubMed] [Google Scholar]
- 17.Aldred N, Ekblad T, Andersson O, Liedberg B, Clare AS. 2011. Real-time quantification of microscale bioadhesion events in situ using imaging surface plasmon resonance (iSPR). ACS Appl. Mater. Interfaces 3, 2085–2091. ( 10.1021/am2003075) [DOI] [PubMed] [Google Scholar]
- 18.Petrone L, Di Fino A, Aldred N, Sukkaew P, Ederth T, Clare AS, Liedberg B. 2011. Effects of surface charge and Gibbs surface energy on the settlement behaviour of barnacle cyprids (Balanus amphitrite). Biofouling 27, 1043–1055. ( 10.1080/08927014.2011.625474) [DOI] [PubMed] [Google Scholar]
- 19.Petrone L, Lee SSC, Teo SLM, Birch WR. 2013. A novel geometry for a laboratory-based larval settlement assay. Biofouling 29, 213–221. ( 10.1080/08927014.2012.762643) [DOI] [PubMed] [Google Scholar]
- 20.Di Fino A, Petrone L, Aldred N, Ederth T, Liedberg B, Clare AS. 2013. Correlation between surface chemistry and settlement behaviour in barnacle cyprids (Balanus improvisus). Biofouling 30, 143–152. ( 10.1080/08927014.2013.852541) [DOI] [PubMed] [Google Scholar]
- 21.Matsumura K, Nagano M, Fusetani N. 1998. Purification of a larval settlement-inducing protein complex (SIPC) of the barnacle, Balanus amphitrite. J. Exp. Zool. 281, 12–20. () [DOI] [Google Scholar]
- 22.Clare AS, Høeg JT. 2008. Balanus amphitrite or Amphibalanus amphitrite? A note on barnacle nomenclature. Biofouling 24, 55–57. ( 10.1080/08927010701830194) [DOI] [PubMed] [Google Scholar]
- 23.Dreanno C, Kirby RR, Clare AS. 2006. Locating the barnacle settlement pheromone: spatial and ontogenetic expression of the settlement-inducing protein complex of Balanus amphitrite. Proc. R. Soc. B 273, 2721–2728. ( 10.1098/rspb.2006.3649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liedberg B, Lundstrom I, Stenberg E. 1993. Principles of biosensing with an extended coupling matrix and surface plasmon resonance. Sens. Actuators B Chem. 11, 63–72. ( 10.1016/0925-4005(93)85239-7) [DOI] [Google Scholar]
- 25.Höök F, Kasemo B, Nylander T, Fant C, Sott K, Elwing H. 2001. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: a quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem. 73, 5796–5804. ( 10.1021/ac0106501) [DOI] [PubMed] [Google Scholar]
- 26.Ramsden JJQ. 1994. Experimental methods for investigating protein adsorption kinetics at surfaces. Q. Rev. Biophys. 27, 41–105. ( 10.1017/S0033583500002900) [DOI] [PubMed] [Google Scholar]
- 27.Chapman RG, Ostuni E, Takayama S, Holmlin RE, Yan L, Whitesides GM. 2000. Surveying for surfaces that resist the adsorption of proteins. Langmuir 122, 8303–8304. ( 10.1021/ja000774f) [DOI] [Google Scholar]
- 28.Back SA, Alhadeff JA. 1983. Differential isoelectric focusing properties of crude and purified human alpha 2-macroglobulin and alpha 2-macroglobulin-proteinase complexes. J. Chromatogr. B 278, 43–51. ( 10.1016/S0378-4347(00)84754-4) [DOI] [PubMed] [Google Scholar]
- 29.Stewart RJ, Wang CS, Shao H. 2011. Complex coacervates as a foundation for synthetic underwater adhesives. Adv. Colloid Interface Sci. 167, 85–93. ( 10.1016/j.cis.2010.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrone L. 2013. Molecular surface chemistry in marine bioadhesion. Adv. Colloid Interface Sci. 195–196, 1–18. ( 10.1016/j.cis.2013.03.006) [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Wei W, Danner E, Ashley RK, Israelachvili JN, Waite JH. 2011. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat. Chem. Biol. 7, 588–590. ( 10.1038/nchembio.630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonker JL, von Byern J, Flammang P, Klepal W, Power AM. 2012. Unusual adhesive production system in the barnacle Lepas anatifera; an ultrastructural and histochemical investigation. J. Morphol. 273, 1377–1391. ( 10.1002/jmor.20067) [DOI] [PubMed] [Google Scholar]
- 33.Rabe M, Verdes D, Seeger S. 2011. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 162, 87–106. ( 10.1016/j.cis.2010.12.007) [DOI] [PubMed] [Google Scholar]
- 34.Wertz CF, Santore MM. 2002. Adsorption and reorientation kinetics of lysozyme on hydrophobic surfaces. Langmuir 18, 1190–1199. ( 10.1021/la0108813) [DOI] [Google Scholar]
- 35.Daly SM, Przybycien TM, Tilton RD. 2003. Coverage-dependent orientation of lysozyme adsorbed on silica. Langmuir 19, 3848–3857. ( 10.1021/la026690x) [DOI] [Google Scholar]
- 36.Ohshima H, Fujita N, Kondo T. 1992. Adsorption kinetics with time delay. Colloid Polym. Sci. 270, 707–710. ( 10.1007/BF00654047) [DOI] [Google Scholar]
- 37.Karlsson M, Ekeroth J, Elwing H, Carlsson U. 2005. Reduction of irreversible protein adsorption on solid surfaces by protein engineering for increased stability. J. Biol. Chem. 280, 25 558–25 564. ( 10.1074/jbc.M503665200) [DOI] [PubMed] [Google Scholar]
- 38.O'Shannessy DJ, Brigham-Burke M, Soneson KK, Hensley P, Brooks I. 1993. Determination of rate and equilibrium binding constants for macromolecular interactions using surface plasmon resonance: use of nonlinear least squares analysis methods. Anal. Biochem. 212, 457–468. ( 10.1006/abio.1993.1355) [DOI] [PubMed] [Google Scholar]
- 39.Schuck P. 1997. Use of surface plasmon resonance to probe the equilibrium and dynamic aspects of interactions between biological macromolecules. Annu. Rev. Biophys. Biomol. Struct. 26, 541–566. ( 10.1146/annurev.biophys.26.1.541) [DOI] [PubMed] [Google Scholar]
- 40.Gohad NV, Aldred N, Hartshorn CM, Lee YJ, Cicerone MT, Orihuela B, Clare AS, Rittschof D, Mount AS. 2014. Synergistic roles for lipids and proteins in the permanent adhesive of barnacle larvae. Nat. Commun. 5, 4414 ( 10.1038/ncomms5414) [DOI] [PubMed] [Google Scholar]
- 41.Guerette PA, et al. 2013. Accelerating the design of biomimetic materials by integrating RNA-seq with proteomics and materials science. Nat. Biotechnol. 31, 908–915. ( 10.1038/nbt.2671) [DOI] [PubMed] [Google Scholar]
- 42.Guerrette PA, Hoon S, Ding D, Amini S, Masic A, Ravi V, Venkatesh B, Weaver JC, Miserez A. 2014. Nanoconfined β-sheets mechanically reinforce the supra-biomolecular network of robust squid sucker ring teeth. ACS Nano 8, 7170–7179. ( 10.1021/nn502149u) [DOI] [PubMed] [Google Scholar]