Abstract
While adult green dock leaf beetles Gastrophysa viridula use tarsal adhesive setae to attach to and walk on smooth vertical surfaces and ceilings, larvae apply different devices for similar purposes: pretarsal adhesive pads on thoracic legs and a retractable pygopod at the 10th abdominal segment. Both are soft smooth structures and capable of wet adhesion. We studied attachment ability of different larval instars, considering the relationship between body weight and real contact area between attachment devices and the substrate. Larval gait patterns were analysed using high-speed video recordings. Instead of the tripod gait of adults, larvae walked by swinging contralateral legs simultaneously while adhering by the pygopod. Attachment ability of larval instars was measured by centrifugation on a spinning drum, revealing that attachment force decreases relative to weight. Contributions of different attachment devices to total attachment ability were investigated by selective disabling of organs by covering them with melted wax. Despite their smaller overall contact area, tarsal pads contributed to a larger extent to total attachment ability, probably because of their distributed spacing. Furthermore, we observed different behaviour in adults and larvae when centrifuged: while adults gradually slipped outward on the centrifuge drum surface, larvae stayed at the initial position until sudden detachment.
Keywords: adhesion, biomechanics, gait pattern, insects, pygopod, walking
1. Introduction
Many different groups of insects have developed locomotory attachment systems, making walking on vertical or overhanging surfaces possible. In adult insects, locomotory attachment devices are usually hairy or smooth surfaces located at the tarsus or pretarsus [1]. Despite their different structure, both types are highly deformable, therefore able to maximize contact to the substrate and thereby attachment force, which is an interplay between van der Waals forces, capillary forces and friction, contributing differently in various behavioural situations and on different substrates. Hairy pads employ densely packed, flexible setae with specialized terminal elements forming many individual contact sites [2], which enable them to conform to surface topography. Additionally, an adhesion-mediating fluid is released, employing fluid coverage on the terminal setae, as previously reported, e.g. in flies [3] and beetles [4]. Smooth pads can be encountered, for example, in adult representatives of Hymenoptera [5], Hemiptera [6], Lepidoptera [7] and Orthoptera [8,9]. Like hairy pads they are supported by adhesion-mediating fluid, and use the combined effect of van der Waals forces, capillarity and friction in their functioning, but their surface structure is composed of a very thin and easily deformable cuticle underlain by thin filaments instead of individual setae [10]. This provides an alternative strategy to conform to challenging surface topographies, such as finely grained textures [9].
The highly effective setal attachment system of the leaf beetle Gastrophysa viridula has been investigated in a number of studies in recent years [11–14]. These beetles are oligophagous, living and feeding on species of the plant family Polygonaceae [15]. Beetles and larvae may attach to host plants at any position of the substrate. Females lay eggs on the underside of leaves, where the oligopod larvae remain grouped, almost motionless after hatching [16–18]. They pass three instars before dropping off to pupate and develop to adult in the soil underneath the plant [19]. Larvae of 2nd instar (L2) start to disperse, settling on the adaxial and abaxial leaf sides [16]. While young larvae move only a few millimetres, older ones (3rd instar, L3) may walk more than 50 cm per day [18]. To walk a distance of 10 cm vertically upwards on a plant stem, 1st instar (L1) take 3.8–6 min, L2 1.4 min, L3 44 s and adult males 14 s [15]. Thus, compared with larvae, adults are more mobile. L1 and L2 were reported to strongly attach to leaves because they would die when falling to the ground. In contrast, although L3 strongly attach to the host plant during feeding and cold weather, they can deliberately drop down during danger (thanatosis) or strong solar radiation, and climb back onto the plant within 10–20 s [18]. Despite the fact that larvae share the same host plant species as their hairy-footed parents, they attach by smooth pads to the same substrate structure. On the ventral thorax, the leaf beetle larvae bear six short legs with a single claw and pulvillus [20,21]. As the body shape and the centre of body mass of elongated larvae are totally different from those of adults, larvae are additionally equipped with a seventh attachment point in the form of a smooth pad at the posterior end of the abdomen, a uniform ring-shaped soft area surrounding the anal opening [21,22]. It is supposed to be a part of the ‘body skin’ and was observed to be retracted and extracted by contraction of the body musculature [23,24]. During contact with the substrate, a white-yellowish mass of irregular shape comes out of the abdominal segment, which adapts well to the irregularities of the substrate and forms intimate contact, supporting attachment and locomotion [23]. Abdominal pads are called anal prolegs, postpedes or pygopods [21,22,25–27] and can be also found in larvae of insect orders other than Coleoptera, such as Lepidoptera [27–32], Diptera (only Chironomidae) [27,33,34], Hymenoptera [32], also Mecoptera, Neuroptera and Raphidioptera [35]. In freely, terrestrially living beetle larvae, the involvement of the abdominal terminal as ‘locomotory supporting organ’ seems to occur generally [35]. Besides in Chrysomelidae, pygopods were verified in numerous families of Coleoptera (Carabidae, Staphilinidae, Coccinellidae, Silphidae, Cantharidae, Lampyridae, Cleridae, Byturidae, Cryptophagidae, Elateridae, Tenebrionidae) [20,22–24,35–37].
To move ahead, larvae bend their abdominal tip forward below up to the 5th abdominal segment, bring the pygopod in contact with the substrate, push their body forward moving the thoracic legs, detach the pygopod leaving small fluid droplets on the surface and repeat the locomotion cycle [23,35]. Beetle larvae of several species are able to adhere properly by only the pygopod, even when hanging upside down. This enables them to re-orient by raising the body and rotating it around, or to fix the body to the substrate for moulting [30,38,39]. The extremely strong bond was supposed to be only achievable by the release of a sticky secretion from the malpighian tubules [23]. So far, the strength of the bond has not been measured. Previous investigations focused on comparative, qualitative analyses of beetle larva attachment and locomotion. The present study proceeds on quantitative observations and measurements, using the dock leaf beetle G. viridula as a model species.
Having seven instead of six attachment points, beetle larvae do not walk using the alternating tripod gait pattern of adults [40]. Thus, we describe the locomotion of G. viridula larvae and experimentally test the attachment ability conferred by their two types of attachment devices. Specifically, we compare attachment ability between larval instars, contributions of pretarsal pads and pygopod, as well as their performance in situations emphasizing frictional or adhesion components of attachment. Additionally, we show how larvae and adult beetles behave and suddenly detach when external lateral force reaches its critical value.
2. Material and methods
2.1. Insects
In order to rear isolated groups of larvae of known age and instar, freshly laid egg patches (20–40 eggs) on Rumex obtusifolius leaves were obtained from laboratory stock rearings [22] and transferred to transparent, ventilated 175 ml polystyrene tubes plugged with Ceaprene stoppers (Greiner Bio-One, Frickenhausen, Germany). To prevent plant leaves and beetle larvae from drying out, moist cotton wool covered with a filter paper disc was placed at the bottom of each tube. Larval instar was identified by the width of the head capsule, which remains nearly constant while the larva gains weight (L1: 480 ± 14 µm, L2: 794 ± 32 µm, L3: 1137 ± 28 µm). The first two instars last 2–3 days, the third 6 days (laboratory temperature 23.1 ± 1.6°C). Larval weight increases exponentially from ca 0.1 mg in freshly hatched L1 to ca 10 mg before pupation.
2.2. Locomotion analysis
Larvae of all three instars one day after hatching respectively moulting were recorded while walking upside down at the underside of a cleaned glass slide (contact angle of Aqua Millipore water: 40°). Videos were taken at 250 frames per second (fps) using a Photron Fastcam 1024 PCI (Photron Ltd, Tokyo, Japan) high-speed video system, mounted on a Leica MZ 12.5 stereo microscope (Leica Mikrosysteme Vertrieb GmbH, Wetzlar, Germany). For each larval instar, contact and swing phases during one straight walk from three individuals was recorded.
2.3. Contact site imaging and measurements
The ventral side of larvae of all instars was photographed while they attached upside down to a glass slide. For this purpose, a digital photo camera Nikon Coolpix E995 (Nikon Corporation, Tokyo, Japan) was mounted on a Leica MZ 12.5 stereo microscope with coaxial incident illumination allowing high contrast imaging of the contact area between pads and glass. Four larvae of each instar were separately imaged 25 times, during different gait phases. We measured the area and perimeter of contact sites of pretarsal pads and pygopods, using SigmaScan Pro 5.0 (SPSS Inc.); in total, 16 pretarsal pads and four pygopods per instar were considered. Contact area of pooled pretarsal pads and pygopods within instar were compared with one-way ANOVA (JMP Pro 11.0.0, SAS Institute Inc., Cary, NC, USA).
Cryo-scanning electron microscopy (cryo-SEM) was carried out with a Hitachi S-4800 SEM (Hitachi High-Technologies Corp., Tokyo, Japan), supplemented with a Gatan ALTO 2500 cryo-preparation system (Gatan Inc., Abingdon, UK). Living specimens were mounted on metal sample holders with Tissue-Tec O.C.T. Compound mounting fluid (Sakura Fine Technical Co. Ltd, Tokyo, Japan) and shock-frozen by dipping them into liquid nitrogen. Then, they were transferred to the cryo-preparation chamber and kept at −140°C. Contamination by ice crystals on the surface of the specimens was removed using sublimation by raising the temperature of the sample to −95°C for 20 min. Subsequently, samples were sputter-coated with a 6 nm thick layer of gold–palladium. Specimens were examined at 5 kV accelerating voltage and −120°C within the chamber of the microscope.
2.4. Force measurements
Attachment ability was tested with a Tetra Zentri-01-P centrifugal force tester (Tetra GmbH, Ilmenau, Germany) [41]. Animals were individually weighed using an analytical balance AG 204 Delta Range (Mettler Toledo GmbH, Greifensee, Switzerland) and placed on either the horizontal surface or the vertical side of a rotating drum made of polished Plexiglas (rotation radius r = 5 cm, contact angle of Aqua Millipore water: 74° [7]). Starting positions of animals and their orientations were arbitrary. The centrifuge drum rotation speed was accelerated to 2000 r.p.m. within 10 s. At every revolution, fibre-optic sensors detected the animal's position which was recorded as a function of rotation speed using the data acquisition software PC Fly (Tetra GmbH). Forces, at which animals were detached from the surface, and safety factors (force divided by body weight) were calculated considering the body weight, rotation speed and animal position at detachment. We used only second and third instar larvae (L2, L3), as L1 were too small to reliably monitor them by the fibre-optic sensors. Twenty individuals of 2nd and 3rd larval instars were tested on both horizontal and vertical surfaces of the drum, with 10 repetitions per individual. Additionally, separate groups of L3 larvae (N = 10, n = 50) were tested on normal (contact angle of Aqua Millipore water: 39° [7]) and silanized (contact angle of Aqua Millipore water: 109° [7]) glass discs mounted on the horizontal surface rotating drum, in order to study the influence of the surface wettability by water (level of hydrophobicity) on the attachment ability of beetle larvae.
The contribution of pretarsal pads and pygopods to the total attachment ability was investigated in two different experiments: (i) inversion test and (ii) centrifugal force measurements. In the first experiment, two groups of L3 larvae (N = 10 each) were placed on a normal glass plate as control, one larva at a time. Then the plate was inverted by 180° (manually, ca 60° s−1) and attachment success was recorded. Rotation was repeated 10 times per each larva. Afterwards the larvae were anaesthetized with CO2, and the pygopods of the larvae in one group were covered with a thin layer of bee's wax. In the other group, the pretarsal pads were covered. The animals were allowed to recover for 2 h before continuing the inversion test.
In the second experiment, L3 larvae were first tested on horizontal and vertical Plexiglas surfaces (groups of 10 larvae per each surface) using the centrifugal force tester as described above. Then, we covered the tarsal pads with wax in five larvae of each group and the pygpods in the other five ones of each group, and repeated the centrifugation tests.
Effects of selectively covered attachment devices during centrifugation in horizontal and vertical orientation were compared using a linear mixed model (Standard Least Squares, REML fitting method as implemented in JMP Pro 11.0.0, SAS Institute Inc.) with safety factor as response variable and pretarsal adhesive pads/pygopod treatments, orientation and their factorial interactions as fixed nominal effects. Individual was treated as a random effect and repetition as a nested effect within individual.
3. Results
3.1. Morphology of the larval adhesion system
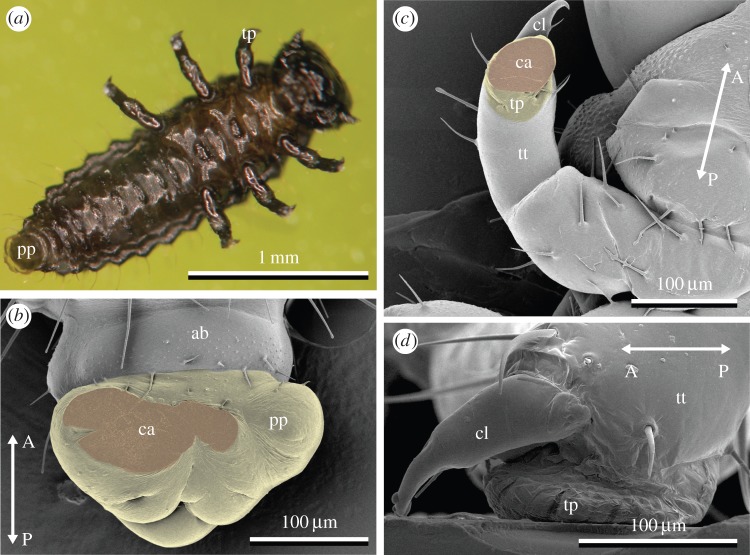
During locomotion, larvae use seven possible attachment points, one on each pretarsus of thoracic legs and an additional one at the posterior end of the abdomen (figure 1a). The pygopod's lobe surrounds the rectum and forms a soft, extendable ring (figure 1b). Our observations showed that only the ventral part of the pygopod was brought in contact with the substrate for attachment; the rectal opening did not come into contact, making a suction effect unlikely. Each leg possesses a single curved, tapered claw on the dorsal side of the pretarsus and a soft smooth adhesive pad on its ventral side. This pretarsal adhesive pad device was deformed, when coming in contact with a surface (figure 1c). Both pygopod and pretarsal pads left liquid footprints and achieved attachment by maximizing substrate contact with their soft, deformable surface and fluid layer in between. The contact area of pretarsal pads of different leg pairs within the same instar was of similar size, only the third leg pair was slightly larger in L3 (figure 2, one-way ANOVA; L1: F2,9 = 0.7, p = 0.52; L2: F2,9 = 2.9, p = 0.10; L3: F2,9 = 4.6, p = 0.04). As larval body mass increased during development (L1: 0.3 ± 0.14 mg, L2: 1.5 ± 0.75 mg, L3: 7.9 ± 2.40 mg, N = 12), contact area of tarsal pads and pygopods increased. Consequently, between instars, contact area of individual tarsal pads, summed tarsal pads and pygopod were significantly different (one-way ANOVA; tarsus 1: F2,9 = 276, p < 0.0001; tarsus 2: F2,9 = 346, p < 0.0001; tarsus 3: F2,9 = 90, p < 0.0001; sum of tarsi: F2,9 = 750, p < 0.0001; pygopod: F2,9 = 507, p < 0.0001). Contact area of the pygopod increased at a greater rate than that of the tarsi (linear regression of log-transformed values of weight and area: log10(areatarsi) = 4.16 + (0.48 ± 0.06) × log10(mass), R2 = 0.97, p < 0.0001; log10(areapygopod) = 4.38 + (0.77 ± 0.18) × log10(mass), R2 = 0.90, p < 0.0001; t-test of both slopes: t(20) = 3.35, p = 0.003). Across instars, this resulted in an increasing contact area ratio of tarsi to pygopod (figure 2). However, total contact area and perimeter of all attachment devices pooled together scaled isometrically (log10(area) = 4.59 + 0.66 × log10(mass), R2 = 0.93, p < 0.0001; log10(perimeter) = 3.35 + 0.35 × log10(mass), R2 = 0.96, p < 0.0001), i.e. close to the expected slopes of two-thirds for relationships between area and volume (log10(length2)/log10(length3) = 2/3), and one-third for length and volume (log10(length1)/log10(length3) = 1/3).
Figure 1.
Attachment pads of Gastrophysa viridula larvae. (a) L1 attached to the underside of a glass slide. (b,c) Cryo-SEM images of pads frozen in contact with a glass cover slide; cover slide is removed after freezing of pygopod (b) and pretarsal adhesive pad of thoracic leg (c). (d) Lateral view of tarsal pad of L3, frozen in contact with glass slide. Arrows indicate anterior–posterior body axis. Labels: ab, terminal abdominal segment; ca, pad contact area; cl, claw; pp, pygopod; tp, tarsal pad; tt, terminal tarsomere.
Figure 2.
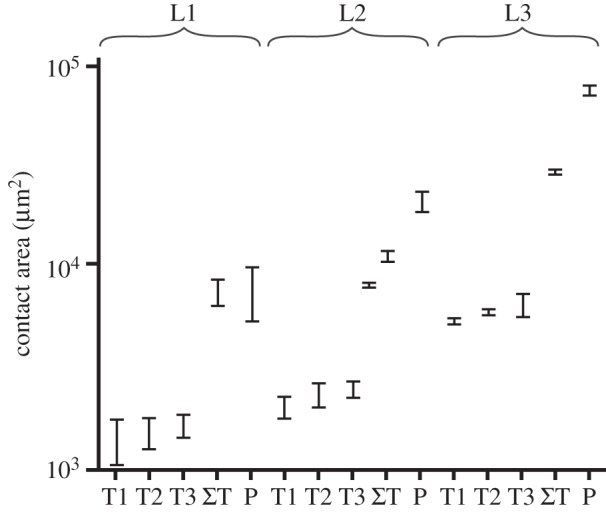
Contact area of pretarsal adhesive pads in thoracic leg pairs T1–T3 (left and right leg pooled together, means ± s.d.) and pygopods (P) of larval instars (L1–L3).
3.2. Locomotion
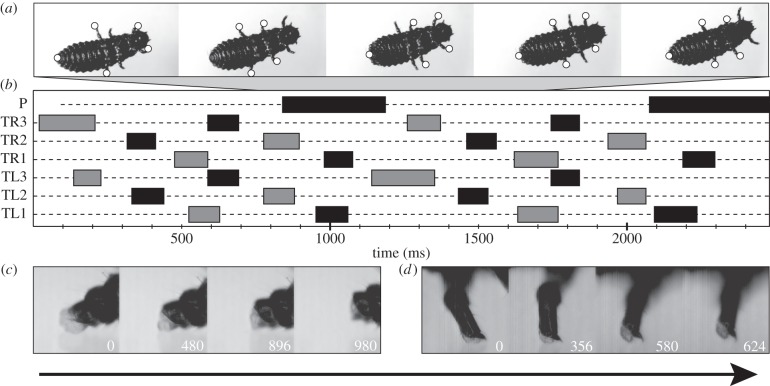
Adults of G. viridula used a typical alternating tripod gait for locomotion, attaching to smooth and slightly rough substrates with their setal attachment pads. Larvae likewise attached with pretarsal adhesive pads during locomotion, and also used the pygopod as an additional attachment site (figure 3). This behaviour was reflected by a characteristic gait pattern, which was observed for all larval stages studied (figure 3a,b). One pair of contralateral legs detached and simultaneously moved forwards. After five to six swing phases of the legs, the pygopod detached (figure 3b), upon which the stretched abdomen was contracted and pulled forward. In all instars, contact phase duration of the tarsal pads was similar for all three leg pairs (one-way ANOVA, F2,95 = 0.93, p = 0.40), and the contact phase of the pygopod was ca three times longer (L1: × 3.48, L2: × 2.64, L3: × 3.09). At detachment, the pygopod was actively retracted into the abdomen, which caused the contact area to peel off from back to front (figure 3c). Detachment of the round tarsal adhesive pads was accomplished by rolling them off in the direction of the motion, peeling off the contact area from hind to front. The final detachment was complemented by pushing with a single claw (figure 3d).
Figure 3.
Locomotion of Gastrophysa viridula larvae. Arrow indicates direction of motion. (a) L2 walking upside down on a glass slide. Contact sites are marked with white dots. (b) Example gait diagram, TL1–TL3, left legs; TR1–TR3, right legs; P, pygopod. Bars mark swinging phases, dotted lines contact/stance phases. (c) Lateral view of pygopod detachment sequence. (d) Lateral view of pretarsal adhesive pad detachment (front leg). Numbers in (c) and (d) indicate elapsed time in milliseconds.
3.3. Attachment ability of larval instars
Attachment ability may be comparatively described by a safety factor, defined as the measured attachment force divided by the body weight of the animal. Horizontal centrifugation provides information about friction forces of attachment devices, and this experimental situation is partially comparable to the situation when the animal attaches to vertical substrate in the field. Likewise, vertical centrifugation can be compared to the situation of the animal on a ceiling, predominantly relying on adhesion forces. L2 achieved significantly higher safety factors than the significantly heavier L3 in both experimental situations (figure 4). Attachment force of L2 was lower than for L3 due to the weight difference (horizontal surface, L2: 0.48 ± 0.19 mN versus L3: 1.18 ± 0.62 mN, F1,38 = 23.5, p < 0.0001; vertical surface, L2: 0.28 ± 0.09 mN versus L3: 0.64 ± 0.14 mN, F1,35 = 93.1, p < 0.0001). Hydrophobicity of the substrate affected attachment ability: safety factors of L3 were significantly lower on hydrophobic silanized glass than on normal glass (mean ± s.d. 27.05 ± 8.14 versus 32.4 ± 11.85, paired t-test, t(9) = −3.17, p = 0.011).
Figure 4.
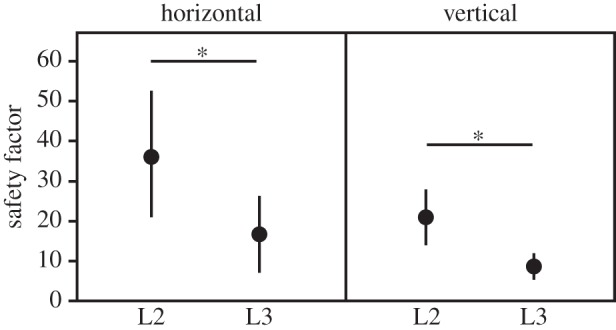
Safety factors (attachment force divided by body weight) generated by L2 and L3 instars of Gastrophysa viridula, attached horizontally and vertically to a Plexiglas drum during centrifugal force tests (mean ± s.d., each instar and position N = 20 individuals, n = 200 runs). Asterisks indicate statistical differences (one-way ANOVA, horizontal: F1,38 = 23.2, p < 0.0001; vertical: F1,35 = 44.1, p < 0.0001).
3.4. Contribution of larval thoracic and abdominal adhesive devices to attachment ability
In the inversion tests, untreated L3 always remained attached to the surface. After covering all six pretarsal pads with wax, 96% of larvae fell off when inverted. When only the pygopod was covered, 63% of larvae fell off. Identically treated groups of L3 were tested using the centrifugal force tester. Covering either type of attachment device led to reduction in safety factors, more so when tarsi were covered (table 1 and figure 5). Safety factors were higher in horizontal than vertical centrifugation orientation. Contribution to attachment ability of the pygopod appears to be influenced by orientation (figure 5), but this was not a significant effect (table 1).
Table 1.
Linear mixed model effects of attachment device type and centrifugation orientation on attachment ability (safety factors), tested in L3 of Gastrophysa viridula (20 unimpaired controls, five treatments per condition). Asterisks indicate statistically significant effects.
| effect | d.f. | d.f. in denominator | F | p-value |
|---|---|---|---|---|
| pretarsal pads | 1 | 173.8 | 42.0 | <0.0001* |
| pygopod | 1 | 173.8 | 8.2 | 0.0046* |
| orientation | 1 | 27.82 | 7.0 | 0.0133* |
| orientation × pretarsal pads | 1 | 173.8 | 2.2 | 0.1386 |
| orientation × pygopod | 1 | 173.8 | 3.9 | 0.0508 |
Figure 5.
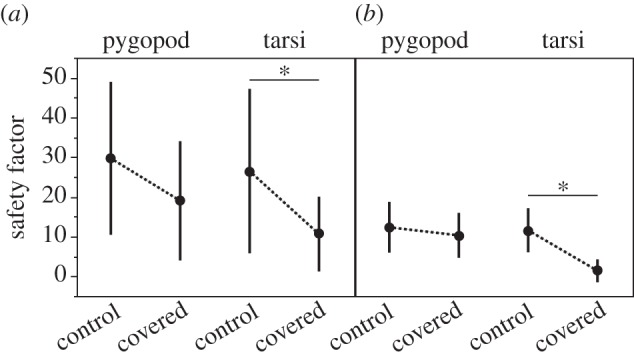
Safety factors (attachment force divided by body weight) generated by Gastrophysa viridula L3 with partially bee's wax-covered, non-usable attachment devices at (a) horizontal and (b) vertical position during centrifugal force tests (means ± s.d., N = 5 larvae in each of four treatment combinations). Asterisks indicate statistical differences (one-way ANOVA with Tukey HSD post hoc comparison, horizontal: F3,96 = 6.5, p < 0.0005; vertical: F3,96 = 23.1, p < 0.0001).
3.5. Sliding during centrifugation
We observed different behaviour of larvae and adults while being tested on a horizontal centrifugal drum surface. Adult beetles gradually slid outwards to the edge of the drum with increasing force, until they lost contact with the substrate (figure 6a,b). By contrast, L3 stayed in the initial position despite increasing rotation speed (figure 6c,d). They did not slide, but remained statically attached until sudden loss of contact.
Figure 6.
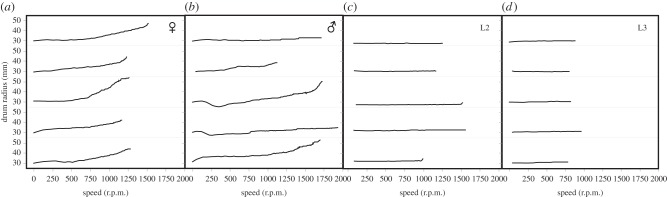
Position of adult and larval Gastrophysa viridula on the horizontal Plexiglas drum surface during centrifugation (five representative sequences with initial distance from the rotation centre of ca 30 mm are plotted for each group of individuals). The end of the curve corresponds to the contact loss between attachment devices of the insect and the drum. As drum rotation speed and centrifugal force increase, adult beetles gradually slide outward (a,b), while larvae stay in a similar position until detachment (c,d).
4. Discussion
Larvae of the leaf beetle G. viridula employ smooth attachment devices on their six walking legs and a seventh smooth contact point in the form of a pygopod to attach to the surface of the same host plant species as their adults, which possess hairy attachment pads on their six legs. There are numerous studies on attachment systems of adult chrysomelids, e.g. [11,42], but almost no quantitative details are known on attachment of chrysomelid larvae [20,22–24,35,36]. In this study, for the first time, we analysed the performance of attachment systems of beetle larvae, described larval gait patterns and measured larval attachment ability in different situations.
The phenotype of dock leaf beetle larvae differs totally from the compact body of the adult beetles. In some respects, the larval body resembles that of caterpillars; however, they do not share the numerous abdominal prolegs with the exception of the pygopod [25,27,29–31,43]. Similar to caterpillars, G. viridula larvae are soft-bodied, suggesting the presence of a hydrostatic skeleton without levers (long legs) and generating analogue locomotion patterns by body waves using a suitable substrate for force transmission [44,45]. There are parallels in the functional morphology of smooth pads (pretarsal ones and pygopods) between beetle larvae and caterpillars. The latter may effectively climb and strongly grip applying the pygopod as anchor, serving the lateral stability [46]. Those effects result in limited crawling speed. Although they crawl up and down more slowly than horizontally, caterpillars perform extremely well on vertical surfaces because they maintain continuous contact with the substrate, and because of the strong ‘grip’ and lateral stability provided by their pygopods [46,47].
The smooth attachment devices of leaf beetle larvae resemble the arolia found in other insect groups, such as cicadas [1,48], stick insects [9,49] and locusts [50], and similarly, they all rely on an adhesion-mediating fluid. In some situations, hairy pads can attach more strongly than smooth pads because splitting the contact area into many small contacts increases the total perimeter of the solid–fluid–air interface, which in turn may increase adhesion force [51]. Smooth pretarsal pads, like those of G. viridula larvae, have a relatively low contact perimeter, which may not be a disadvantage owing to the contribution of area-dependent Laplace pressure [52]. A possible advantage of such smooth pads lies in their malleable construction, which might be energy-efficient and beneficial for fast-growing larvae, as the attachment surface can expand between moults and thereby kept up with rapid increase in weight. Smooth pads may also be easier to shed during moults. However, safety factors decrease as larvae grow. The obtained safety factors correspond to previous reports that young larvae properly attach almost motionlessly to the abaxial leaf without falling down, while older larvae move on and fall off the plant more frequently, but quickly return onto the plant [16,18]. Contact sites grow isometrically during larval development; possible differences in muscle strength, wind resistance, load sharing or secretion composition between instars may contribute to lessened attachment ability in older larvae. Moreover, the attachment ability seems to correspond to behavioural particularities of larvae. L2 have to keep properly in contact with the host plant in order to feed and develop successfully. They are less agile than L3, and could not get back easily to the plant after dropping down. By contrast, L3 move faster and over longer distances and must drop down for pupation in the soil after a certain period of ingestion.
Locomotory attachment systems are not necessarily optimized for strong adhesion, but rather for efficient dynamic attachment and detachment during locomotion under difficult circumstances. Larvae use not only different types of attachment devices, but also a different type of gait pattern from adults. Larval locomotion appears as a combination of walking (thoracic legs) and crawling (pygopod). While locomoting adult beetles have only three contact sites with the surface while running even on the ceiling [53,54], the smooth attachment system of larvae is less effective, necessitating more simultaneous contact points to avoid falling off the host plant (hairy pads of adult G. viridula achieve friction force of ca 6.42 × 10−4 mN × μm−2 (male) and 6.13 × 10−4 mN × μm−2 (female), and adhesion force of 1 × 10−4 mN × μm−2 (male) and 5.3 × 10−5 mN × μm−2 (female) [55], while smooth larval pads only produced ca 1.0 × 10−5 mN × μm−2 friction and 5.7 × 10−6 mN × μm−2 adhesion). However, it is used in different behavioural contexts from attachment pads of adults, such as anchoring during moulting and feeding on leaf edges.
The pygopod is usually used as a long-term attachment device. For example, during moulting, the pygopod is used as the sole adhesion point of the larva to the underside of a leaf, but in this situation, a sticky secretion is exuded that firmly attaches the larva to the leaf [22]. This secretion then hardens (D. B. Zurek 2007, personal observation), a process that may require more time to anchor the abdomen than the sudden onset of applied force in centrifugal tests allows. According to previous findings [22], the role of the pygopod during locomotion is a combination of anchoring the long, heavy abdomen and assisting forward motion, especially when climbing upwards. Friction is emphasized in these situations, and such a role is supported by our observation that covering the pygopod with wax impacts the friction component of attachment more than the adhesion component. Although the contact area of the pygopod is larger than the summed contact areas of the tarsal pads, the distribution of all synergistically acting seven contact points around the larva's body might be of greater importance for keeping attachment stability and for preventing peeling off, as likewise suggested for caterpillars [30]. Similar results were recently described in spiders, where the collective effect of several legs during simultaneous attachment was stronger than the mathematical sum of adhesive forces generated by individual legs [56]. In centrifugation tests, larvae could withstand greater centrifugal forces on the horizontal than on the vertical Plexiglas drum surface, consistent with experiments performed with other insect species [57].
The friction–adhesion value ratio in dock leaf beetle larvae (L2 1.9 : 1, L3 1.7 : 1) is lower than that recently published for sawfly larvae 3.1 : 1 [58], but similar to those found in smooth attachment device-bearing adult codling moths on Plexiglas (1.6 : 1) [7] and specialized ants on Perspex (2.2 : 1) [59]. Thus, similar to adult insects in centrifugal force experiments, G. viridula larvae seem to attach predominantly by their six thoracic legs, differing from sawfly larvae for which the abdominal legs were supposed to contribute essentially to successful contact formation with the substrate and to enhance friction [58]. Unlike sawfly larvae, which bear seven pairs of abdominal legs in addition to three pairs of thoracic legs and a pygopod, G. viridula larvae have only one additional attachment point more than adult insects.
Attachment systems of animals usually have high attachment strength in the stance phase without impairing locomotion dynamics. Strong physical interaction between the pad and substrate has to be easily undone during the swing phase. Detachment of hairy pads is done by twisting, pulling or lifting the pretarsus. During these movements, only a portion of setae are detached at once [60]. Smooth aroliae of Hymenoptera can be folded or deflated [5,61,62] at detachment, which will gradually reduce the contact area between the pad and substrate. The ‘rolling-off’ movements of larval G. viridula, described in this study, are reminiscent of tree frogs, which detach their smooth pads by pivoting the toes forward [63].
Observation of sliding behaviour along the surface in larvae and adults at an increasing centrifugal force further underlines differences between their attachment systems. Adult G. viridula slid continuously when centrifuged, suggesting either an aquaplaning-like effect [64] or stick-and-slip reduction owing to the presence of multiple minute contacts instead of one or few larger ones [65,66]. Owing to multiple minute contacts, stick-and-slip events happen at each single contact, but at different times. That is why the entire array of contacts did not demonstrate a pronounced stick-and-slip, but rather continuous slipping motion. Similar to sawfly larvae [58] and adult codling moths [7], beetle larvae did not slide continuously: they rather stayed at the same position until they suddenly lost contact at certain critical centrifugal force. While hairy pads [66] or sheet-like aroliae [64] start slipping when lateral forces are applied, round larval pads are more likely to tilt and roll. This would lead to sudden detachment, when the muscle strength, required for keeping the leg in position, is surpassed by centrifugal force. Another possible reason is that the pygopod might help to anchor the larva in a certain position owing to additional effect of adhesion-mediating fluid released through the pad and pygopod cuticle [23,31]. While this contribution is unlikely to be large here, a predominant anchor effect of pygopods was experimentally confirmed for caterpillars [44]. Moreover, adhesion-mediating fluid was previously indicated by the visualization of foot- and pygopod prints in sawfly larvae and caterpillars [31,58]. For the latter, Hasenfuss [31] suggested an attachment mechanism based on capillary and meniscus forces, caused by a mobile liquid containing hydrocarbons and traces of more polar liquids [31]. Such fluid-mediated adhesion (meniscus) is likely to form an important contribution also to larval attachment.
Larvae and adults of the beetle species studied live on host plants of the same species having similar surface structures, and their different attachment systems encounter similar substrates. However, young larvae are more often found on the underside of the leaves while adults do not show such a preference [67]. Adaxial and abaxial leaf surfaces have different surface roughness owing to the stronger presence of stomata and trichomes, and pronounced venation on the abaxial side [11,68,69]. Hosoda & Gorb [70] showed greater attachment ability of adult beetles to the adaxial than the abaxial side of dock leaves (R. obtusifolius) [70]. It is possible that the smooth devices of larvae can attach stronger to the abaxial than the adaxial side. In this context, claws also have to be considered. The average diameter of an ideal circle fitting the concavity of the larval claw is approximately 54 µm, corresponding to the diameter of trichomes (55 µm) on the abaxial leaf of R. obtusifolius (D. Voigt 2006, unpublished data). A comparative study considering the attachment ability of larvae and adults on different roughness profiles might shed light on situational advantages of smooth versus hairy pads and the significance of claws in respect to their adaptation to certain conditions and/or specific functions. Interestingly, larvae frequently pinch the leaf between their thoracic legs, simultaneously adhering to both leaf sides, sitting and feeding at the margin of holes they ate [14,15,17]. Their mission consists of sitting on leaves, moving little, feeding and developing while protecting themselves against predators. By contrast, adult beetles have to be much more mobile; besides feeding, copulation and oviposition, they explore new habitats to ensure reproduction as well as dispersal of the progeny. This is reflected by higher walking speeds and covered distances of beetle imagines [15,18]. These different tasks of different developmental stages are supported by the different attachment systems that larvae and adults are equipped with.
Acknowledgements
We thank Jan Schuppert (Max Planck Institute for Intelligent Systems, Stuttgart, Germany) for valuable discussions and support of cryo-SEM and leaf beetle stock rearing.
Funding statement
This work was supported by German Science Foundation to S.N.G. (DFG, No. GO 995/10-1 and Project No. C-10 within SFB 677).
References
- 1.Beutel RG, Gorb SN. 2001. Ultrastructure of attachment specializations of hexapods (Arthropoda): evolutionary patterns inferred from a revised ordinal phylogeny. J. Zool. Syst. Evol. Res. 39, 177–207. ( 10.1046/j.1439-0469.2001.00155.x) [DOI] [Google Scholar]
- 2.Federle W. 2006. Why are so many adhesive pads hairy? J. Exp. Biol. 209, 2611–2621. ( 10.1242/jeb.02323) [DOI] [PubMed] [Google Scholar]
- 3.Walker G, Yulf AB, Ratcliffe J. 1985. The adhesive organ of the blowfly, Calliphora vomitoria: a functional approach (Diptera: Calliphoridae). J. Zool. 205, 297–307. ( 10.1111/j.1469-7998.1985.tb03536.x) [DOI] [Google Scholar]
- 4.Ishii S. 1987. Adhesion of a leaf feeding ladybird Epilachna vigintioctomaculta (Coleoptera: Coccinellidae) on a vertically smooth surface. Appl. Ent. Zool. 22, 222–228. [Google Scholar]
- 5.Federle W, Brainerd EL, McMahon TA, Hölldobler B. 2001. Biomechanics of the movable pretarsal adhesive organ in ants and bees. Proc. Natl Acad. Sci. USA 98, 6215–6220. ( 10.1073/pnas.111139298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemente CJ, Federle W. 2008. Pushing versus pulling: division of labour between tarsal attachment pads in cockroaches. Proc. R. Soc. B 275, 1329–1336. ( 10.1098/rspb.2007.1660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Bitar L, Voigt D, Zebitz CPW, Gorb SN. 2009. Tarsal morphology and attachment ability of the codling moth Cydia pomonella L. (Lepidoptera, Tortricidae) to smooth surfaces. J. Insect Physiol. 55, 1029–1038. ( 10.1016/j.jinsphys.2009.07.008) [DOI] [PubMed] [Google Scholar]
- 8.Gorb SN. 2000. Biological microtribology: anisotropy in frictional forces of orthopteran attachment pads reflects the ultrastructure of a highly deformable material. Proc. R. Soc. Lond. B 267, 1239–1244. ( 10.1098/rspb.2000.1133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drechsler P, Federle W. 2006. Biomechanics of smooth adhesive pads in insects: influence of tarsal secretion on attachment performance. J. Comp. Physiol. A 192, 1213–1222. ( 10.1007/s00359-006-0150-5) [DOI] [PubMed] [Google Scholar]
- 10.Lees AD, Hardie J. 1988. The organs of adhesion in the aphid Megoura viciae. J. Exp. Biol. 136, 209–228. [Google Scholar]
- 11.Gorb EV, Gorb SN. 2009. Effects of surface topography and chemistry of Rumex obtusifolius leaves on the attachment of the beetle Gastrophysa viridula. Entomol. Exp. Appl. 130, 222–228. ( 10.1111/j.1570-7458.2008.00806.x) [DOI] [Google Scholar]
- 12.Voigt D, Schweikart A, Fery A, Gorb SN. 2012. Leaf beetle attachment on wrinkles: isotropic friction on anisotropic surfaces. J. Exp. Biol. 215, 1975–1982. ( 10.1242/jeb.068320) [DOI] [PubMed] [Google Scholar]
- 13.Bullock JMR, Federle W. 2010. The effect of surface roughness on claw and adhesive hair performance in the dock beetle Gastrophysa viridula. Insect Sci. 18, 298–304. ( 10.1111/j.1744-7917.2010.01369.x) [DOI] [Google Scholar]
- 14.Hosoda N, Gorb SN. 2012. Underwater locomotion in a terrestrial beetle: combination of surface de-wetting and capillary forces. Proc. R. Soc. B 279, 4236–4242. ( 10.1098/rspb.2012.1297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renner K. 1970. Zum Nahrungsaufnahme-Verhalten von Gastroidea viridula Deg. (Col., Chrysomelidae). Z. Pflanzenkh. Pflanzen. 77, 228–234. [Google Scholar]
- 16.Kleine R. 1911. Biologische Betrachtungen an Gastroidea (Gastrophysa) viridula Deg. (Col.). Int. Ent. Z. 5, 63–72. [Google Scholar]
- 17.Remaudière G. 1948. Éthologie de Gastroidea viridula De Geer (Col. Chrysomelidae). Rev. Pathol. Vegetale Entomol. Agric. 27, 38–53. [Google Scholar]
- 18.Engel H. 1956. Beiträge zur Lebensweise des Ampferblattkäfers (Gastrophysa viridula Deg.). Z. Angew. Entomol. 38, 322–354. ( 10.1111/j.1439-0418.1956.tb01609.x) [DOI] [Google Scholar]
- 19.Swatonek F. 1972. Ein Beitrag zur Biologie des Ampferblattkäfers (Gastroidea viridula Deg.). Anz. Schädlingskd. 45, 116–119. [Google Scholar]
- 20.Meyer VE. 1934. Beobachtungen über die Larve von Phyllotreta vittula Redtenbacher. Arb. Morph. Taxon. Ent. 20, 158–166. ( 10.1111/j.1420-9101.2007.01335.x) [DOI] [Google Scholar]
- 21.Klausnitzer B. 1996. Käfer Mitteleuropas , 1st edn Jena, Germany: Spektrum Akademischer Verlag. [Google Scholar]
- 22.Kemner NVA. 1918. Vergleichende Studien über das Analsegment und das Pygopodium einiger Koleopterenlarven. Dissertation Lunds Universitet, Sweden. [Google Scholar]
- 23.Brass P. 1914. Das 10. Abdominalsegment der Käferlarven als Bewegungsorgan. Zool. Jahrb. 37, 65–124. ( 10.1007/BF00424727.pdf) [DOI] [Google Scholar]
- 24.Erber D. 1968. Bau, Funktion und Bildung der Kotpresse mitteleuropäischer Clytrinen und Cryptocephalinen (Coleoptera, Chrysomelidae). Z. Morph. Tiere 62, 245–306. ( 10.1007/BF00401486) [DOI] [Google Scholar]
- 25.Leisewitz W. 1906. Über chitinöse Fortbewegungs-Apparate einiger (insbesondere fussloser) Insektenlarven. Dissertation, Königliche Ludwig-Maximilians-Universität zu München, Munich, Germany. [Google Scholar]
- 26.Snodgrass RE. 1935. Principles of insect morphology. New York, NY: McGraw-Hill Book Company Inc. [Google Scholar]
- 27.Hinton HE. 1955. On the structure, function, and distribution of the prolegs of the Panorpoidea, with a criticism of the Berlese-Imms theory. Ecol. Entomol. 106, 455–540. ( 10.1111/j.1365-2311.1955.tb01265.x) [DOI] [Google Scholar]
- 28.Barbier R. 1985. Morphogenese et evolution de la cuticle et des crochets des fauses-pattes, au cours du developpement larvaire de la Galleria mellonella (Lepidoptera, Pyralidae). Bull. Soc. Zool. France 110, 205–221. [Google Scholar]
- 29.Snodgrass RE. 1961. The caterpillar and the butterfly. Smithson. Misc. Collect. 143, 1–51. [Google Scholar]
- 30.Brackenbury J. 1999. Fast locomotion in caterpillars. J. Insect Physiol. 45, 525–533. ( 10.1016/S0022-1910(98)00157-7) [DOI] [PubMed] [Google Scholar]
- 31.Hasenfuss I. 1999. The adhesive devices in larvae of Lepidoptera (Insecta, Pterygota). Zoomorphology 119, 143–162. ( 10.1007/s004350050088) [DOI] [Google Scholar]
- 32.Suzuki Y, Palopoli MF. 2001. Evolution of insect abdominal appendages: are prolegs homologous or convergent traits? Dev. Genes Evol. 211, 486–492. ( 10.1007/s00427-001-0182-3) [DOI] [PubMed] [Google Scholar]
- 33.Brodsky K. 1930. Zur Kenntnis der Wirbellosenfauna der Bergströme Mittelasien. II. Deuterophlebia mirabilis EDW . Z. Morphol. Ökol. Tiere 18, 289–321. ( 10.1007/BF00419212) [DOI] [Google Scholar]
- 34.Nachtigall W. 1974. Biological mechanisms of attachment. Berlin, Germany: Springer. [Google Scholar]
- 35.Müller GW. 1912. Der Enddarm einiger Insectenlarven als Bewegungsorgan. Zool. Jahrb. 3, 219–240. [Google Scholar]
- 36.Wendler G. 1964. Über die Fortbewegung der Larven von Cantharis fusca. J. Comp. Physiol. A 48, 283–294. ( 10.1007/BF00339457) [DOI] [Google Scholar]
- 37.Domagala P, Ghirardella H. 1984. Structure and function of the terminal abdominal appendages (pygypodia) of photurid firefly larvae. Biol. Bull. 166, 299–309. ( 10.2307/1541218) [DOI] [Google Scholar]
- 38.Dixon AFG. 2000. Insect predator-prey dynamics: ladybird beetles and biological control. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 39.Wyss U. 2009. Behaviour and development of Clithostethus arcuatus (Coccinellidae, Scymninae). DgaaE-Nachr 23, 33. [Google Scholar]
- 40.Wilson DM. 1966. Insect walking. Annu. Rev. Entomol. 11, 103–122. ( 10.1146/annurev.en.11.010166.000535) [DOI] [PubMed] [Google Scholar]
- 41.Gorb SN, Gorb EV, Kastner V. 2001. Scale effects on the attachment pads and friction forces in syrphid flies (Diptera, Syrphidae). J. Exp. Biol. 204, 1421–1431. [DOI] [PubMed] [Google Scholar]
- 42.Voigt D, Schuppert JM, Dattinger S, Gorb SN. 2008. Sexual dimorphism in the attachment ability of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera : Chrysomelidae) to rough substrates. J. Insect Physiol. 54, 765–776. ( 10.1016/j.jinsphys.2008.02.006) [DOI] [PubMed] [Google Scholar]
- 43.Mezoff S, Papastathis N, Takesian A, Trimmer B. 2004. The biomechanical and neural control of hydrostatic limb movements in Manduca sexta. J. Exp. Biol. 207, 3043–3053. ( 10.1242/jeb.01136) [DOI] [PubMed] [Google Scholar]
- 44.Lin H-T, Trimmer B. 2010. The substrate as a skeleton: ground reaction forces from a soft-bodied legged animal. J. Exp. Biol. 213, 1133–1142. ( 10.1242/jeb.037796) [DOI] [PubMed] [Google Scholar]
- 45.Lin H-T, Slate DJ, Paetsch CR, Dorfmann AL, Trimmer B. 2011. Scaling of caterpillar body properties and its biomechanical implications for the use of a hydrostatic skeleton. J. Exp. Biol. 214, 1194–1204. ( 10.1242/jeb.051029) [DOI] [PubMed] [Google Scholar]
- 46.van Griethuijsen LI, Trimmer B. 2009. Kinematics of horizontal and vertical caterpillar crawling. J. Exp. Biol. 212, 1455–1462. ( 10.1242/jeb.025783) [DOI] [PubMed] [Google Scholar]
- 47.Trimmer B, Issberner J. 2007. Kinematics of soft-bodied, legged locomotion in Manduca sexta larvae. Biol. Bull. 212, 130–142. ( 10.2307/25066590) [DOI] [PubMed] [Google Scholar]
- 48.Frantsevich L, Ji A, Dai Z, Wang J, Frantsevich L, Gorb SN. 2008. Adhesive properties of the arolium of a lantern-fly, Lycorma delicatula (Auchenorrhyncha, Fulgoridae). J. Insect Physiol. 54, 818–827. ( 10.1016/j.jinsphys.2008.03.005) [DOI] [PubMed] [Google Scholar]
- 49.Busshardt P, Gorb SN, Wolf H. 2011. Activity of the claw retractor muscle in stick insects in wall and ceiling situations. J. Exp. Biol. 214, 1676–1684. ( 10.1242/jeb.051953) [DOI] [PubMed] [Google Scholar]
- 50.Perez Goodwyn P, Peressadko A, Schwarz H, Kastner V, Gorb SN. 2006. Material structure, stiffness, and adhesion: why attachment pads of the grasshopper (Tettigonia viridissima) adhere more strongly than those of the locust (Locusta migratoria) (Insecta: Orthoptera). J. Comp. Physiol. A 192, 1233–1243. ( 10.1007/s00359-006-0156-z) [DOI] [PubMed] [Google Scholar]
- 51.Varenberg M, Peressadko A, Gorb SN, Arzt E. 2006. Effect of real contact geometry on adhesion. Appl. Phys. Lett. 89, 121905 ( 10.1063/1.2356099) [DOI] [Google Scholar]
- 52.Barnes WJP. 2007. Functional morphology and design constraints of smooth adhesive pads. MRS Bull. 32, 479–485. ( 10.1557/mrs2007.81) [DOI] [Google Scholar]
- 53.Gorb SN. 2011. Biological fibrillar adhesives: functional principles and biomimetic applications. In Handbook of adhesion technology (eds da Silva LFM, Öchsner A, Adams RD.), pp. 1409–1436. Berlin, Germany: Springer. [Google Scholar]
- 54.Hughes GM. 1952. The co-ordination of insect movements. J. Exp. Biol. 29, 267–285. [Google Scholar]
- 55.Bullock JMR, Federle W. 2009. Division of labour and sex differences between fibrillar, tarsal adhesive pads in beetles: effective elastic modulus and attachment performance. J. Exp. Biol. 212, 1876–1888. ( 10.1242/jeb.030551) [DOI] [PubMed] [Google Scholar]
- 56.Wohlfart E, Wolff JO, Arzt E, Gorb SN. 2014. The whole is more than the sum of all its parts: collective effect of spider attachment organs. J. Exp. Biol. 217, 222–224. ( 10.1242/jeb.093468) [DOI] [PubMed] [Google Scholar]
- 57.Gorb SN, et al. 2002. Structural design and biomechanics of friction-based releasable attachment devices in insects. Integr. Comp. Biol. 42, 1127–1139. ( 10.1093/icb/42.6.1127) [DOI] [PubMed] [Google Scholar]
- 58.Voigt D, Gorb SN. 2012. Attachment ability of sawfly larvae to smooth surfaces. Arthropod. Struct. Dev. 41, 145–153. ( 10.1016/j.asd.2011.10.001) [DOI] [PubMed] [Google Scholar]
- 59.Federle W, Baumgartner W, Hölldobler B. 2004. Biomechanics of ant adhesive pads: frictional forces are rate- and temperature-dependent. J. Exp. Biol. 207, 67–74. (doi10.1242/jeb.00716) [DOI] [PubMed] [Google Scholar]
- 60.Niederegger S, Gorb SN. 2003. Tarsal movements in flies during leg attachment and detachment on a smooth substrate. J. Insect Physiol. 49, 611–620. ( 10.1016/S0022-1910(03)00048-9) [DOI] [PubMed] [Google Scholar]
- 61.Snodgrass RE. 1956. Anatomy of the honey bee. Ithaca, NY: Cornell University Press. [Google Scholar]
- 62.Frantsevich L, Gorb SN. 2002. Arcus as a tensegrity structure in the arolium of wasps (Hymenoptera: Vespidae). Zoology 105, 225–237. ( 10.1078/0944-2006-00067) [DOI] [PubMed] [Google Scholar]
- 63.Hanna G, Barnes WJP. 1991. Adhesion and detachment of the toe pads of tree frogs. J. Exp. Biol. 155, 103–125. [Google Scholar]
- 64.Bohn HF, Federle W. 2004. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl Acad. Sci. USA 101, 14 138–14 143. ( 10.1073/pnas.0405885101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varenberg M, Gorb SN. 2009. Hexagonal surface micropattern for dry and wet friction. Adv. Mater. 21, 483–486. ( 10.1002/adma.200802734) [DOI] [Google Scholar]
- 66.Gravish N, et al. 2010. Rate-dependent frictional adhesion in natural and synthetic gecko setae. J. R. Soc. Interface 7, 259–269. ( 10.1098/rsif.2009.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voigt D, Hosoda N, Schuppert J, Gorb SN. 2010. On the laboratory rearing of green dock leaf beetles Gastrophysa viridula (Coleoptera: Chrysomelidae). Insect Sci. 18, 379–384. ( 10.1111/j.1744-7917.2010.01355.x) [DOI] [Google Scholar]
- 68.Voigt D, Gorb EV, Gorb SN. 2005. Plant surface–bug interactions: Dicyphus errans stalking along trichomes. Arthropod-Plant Interact. 1, 221–243. ( 10.1007/s11829-007-9021-4) [DOI] [Google Scholar]
- 69.Voigt D, Gorb SN. 2010. Locomotion in a sticky terrain. Arthropod-Plant Interact. 4, 69–79. ( 10.1007/s11829-010-9088-1) [DOI] [Google Scholar]
- 70.Hosoda N, Gorb SN. 2005. Critical roughness for insect attachment: experimental evidences for the beetle Gastrophysa viridula. In Society for Experimental Biology Annual Main Meeting, abstract A7.34, p. S144. [Google Scholar]