Abstract
Cadherins are mediators of cell–cell adhesion in epithelial tissues. E-cadherin is a known tumour suppressor and plays a central role in suppressing the invasive phenotype of cancer cells. However, the abnormal expression of N- and P-cadherin (‘cadherin switching’, CS) has been shown to promote a more invasive and m̀alignant phenotype of cancer, with P-cadherin possibly acting as a key mediator of invasion and metastasis in bladder cancer. Cadherins are also implicated in numerous signalling events related to embryonic development, tissue morphogenesis and homeostasis. It is these wide ranging effects and the serious implications of CS that make the cadherin cell adhesion molecules and their related pathways strong candidate targets for the inhibition of cancer progression, including bladder cancer. This review focuses on CS in the context of bladder cancer and in particular the switch to P-cadherin expression, and discusses other related molecules and phenomena, including EpCAM and the development of the cancer stem cell phenotype.
Keywords: bladder cancer, cadherin, catenin, cell adhesion, stem cells, EpCAM
1. Introduction
Urothelial bladder cancer (UBC) is the fifth most common cancer in the Western society, with a global incidence of over 356 000 and a prevalence estimated at 2.7 million [1,2]. The burden of the disease is predicted to increase significantly in the foreseeable future as a result of population ageing and the increasing world population, together with the progression of the tobacco epidemic and increasing exposure to occupational carcinogens in developing countries [2]. In the UK, there are approximately 10 200 new cases and 5000 deaths attributed to bladder cancer per year [3]. In the Western populations, over 90% of bladder cancers are transitional cell carcinomas of urothelial origin (urothelial cancers, UCs), and at presentation 75–85% will be non-muscle-invasive tumours (NMIBC, stages Ta/T1/Tis), with the remainder being muscle-invasive (MIBC, stages T2–4) [1,4–6].
NMIBC is a heterogeneous disease typified by a high rate of recurrence (15–61% at one year, depending upon risk category [7]) and so long-term, even lifelong, surveillance with outpatient flexible cystoscopy is the mainstay of subsequent management [6,8]. Progression to MIBC is also a concern for high-risk NMIBC patients, occurring in up to 17% of patients at one year [7]. However, the overall prognosis is good with 65–85% of patients surviving for 5 years or more [5].
Progression to (or presentation with) MIBC represents the critical step in the disease course, necessitating more radical therapies and carrying a 5-year survival rate of only 25–50% [5,9]. For curative intent, patients who present with or progress to MIBC are treated by radiotherapy [6,10], chemoradiotherapy [11], radical cystectomy or neoadjuvant chemotherapy followed by radical cystectomy [6,9,10].
The cumulative cost of treating UBC exceeds all other forms of human cancer, the majority of which is attributable to the long-term treatment and surveillance of NMIBC [12–14]. Despite this, there is only modest research funding for UBC compared with other malignancies [15], and as a result, there has been a lack of scientific advancement in the field [15–17], with no major new drugs approved for UBC in over 10 years [17,18].
Cadherins are mediators of cell–cell adhesion in epithelial tissues [19,20]. We have previously demonstrated that the abnormal expression of P-cadherin (an example of ‘cadherin switching’) is associated with an invasive and aggressive phenotype of UBC [21], and have hypothesized that P-cadherin may act as a key effector of muscle invasion [22]. The cadherins are involved in a number of important phenomena related to cancer progression, including epithelial-to-mesenchymal transition (EMT) and the development of a cancer stem cell phenotype [22,23]. It is these wide ranging effects and the serious implications of cadherin switching (CS) that make the cadherins and their related pathways strong candidate targets for the inhibition of cancer progression, including UBC. This review focuses on cadherin-based cell adhesion in the context of UBC and the switch to P-cadherin expression, and discusses other related molecules and phenomena, including EpCAM and the development of the cancer stem cell phenotype.
2. Methods
Our group has been working in the field of cadherin biology for a number of years [21,24], and we regularly review the literature on these molecules and their associated pathways [22]. Specifically, this review was written using papers obtained following PubMed searches and with the following structure: bladder cancer background, epidemiology and molecular pathogenesis; cadherin background and biology; cadherins in epithelial malignancies, CS and cadherins in bladder cancer. The background to cadherins and cadherin biology presented here has been derived from key papers by workers who initially characterized and described these molecules, and then who subsequently investigated cadherin expression and function in various epithelial malignancies and model systems. We updated the field for CS to describe this process in the context of malignancy and related phenomena (e.g. EMT, cell migration, metastasis, cancer stem cells (CSCs), EpCAM signalling), using papers written by significant workers in this field. The data, findings and information contained within these publications were then assimilated to create a review of CS in bladder cancer and including some of our own interpretations.
3. Molecular pathways to non-muscle-invasive and muscle-invasive bladder cancer
Different approaches have been taken to describe the molecular alterations involved in bladder tumorigenesis [25–30]. We have previously described such pathways based upon the six ‘hallmarks of cancer’ described by Hanahan & Weinberg in 2000 [31–34]. In 2011, Hanahan & Weinberg [34] updated their original landmark review, describing genome instability and inflammation as underlying these hallmark changes, and proposed ‘reprogramming of energy metabolism’ and ‘evading immune destruction’ as two emerging hallmarks with potential for generality. In addition, they described that tumours exhibit another dimension of complexity by containing a repertoire of recruited, ostensibly normal cells that contribute to the acquisition of hallmark traits by creating the ‘tumour microenvironment’ [34], and our own research has demonstrated the apparent importance of the immunological milieu of the bladder tumour microenvironment (R. T. Bryan et al. 2013, unpublished data). In their 2011 update, Hanahan & Weinberg [34] also introduced the concept of ‘CSCs', a concept that has existed for a number of years in haematopoietic malignancies [35,36]. CSCs are a subset of tumour cells that have the ability to self-renew and to generate all of the heterogeneous cells that comprise a tumour (properties that are analogous to a stem cell, the original cell of an organ and responsible for organogenesis and organ maintenance) [23,35,37–39]. In the setting of UBC, CSCs appear to play a role in a subset of tumours, but their true significance is yet to be clarified [23].
Other authors have reviewed the field of UBC molecular pathogenesis in detail [25–30], and there has been general consensus on a divergent pathway for the development of Ta/T1 disease and Tis/T2+ disease [28,40–45]. However, Dancik et al. [46] recently identified a cell of origin gene signature for basal cells and umbrella cells of the urothelium. By using this cell of origin signature in UBCs from 874 patients, it appeared that NMIBCs and MIBCs developed from distinct progenitor cells [46], possibly shifting our understanding of urothelial carcinogenesis away from the classical two pathway models. Further detailed genomic and epigenomic studies of both MIBCs and NMIBCs are thus required to clarify our understanding of the pathogenesis of these tumours [47].
Although a detailed examination of these pathways is beyond the scope of this review, this is a rapidly changing field, and new developments appear frequently with the advent of high-throughput experimental platforms, including ‘deep sequencing’ [48], proteomics [49–51] and metabolomics [52]. Most recently, The Cancer Genome Atlas (TCGA) Research Network undertook the comprehensive molecular characterization of 131 MIBCs [48]. With regard to somatic DNA mutations, a notable finding was the significant enrichment of non-silent mutations in chromatin regulatory genes compared with other epithelial cancers studied: 76% of the tumours (MIBCs) had an inactivating mutation in one or more of these genes, and 41% had at least two such mutations [48]. TP53 mutations were also common (49%), as were amplification and overexpression of MDM2, suggesting that TP53 function was inactivated in 76% of tumours [48]. There were a large number of previously undescribed mutations, and viral DNAs and transcripts were also indentified [48]. RNA-seq data identified four tumour clusters and pathway analysis demonstrated three frequently dysregulated pathways [48]: cell cycle regulation (altered in 93% of cases); kinase and phosphatidylinositol-3-OH kinase (PI(3)K) signalling (72%) and chromatin remodelling (89%). A number of the genomic alterations identified are theoretically amenable to therapeutic targeting [48], and such new therapeutics are desperately needed for UBC [17,18,53].
Choi et al. [54] also used whole genome mRNA expression profiling to cluster MIBCs into three distinct groups, based upon the established molecular subtypes of breast cancer: basal MIBCs shared biomarkers with basal breast cancers and were characterized by p63 activation, squamous differentiation and more aggressive disease; luminal MIBCs contained features of active PPARγ and oestrogen receptor transcription and were enriched with activating FGFR3 mutations and potential FGFR inhibitor sensitivity; p53-like MIBCs were consistently resistant to a number of chemotherapeutics, including cisplatin; and all chemoresistant tumours adopted a p53-like phenotype after therapy [54]. These findings have important implications for the clinical management of MIBC: they include not only prognostic information, but also suggestions for subtype-directed targeted therapy and potential to predict response to cisplatin-based chemotherapy (although further work is needed to elucidate other biomarkers of resistance) [55]. It is, however, disappointing that NMIBCs were not analysed in the same way by either the TCGA Research Network or Choi et al. [47], especially as these tumours represent the vast majority (more than 75%) of bladder cancer patients [56,57].
4. Cadherins
The classical cadherins are calcium-dependent transmembrane glycoproteins found at the adherens junction and are mediators of cell–cell adhesion in epithelial tissues [19,20]. E-cadherin is a tumour suppressor, playing a central role in suppressing the invasive phenotype of UBC cells [58]. The abnormal expression of other ‘classical’ cadherins (P- and N-cadherin) has been shown to promote a more invasive and malignant phenotype of UBC [21,22], possibly acting as key mediators of invasion and metastasis. With such a large difference in UBC outcomes between early-stage disease (stage Ta) versus MIBC (stages T2+), it is reasonable to assume that cell adhesion molecules, and in particular cadherins, play a fundamental role in the spread of bladder tumours, initially from the urothelium into the lamina propria (through the basement membrane) and subsequently into the detrusor muscle [22]. Therefore, the classical cadherins and their related molecular pathways represent attractive therapeutic targets for the inhibition of progression in bladder cancer patients [19,58–60].
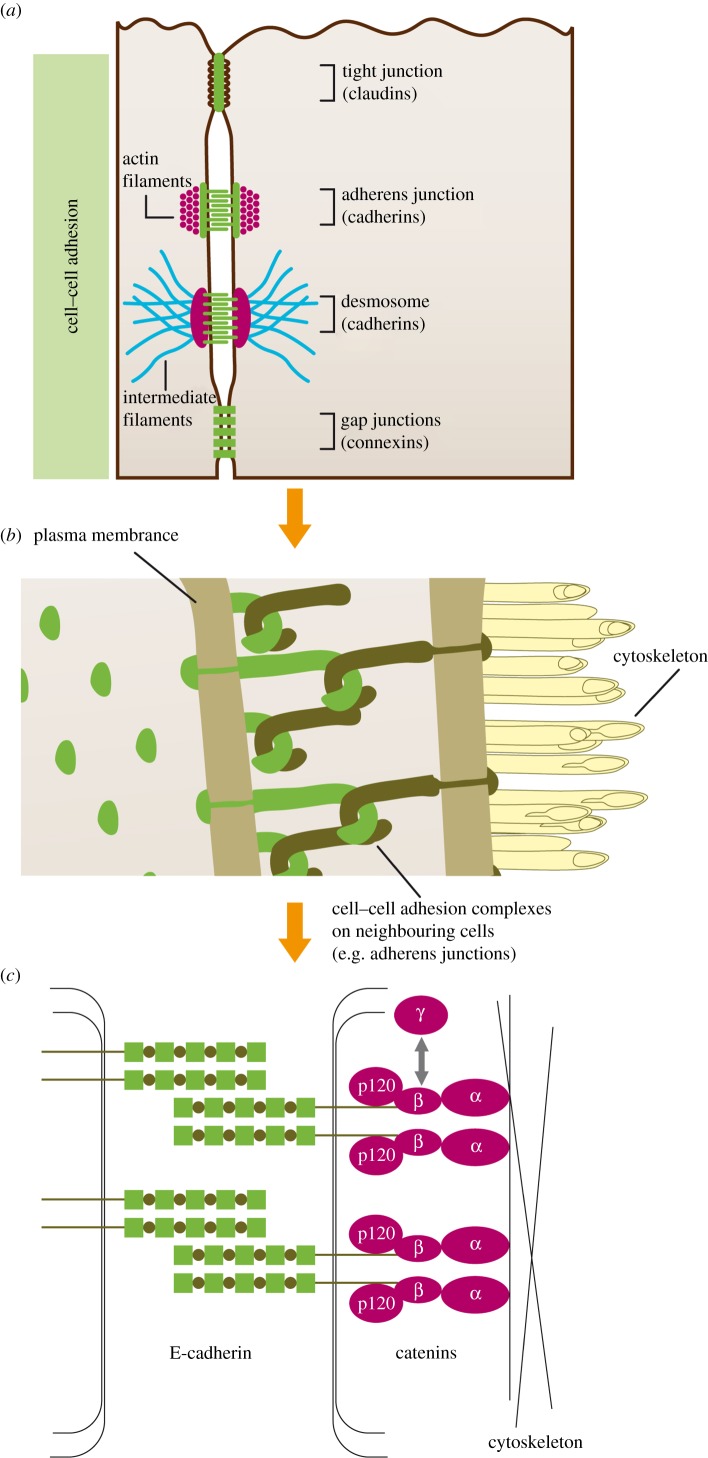
Cadherins comprise extracellular (EC1–5), transmembranous and cytoplasmic domains, with the cytoplasmic domain anchored to the cell cytoskeleton by catenin family members (α-, β-, γ-catenin and p120) [19,60–64]. P21-activated kinase 5 (PAK5) also appears to associate with β-catenin and p120 to stabilize the adherens junction in order to maintain normal cell–cell adhesion [65]. Traditionally, cell–cell adhesion is described as being achieved by the symmetric interactions of the first extracellular domains (EC1) of cadherins on neighbouring cells (trans-interaction) [63,66]; cadherins on the same cell also interact with each other (cis-interaction) through the EC1 domain of one and the EC2 domain of the other [63,66,67]. More recently, it has been described that optimal cell–cell adhesion (50–70 pN) is achieved by all five EC domains of E-cadherin, and with a cell–cell separation of 5–11 nm [64] (figure 1). E-, P- and N-cadherin were the first cadherins identified, and can all mediate cell–cell adhesion in this fashion [62,68]:
— E-cadherin (cadherin-1, 120 kDa): the main mediator of cell–cell adhesion in epithelial tissues and expressed by most normal epithelial cells [19,60,61,68–70].
— N-cadherin (cadherin-2, 130 kDa): expressed by neural, endothelial and muscle cells, but not normally by epithelial cells [61,68].
— P-cadherin (cadherin-3, 118 kDa): normally only weakly expressed in the basal layers of stratified epithelia such as oesophagus, bronchus and bladder [21,68,70].
Figure 1.
Cell–cell adhesion in epithelial tissues (taken from reference [22] with permission). (a) Overview of cell–cell adhesion complexes; (b) pictorial representation of cell–cell interactions on neighbouring cells; (c) molecular structure of the adherens junction, showing the relationship between E-cadherin molecules on neighbouring cells, and between E-cadherin, the catenins (α, β, γ, p120) and the cell cytoskeleton. Traditionally, cadherins on neighbouring cells adhere via EC1 domains, although more recent research suggests that all five EC domains are required for optimal adhesion [64].
Epithelial malignancies, including bladder cancer, typically show loss of E-cadherin expression as grade and stage progress, and this is often accompanied by increased expression of N- or P-cadherin. This phenomenon is described as ‘CS’ [19,60,68,70–72], illustrated in the bladder cancer setting in figure 2. Excellent reviews of the field have been published recently [73,74], and we have previously reviewed this field for bladder cancer [22]; we provide an overview below.
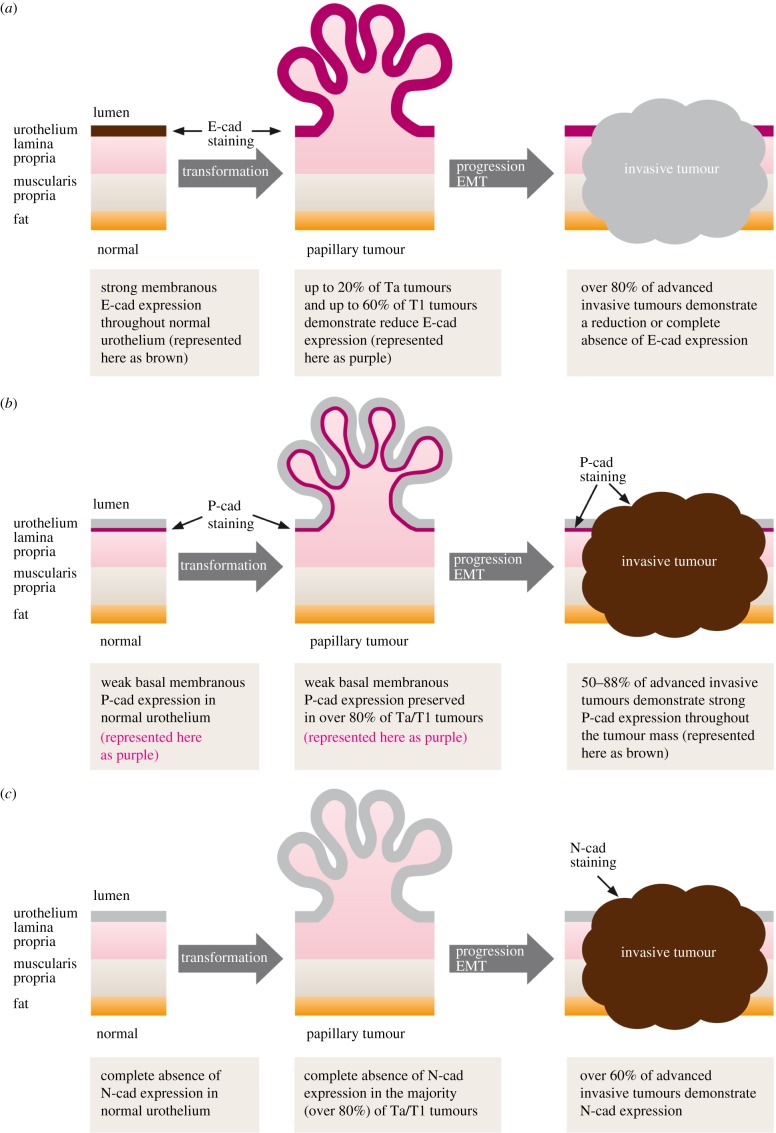
Figure 2.
Cadherin switching in bladder UCs (taken from reference [22] with permission). (a) E-cadherin is strongly expressed at the cell membrane throughout the normal urothelium. Reduced expression is observed in a proportion of NMIBCs, and the majority of MIBCs demonstrate either reduced expression or a complete absence of E-cadherin; (b) P-cadherin is expressed in the basal one to two layers of normal urothelium, and this pattern is preserved in the majority of NMIBCs. The majority of MIBCs demonstrate strong P-cadherin expression throughout the tumour mass; (c) N-cadherin is not expressed in normal urothelium or the majority of NMIBCs. However, the majority of muscle-invasive UCs express N-cadherin throughout the tumour mass.
5. Cadherin switching
CS is a hallmark of EMT [75], the process by which epithelial cells lose their characteristic polarity, disassemble cell junctions, and become more migratory as a precursor to invasion and metastasis (they acquire properties analogous to mesenchymal cells) [19,24,60,76–81]. In this setting, CS typically describes a process where the normal expression of E-cadherin is replaced by the abnormal expression of N-cadherin, or where N-cadherin expression is increased and E-cadherin levels remain unchanged [19,60,75]. CS appears to play a role late in many malignancies (including breast, prostate, pancreas, ovarian, bladder and melanoma), resulting in a more invasive and malignant phenotype of disease with a worse outcome [19,21,60,70,73–75,82–87]. The regulation of CS is yet to be fully elucidated, but most likely involves transcriptional and post-transcriptional events, possibly influenced by cytokines or growth factors [19,60]. Recently, Slug (SNAI2, a member of the Snail family of zinc-finger transcription factors) has been identified to play a critical role in EMT by control of the E-cadherin to N-cadherin switch in UBC [88].
In UBC, ourselves and others have described CS, demonstrating increased expression of both P- and N-cadherin in late stage high-grade disease (figure 2) [21,68,87,89,90]. We studied 153 bladder tumours and used a variety of cell lines and functional in vitro models [21]: increased membranous P-cadherin expression was observed in almost half of all MIBCs and almost 40% of grade 3 UBCs, accompanied by significantly reduced expression of E-cadherin [21]. Increased P-cadherin expression was associated with worse bladder cancer-specific survival, and P-cadherin status was an independent prognostic factor (alongside grade and stage) [21]. Functional in vitro experiments showed that altering the balance of E- and P-cadherin in favour of P-cadherin expression enhanced anchorage-independent growth, and that P-cadherin alone was unable to mediate normal cell–cell adhesion [21]. We concluded that P-cadherin expression promoted a more malignant and invasive phenotype of bladder cancer (even in the presence of E-cadherin), and appeared to have a novel role late in the disease process [21].
Mandeville et al. [90] also demonstrated similar findings. In their in vitro studies, using P-cadherin transfection and knockdown, they demonstrated that P-cadherin induced a significant increase in migratory capacity (although with no accompanying change in invasive potential) [90]. The authors suggested that P-cadherin may have a role in regulating the migration of basal cells to the intermediate cell layer in normal urothelium as well as a role in neoplastic progression [90]. More recently, Wang et al. [87] have demonstrated similar findings.
We and others have postulated that a subgroup of aggressive P-cadherin-expressing tumours may be derived from the normally weakly P-cadherin-expressing basal layer of the urothelium [22]. In support of this hypothesis, Van Batavia et al. [91] recently demonstrated that papillary and carcinoma in situ (CIS) lesions were derived from different urothelial populations, with intermediate cells contributing to non-invasive papillary lesions and basal cells representing the origin of CIS (which ultimately leads to MIBC). These findings support a model in which the heterogeneity observed in bladder cancers is determined both by genetic changes and the cell lineage from which the tumour originates [91].
However, despite P-cadherin expression being associated with a more aggressive phenotype in many cancers, such behaviour is not ubiquitous and is context dependent [74]. For example, in malignant melanoma, which commonly demonstrates a cadherin switch to N-cadheri expression [22], P-cadherin promotes adhesion and inhibits invasion in a similar fashion to E-cadherin [74], and E-cadherin negative breast cancer cells show many similarities when subsequently transfected with E- or P-cadherin [73,92]. Ribeiro et al. [93] investigated these phenomena in detail in a breast cancer model, demonstrating that P-cadherin co-localizes with E-cadherin, and promotes cell invasion by disrupting E-cadherin/catenin interactions. E- and P-cadherin co-expressing tumour cells showed enhanced in vivo tumour growth compared with those expressing only E- or only P-cadherin, and co-expression of E- and P-cadherin in breast tumours correlated with high-grade biologically aggressive tumours accompanied by poor patient survival [93]. It is therefore feasible that P-cadherin only promotes invasion in tissues that endogenously express E-cadherin [73], with heterodimerization between E- and P-cadherin disrupting the formation of functional cadherin–catenin complexes [74].
It is likely that the key mechanisms involved in P-cadherin's deregulation largely occur in the promoter region of CDH3 and not by structural alterations of its coding sequences [73]: in 2005, Paredes et al. demonstrated hypomethylation of the CDH3 gene promoter correlated with P-cadherin overexpression in breast cancer [73,94], and other workers have described this phenomenon in pancreatic [73,95] and colorectal cancers [73,96]. Our own data suggest differential CDH3 promoter methylation between bladder cancer cell lines and tumours, and normal urothelium (R. T. Bryan 2003, unpublished data). Furthermore, the balance of E- and P-cadherin expression impacts the overall genetic programme [73], altering the expression of genes involved in signal transduction and growth factors, cell cycle, cell adhesion and the extracellular matrix, cytokines and inflammation [73,92]. In addition, P-cadherin can provoke the secretion of proinvasive factors such as the matrix metalloproteinases MMP1 and MMP2 [73,74,97]. The role of p120 also appears important, with P-cadherin probably interfering with the normal binding of p120 to E-cadherin at the adherens junction [73,98]. In a pancreatic cancer model, accumulation of p120 in the cytoplasm (and not bound to E-cadherin at the membrane) appeared to induce the increased cell migration seen following P-cadherin expression via the Rho GTPases, Rac1 and Cdc42 [73,99]. P-cadherin-induced increase in Rac1 and Cdc42 activity (mediated via p120) has also been observed in ovarian cancer [73,100]. Specifically, insulin-like growth factor 1 receptor (IGF1R) can seemingly form a complex with P-cadherin, resulting in the tyrosine phosphorylation and activation of cytoplasmic p120 to promote invasion [74,100,101]; this pathway appears specific to P-cadherin and not the other classical cadherins [74,101].
Taken together, all of the above-mentioned data emphasize that P-cadherin represents a very attractive target for novel anti-cancer therapeutics [73], and phase I trials of a P-cadherin inhibitor (PF-03732010, a human monoclonal antibody against P-cadherin) have been undertaken [102], although its development now seems to have stalled.
6. Cadherins and cancer stem cells
Although solid tumours can be reduced in size or eradicated by chemotherapy, radiotherapy or surgery (alone or in combinations), disease relapse or progression often occurs [35,103]. Such relapse or progression may be explained by the persistence of residual tumour-initiating cells and tumour-maintaining cells, and such cells have been reported in a variety of malignancies (breast, brain, prostate, lung, pancreas, etc.) since they were first identified in leukaemia [38,78,103]. Such ‘CSCs’ theoretically have the ability to self-renew and to generate the heterogeneous cells that comprise a tumour [39,103,104], and thus need to be eradicated to provide long-term disease-free survival (although it appears that CSCs are more resistant to conventional therapies) [37,39,105,106]. CSCs may either develop following genetic or epigenetic events in normal stem cells or from differentiated tumour cells that develop the capability for unlimited growth [23,81]. Cellular markers of ‘stemness' are still under debate, but include CD44, CD24, CD133 and EpCAM [81]: in breast, prostate and oral squamous carcinomas, CSCs are likely identified as CD44+/CD24−, whereas CD133 appears to be a CSC marker in gliomas and in colon and pancreatic carcinomas [81].
In a previous review, we suggested that the evidence supports the CSC paradigm for UBC, as in other epithelial malignancies [23]. As discussed in §4, in normal urothelium, P-cadherin is expressed only in the basal cell layer (the assumed urothelial stem cell niche) and in a subset of more aggressive UBCs [21–23,68,90]. It is therefore tempting to assume that P-cadherin is a marker of urothelial stem cells and UBC CSCs. Although E-cadherin intercellular adhesion is considered important for the survival of human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) [81], Kolle et al. [107] recently identified CDH3 (P-cadherin) and TACSTD1 (EpCAM) as genes encoding hESC markers (antibodies for EpCAM were also able to enrich for pluripotent hESCs). Vieira et al. [108] have also demonstrated that P-cadherin mediates stem cell properties in basal-like breast cancer. P-cadherin therefore appears promising as a potential marker of CSCs in UBC, and similar work is required to confirm these findings in UBC [23]. The fact that CDH3 (P-cadherin) did not appear in Dancik et al.'s [46] cell of origin signature described earlier is somewhat surprising because it is normally expressed by basal urothelial cells and in a subset of aggressive UBCs that may also harbour CSCs; however, as described in §5, P-cadherin's deregulation is most likely governed by epigenetic phenomena rather than by structural alterations in its coding sequences [73]. Characterization of the UBC epigenome/methylome may thus be required to elucidate P-cadherin's role in these UBC subtypes.
It is highly feasible that treatment-resistant cells develop via other mechanisms and pathways, with CSCs being responsible only for a minority [103,109]. Heterogeneity within some tumours may result from selective pressure during tumorigenesis [34,106] (figure 3). It has been suggested that UBCs arise from more differentiated cells, and self-renewal capacity may be acquired secondarily by inactivation of p53 and RB1 function [103,109]. The tumour microenvironment may also play an important role [37], potentially inducing a transitory or reversible CSC-like state [111]: although EMT may drive the development of CSCs [34], EMT itself is reversible with mesenchymal-to-epithelial transition (MET) favouring a cell's colonization of distant sites to generate metastases [34]. Whether the CSC state reverses in a similar setting and fashion remains unknown, but such interactions highlight the importance of the tumour microenvironment for all cancer cells, not just CSCs [34].
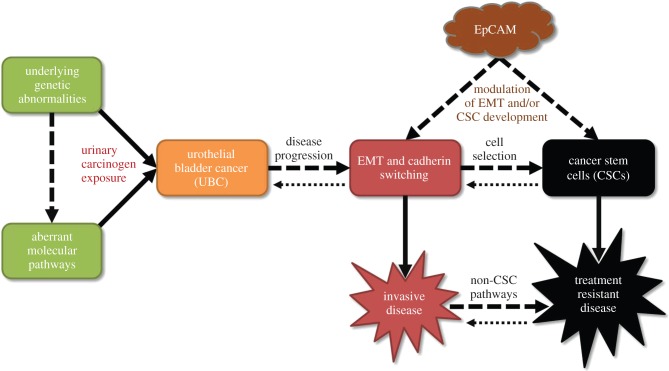
Figure 3.
Proposed pathways for the development of a bladder cancer stem cell phenotype and the relationship with EpCAM (adapted from [23]). Cancer stem cells (CSCs) result in the development of treatment-resistant disease in some cancer settings, and this diagram proposes potential pathways for their development in UBC. There is likely considerable plasticity in these pathways [110], with cells reverting to a less aggressive state by mesenchymal-to-epithelial transition (MET) or by the reversal of the CSC phenotype, and most likely influenced by the tumour microenvironment [23]. We also propose a model whereby EpCAM modulates the development of EMT and/or CSCs (see text).
7. Cadherins and EpCAM
EpCAM is a type 1 membrane protein that functions as a cell adhesion molecule [112]. It is overexpressed in many epithelial malignancies, including bladder CIS [113] and high-grade and advanced stage UBCs [114]. The tumour-specific expression of EpCAM has led to its use for capturing circulating tumour cells by the FDA-approved CELLSEARCH system [115], and also for directing therapies to bladder tumours [116]. High tissue levels of EpCAM are associated with a poor prognosis in UBC [114]. However, the role of EpCAM remains elusive: both tumour suppressor and oncogenic properties have been reported. In 2009, Maetzel et al. [117] demonstrated that EpCAM could be sequentially cleaved to release extracellular and intracellular domains, ‘EpEX’ and ‘EpICD’, respectively; EpICD diffuses into the nucleus and activates oncogenic signalling events by associating with FHL2, β-catenin and Lef-1 [117,118] (figure 4).
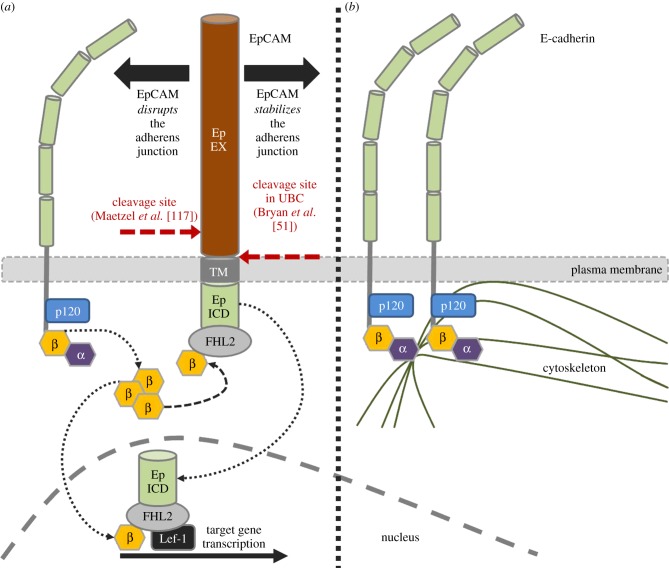
Figure 4.
EpCAM's relationship with E-cadherin (adapted from [117,119]). The dual role of EpCAM in epithelial tissues is demonstrated. EpCAM can either disrupt the adherens junction, resulting in the release of β-catenin (a), or stabilize the adherens junction to maintain E-cadherin's anchorage to the cell cytoskeleton (b). In (a), released β-catenin subsequently forms a complex with EpICD and the transcriptional cofactor FHL2 [120], either at the cell membrane or in the cell nucleus. The EpICD/FHL2/β-catenin complex then interacts with the Lef-1 transcription factor in the cell nucleus to activate the transcription of various target genes, including known oncogenes. In UBC, we demonstrated that the extracellular domain of EpCAM is released by cleavage immediately adjacent to the cell membrane [51]. The exact location of cleavage was not described by Maetzel et al. [117], but the protease involved (TACE or ADAM 17) usually cleaves membrane proteins 10–15 residues away from the membrane surface [121], suggesting atypical cleavage or an alternative mechanism of extracellular domain release in UBC (α, α-catenin; β, β-catenin).
In 2014, as part of our de novo urinary biomarker discovery programme [122], we demonstrated that elevated urinary EpCAM was observed in patients with grade 3 NMIBCs and MIBCs [50,51]. EpCAM was a significant independent prognostic factor for UBC-specific survival, with elevated urinary levels resulting in an increased risk of death from bladder cancer (hazard ratio 1.76). The predominant form of EpCAM in the urine was a soluble and stable form comprising the entire extracellular domain, and not the intact protein [51]. Our data therefore suggested that the cleavage of EpCAM into EpEX and EpICD could also occur in UBC [51,117], and further evidence supports this: Ralhan et al. [123] recently demonstrated that nine of 10 cases of UBC were positive for EpICD. However, our work demonstrated that the extracellular domain of EpCAM was released by cleavage immediately adjacent to the cell membrane [51]; the exact location of cleavage was not described by Maetzel et al. [117], but the protease involved (TACE or ADAM 17) usually cleaves membrane proteins 10–15 residues away from the membrane surface [121], suggesting atypical cleavage or an alternative mechanism of extracellular domain release in UBC [51].
Notably, there are important relationships between EpCAM and classical cadherins, although this relationship appears to be tissue- and tumour-specific [124]. In 1997, Litvinov et al. [125] suggested that EpCAM has a role in the development of a proliferative and malignant phenotype of epithelial cell: increasing the expression of EpCAM in cadherin-positive cells led to the gradual abrogation of adherens junctions [125]. Although EpCAM had no influence on the total amount of cellular cadherin, it affected the interaction of the cadherins with the cytoskeleton and, as cadherin-mediated cell–cell adhesion diminished, EpCAM-mediated intercellular connections predominated [125]. In a murine fibroblast model, Winter et al. [126] subsequently demonstrated that this may occur by disruption of the link between α-catenin and F-actin, probably by EpCAM's disruption of the actin cytoskeleton or possibly via p120. In later work, on human breast epithelial cells, Winter et al. [127] demonstrated that EpCAM cross-signalling with N-cadherin resulted in the abrogation of cadherin adhesion complexes, mediated by PI(3)K. In breast cancer cell lines, Martowicz et al. [128] showed that epithelial cells need EpCAM to promote growth and invasion, yet mesenchymal tumour cells are independent of EpCAM for invasion and progression; Martowicz et al. [129] also demonstrated that overexpression of EpCAM in human mammary epithelial cells led to a more proliferative phenotype and downregulation of E-cadherin.
Conversely, in a zebrafish model, Slanchev et al. [130] demonstrated that EpCAM was indispensable for skin epithelial integrity, and that epcam mutant embryos displayed reduced levels of membranous E-cadherin. Guerra et al. [131] also postulated an important role for EpCAM in the maintenance of normal intestinal architecture and function in congenital tufting enteropathy, using an mTrop1/Epcam knockout mouse model of the disease. Other model systems have also demonstrated a direct association between loss of EpCAM expression and loss of cadherin-mediated adhesion [132].
Seemingly, EpCAM has dual functions in normal and cancerous cells with regard to cadherin regulation, cell–cell adhesion and epithelial integrity: EpCAM may be essential for normal epithelial tissue integrity and cell–cell adhesion, but there also appears to be a role for EpCAM in the disruption of normal cell–cell adhesion to initiate EMT, with the subsequent transformed cells acting independently of EpCAM signalling for invasion and progression. Interestingly, Zeb1 (a known transcription factor inducing EMT) represses both E-cadherin and EpCAM by binding to the EpCAM promoter [133], yet the expression of E-cadherin and EpCAM is related to a stem cell-like phenotype [134,135]; in basal-like breast cancer, EpCAM and P-cadherin both appear to be associated with the CSC phenotype [108]. As described for the hallmarks of cancer [33], the timing and ordering of these events appears to differ between normal and tumourous tissues, between different tissue and tumour types, and most likely within the same tumour. It is feasible that during EMT in some malignancies, EpCAM may stimulate the dissolution of E-cadherin/catenin complexes and so permit P- and N-cadherin complexes to predominate (CS) and β-catenin-mediated oncogene transcription to be upregulated; yet in other tumour types, EpCAM and E-cadherin may be downregulated in parallel, with EMT being driven by alternative pathways. Conversely, EpCAM may stabilize E-cadherin/catenin complexes in some tumours, possibly providing a ‘stable’ and less chaotic cellular milieu unaffected by EMT, in which the development of a CSC phenotype can be ‘nurtured’ by alternative pathways (as described in §6, EpCAM is a cell surface marker of hESCs, and can be used to isolate a pluripotent subpopulation from hESC culture [107]). If the latter model is correct, then the corollary would potentially be the normalization of β-catenin-mediated transcription in CSCs; evidence to date in other malignancies suggests that this is not the case [110,136,137]. However, these are dynamic processes, and even within the same tumour, all of these proposed phenomena may be unfolding simultaneously; in the future, single cell genomics may resolve these issues [138,139]. It is important to note that CSC-like treatment-resistant disease may develop via alternative pathways (figure 3), and there is likely to be considerable plasticity [110], with cells reverting to a less aggressive state by MET or by the reversal of the CSC phenotype. Furthermore, the influence of EpCAM on P-cadherin is yet to be elucidated. Our current research is attempting to resolve some of these mechanisms.
8. Discussion and conclusion
P-cadherin seemingly has a number of fundamental roles in bladder cancer and other malignancies, including mediating the development of CSCs and EMT, both of which lead to more aggressive disease and worse survival. The mechanisms of these phenomena have been well-described in other malignancies, but remain to be elucidated in UBC. Although we have assumed some crossover of P-cadherin's function between tumour and tissue types, we know that many of P-cadherin's actions are tumour- and tissue-specific. Therefore, such findings from other malignancies need to be reproduced in UBC if we are genuinely to understand P-cadherin's role in this setting. However, given the genomic characterizations of MIBC described above [46,48,54], it is unlikely that P-cadherin represents a ‘driver’ of urothelial carcinogenesis [140]; P-cadherin is more likely to represent an important downstream effector of such driver mutations, with multiple influences on important pathways and phenomena that determine outcomes in advanced disease (e.g. EMT, CSCs), probably mediated by PI(3)K [48]. Moreover, it appears that P-cadherin plays a fundamental role in the cell surface and cell adhesion phenomena that permit tumour cells to migrate and invade, and possibly to metastasize.
In conclusion, P-cadherin represents a highly attractive therapeutic target, alongside N-cadherin [141–143]. However, given P-cadherin's complex interactions described above (and undoubtedly many yet to be discovered), P-cadherin inhibition may have far more wide-reaching effects than those directly related to tumour invasion and progression. The difficulties of taking an anti-P-cadherin agent through clinical trials and into clinical use should therefore not be underestimated. Furthermore, the association of classical cadherins with EpCAM is particularly fascinating and requires further elucidation in UBC, and our work in this area is ongoing.
References
- 1.van Rhijn BW, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, Witjes JA, Zlotta AR. 2009. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur. Urol. 56, 430–432. ( 10.1016/j.eururo.2009.06.028) [DOI] [PubMed] [Google Scholar]
- 2.Ploeg M, Aben KK, Kiemeney LA. 2009. The present and future burden of urinary bladder cancer in the world. World J. Urol. 27, 289–293. ( 10.1007/s00345-009-0383-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Research UK 2009. CancerStats key facts - bladder cancer. See http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bladder/.
- 4.Lorusso V, Silvestris N. 2005. Systemic chemotherapy for patients with advanced and metastatic bladder cancer: current status and future directions. Ann. Oncol. 16(Suppl. 4), iv85–iv89. ( 10.1093/annonc/mdi914) [DOI] [PubMed] [Google Scholar]
- 5.Wallace DMA, Bryan RT, Dunn JA, Begum G, Bathers S. 2002. Delay and survival in bladder cancer. BJU Int. 89, 868–878. ( 10.1046/j.1464-410X.2002.02776.x) [DOI] [PubMed] [Google Scholar]
- 6.Kaufman DS, Shipley WU, Feldman AS. 2009. Bladder cancer. Lancet 374, 239–249. ( 10.1016/S0140-6736(09)60491-8) [DOI] [PubMed] [Google Scholar]
- 7.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. 2006. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 49, 466 ( 10.1016/j.eururo.2005.12.031) [DOI] [PubMed] [Google Scholar]
- 8.Babjuk M, et al. 2013. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur. Urol. 64, 639–653. [DOI] [PubMed] [Google Scholar]
- 9.Vale CL. 2005. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data: Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur. Urol. 48, 202–205. ( 10.1016/j.eururo.2005.04.006) [DOI] [PubMed] [Google Scholar]
- 10.Stenzl A, Cowan NC, De SM, Kuczyk MA, Merseburger AS, Ribal MJ, Sherif A, Witjes JA. 2011. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur. Urol. 59, 1009–1018. ( 10.1016/j.eururo.2011.03.023) [DOI] [PubMed] [Google Scholar]
- 11.James ND, et al. 2012. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N. Engl. J. Med. 366, 1477–1488. ( 10.1056/NEJMoa1106106) [DOI] [PubMed] [Google Scholar]
- 12.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. 2003. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics 21, 1315–1330. ( 10.1007/BF03262330) [DOI] [PubMed] [Google Scholar]
- 13.Riley GF, Potosky AL, Lubitz JD, Kessler LG. 1995. Medicare payments from diagnosis to death for elderly cancer patients by stage at diagnosis. Med. Care 33, 828–841. ( 10.1097/00005650-199508000-00007) [DOI] [PubMed] [Google Scholar]
- 14.Simons MP, O'Donnell MA, Griffith TS. 2008. Role of neutrophils in BCG immunotherapy for bladder cancer. Urol. Oncol. 26, 341–345. ( 10.1016/j.urolonc.2007.11.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotan Y, et al. 2009. Key concerns about the current state of bladder cancer: a position paper from the Bladder Cancer Think Tank, the Bladder Cancer Advocacy Network, and the Society of Urologic Oncology. Cancer 115, 4096–4103. ( 10.1002/cncr.24463) [DOI] [PubMed] [Google Scholar]
- 16.Kaplan AL, Litwin MS, Chamie K. 2014. The future of bladder cancer care in the USA. Nat. Rev. Urol. 11, 59–62. ( 10.1038/nrurol.2013.180) [DOI] [PubMed] [Google Scholar]
- 17.Bryan RT, Kirby R, O'Brien T, Mostafid H. 2014. So much cost, such little progress. Eur. Urol. 66, 263–264. [DOI] [PubMed] [Google Scholar]
- 18.Bryan RT, James ND. 2011. Bladder cancer: time for a rethink? Oncol. Williston Park. 25, 965–68. [PubMed] [Google Scholar]
- 19.Cavallaro U, Schaffhauser B, Christofori G. 2002. Cadherins and the tumour progression: is it all in a switch? Cancer Lett. 176, 123–128. ( 10.1016/S0304-3835(01)00759-5) [DOI] [PubMed] [Google Scholar]
- 20.Takeichi M. 1995. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 7, 619–627. ( 10.1016/0955-0674(95)80102-2) [DOI] [PubMed] [Google Scholar]
- 21.Bryan RT, Atherfold PA, Yeo Y, Jones LJ, Harrison RF, Wallace DM, Jankowski JA. 2008. Cadherin switching dictates the biology of transitional cell carcinoma of the bladder: ex vivo and in vitro studies. J. Pathol. 215, 184–194. ( 10.1002/path.2346) [DOI] [PubMed] [Google Scholar]
- 22.Bryan RT, Tselepis C. 2010. Cadherin switching and bladder cancer. J. Urol. 184, 423–431. ( 10.1016/j.juro.2010.04.016) [DOI] [PubMed] [Google Scholar]
- 23.Bryan RT. 2011. Bladder cancer and cancer stem cells: basic science and implications for therapy. Sci. World J. 11, 1187–1194. ( 10.1100/tsw.2011.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy RG, et al. 2002. Aberrant P-cadherin expression is an early event in hyperplastic and dysplastic transformation in the colon. Gut 50, 513–519. ( 10.1136/gut.50.4.513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birkhahn M, Mitra AP, Cote RJ. 2007. Molecular markers for bladder cancer: the road to a multimarker approach. Expert Rev. Anticancer Ther. 7, 1717–1727. ( 10.1586/14737140.7.12.1717) [DOI] [PubMed] [Google Scholar]
- 26.Castillo-Martin M, Domingo-Domenech J, Karni-Schmidt O, Matos T, Cordon-Cardo C. 2010. Molecular pathways of urothelial development and bladder tumorigenesis. Urol. Oncol. 28, 401–408. ( 10.1016/j.urolonc.2009.04.019) [DOI] [PubMed] [Google Scholar]
- 27.Cheng L, Zhang S, MacLennan GT, Williamson SR, Lopez-Beltran A, Montironi R. 2011. Bladder cancer: translating molecular genetic insights into clinical practice. Hum. Pathol. 42, 455–481. ( 10.1016/j.humpath.2010.07.007) [DOI] [PubMed] [Google Scholar]
- 28.Knowles MA. 2008. Molecular pathogenesis of bladder cancer. Int. J. Clin. Oncol. 13, 287–297. ( 10.1007/s10147-008-0812-0) [DOI] [PubMed] [Google Scholar]
- 29.Shariat SF, Karam JA, Lerner SP. 2008. Molecular markers in bladder cancer. Curr. Opin. Urol. 18, 1–8. ( 10.1097/MOU.0b013e3282f1c5c1) [DOI] [PubMed] [Google Scholar]
- 30.Youssef RF, Mitra AP, Bartsch G, Jr, Jones PA, Skinner DG, Cote RJ. 2009. Molecular targets and targeted therapies in bladder cancer management. World J. Urol. 27, 9–20. ( 10.1007/s00345-008-0357-x) [DOI] [PubMed] [Google Scholar]
- 31.Bryan RT, Hussain SA, James ND, Jankowski JA, Wallace DM. 2005. Molecular pathways in bladder cancer: part 1. BJU Int. 95, 485–490. ( 10.1111/j.1464-410X.2005.05325.x) [DOI] [PubMed] [Google Scholar]
- 32.Bryan RT, Hussain SA, James ND, Jankowski JA, Wallace DM. 2005. Molecular pathways in bladder cancer: part 2. BJU Int. 95, 491–496. ( 10.1111/j.1464-410X.2005.05326.x) [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell 100, 57–70. ( 10.1016/S0092-8674(00)81683-9) [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 35.Reya T, Morrison SJ, Clarke MF, Weissman IL. 2001. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111. ( 10.1038/35102167) [DOI] [PubMed] [Google Scholar]
- 36.Bonnet D, Dick JE. 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3, 730–737. ( 10.1038/nm0797-730) [DOI] [PubMed] [Google Scholar]
- 37.Brandt WD, Matsui W, Rosenberg JE, He X, Ling S, Schaeffer EM, Berman DM. 2009. Urothelial carcinoma: stem cells on the edge. Cancer Metastasis Rev. 28, 291–304. ( 10.1007/s10555-009-9187-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta PB, Chaffer CL, Weinberg RA. 2009. Cancer stem cells: mirage or reality? Nat. Med. 15, 1010–1012. ( 10.1038/nm0909-1010) [DOI] [PubMed] [Google Scholar]
- 39.Visvader JE, Lindeman GJ. 2008. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768. ( 10.1038/nrc2499) [DOI] [PubMed] [Google Scholar]
- 40.Goebell PJ, Knowles MA. 2010. Bladder cancer or bladder cancers? Genetically distinct malignant conditions of the urothelium. Urol. Oncol. 28, 409–428. ( 10.1016/j.urolonc.2010.04.003) [DOI] [PubMed] [Google Scholar]
- 41.Knowles MA, Currie GA. 1993. Genetic alterations in bladder cancer. Lancet 342, 1184–1185. ( 10.1016/0140-6736(93)92169-T) [DOI] [PubMed] [Google Scholar]
- 42.Knowles MA. 2001. What we could do now: molecular pathology of bladder cancer. Mol. Pathol. 54, 215–221. ( 10.1136/mp.54.4.215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knowles MA. 2006. Molecular subtypes of bladder cancer: Jekyll and Hyde or chalk and cheese? Carcinogenesis 27, 361–373. ( 10.1093/carcin/bgi310) [DOI] [PubMed] [Google Scholar]
- 44.Knowles MA. 2008. Bladder cancer subtypes defined by genomic alterations. Scand. J. Urol. Nephrol. 218, 116–130. ( 10.1080/03008880802284605) [DOI] [PubMed] [Google Scholar]
- 45.Hurst CD, Platt FM, Knowles MA. 2013. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur. Urol. 65, 367–369. ( 10.1016/j.eururo.2013.08.057) [DOI] [PubMed] [Google Scholar]
- 46.Dancik GM, Owens CR, Iczkowski KA, Theodorescu D. 2014. A cell of origin gene signature indicates human bladder cancer has distinct cellular progenitors. Stem Cells 32, 974–982. ( 10.1002/stem.1625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bryan RT, Kirby R, Mostafid H. 2014. Does the nonurologic scientific community understand urothelial bladder cancer? Eur. Urol. 66, 601–602. ( 10.1016/j.eururo.2014.04.010) [DOI] [PubMed] [Google Scholar]
- 48.The Cancer Genome Atlas Research Network. 2014. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322. ( 10.1038/nature12965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bryan RT, et al. 2011. Assessment of high-throughput high-resolution MALDI–TOF–MS of urinary peptides for the detection of muscle-invasive bladder cancer. Proteomics Clin. Appl. 5, 493–503. ( 10.1002/prca.201100011) [DOI] [PubMed] [Google Scholar]
- 50.Shimwell NJ, Bryan RT, Wei W, James ND, Cheng KK, Zeegers MP, Johnson PJ, Martin A, Ward DG. 2013. Combined proteome and transcriptome analyses for the discovery of urinary biomarkers for urothelial carcinoma. Br. J. Cancer 108, 1854–1861. ( 10.1038/bjc.2013.157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bryan RT, et al. 2013. Urinary EpCAM in urothelial bladder cancer patients: characterisation and evaluation of biomarker potential. Br. J. Cancer 110, 679–685. ( 10.1038/bjc.2013.744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hawkins RD, Hon GC, Ren B. 2010. Next-generation genomics: an integrative approach. Nat. Rev. Genet. 11, 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Svatek RS, Hollenbeck BK, Holmang S, Lee R, Kim SP, Stenzl A, Lotan Y. 2014. The economics of bladder cancer: costs and considerations of caring for this disease. Eur. Urol. 66, 253–262. ( 10.1016/j.eururo.2014.01.006) [DOI] [PubMed] [Google Scholar]
- 54.Choi W, et al. 2014. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25, 152–165. ( 10.1016/j.ccr.2014.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hurst CD, Knowles MA. 2014. Molecular subtyping of invasive bladder cancer: time to divide and rule? Cancer Cell 25, 135–136. ( 10.1016/j.ccr.2014.01.026) [DOI] [PubMed] [Google Scholar]
- 56.Boustead GB, Fowler S, Swamy R, Kocklebergh R, Hounsome L. 2013. Stage, grade and pathological characteristics of bladder cancer in the UK: British Association of Urological Surgeons (BAUS) Urology Tumour Registry . BJU Int. 113, 924–930. [DOI] [PubMed] [Google Scholar]
- 57.Bryan RT, et al. 2013. A comparison of patient and tumour characteristics in two UK bladder cancer cohorts separated by 20 years. BJU Int. 112, 169–175. ( 10.1111/bju.12032) [DOI] [PubMed] [Google Scholar]
- 58.Mao Q, et al. 2008. Up-regulation of E-cadherin by small activating RNA inhibits cell invasion and migration in 5637 human bladder cancer cells. Biochem. Biophys. Res. Commun. 375, 566–570. ( 10.1016/j.bbrc.2008.08.059) [DOI] [PubMed] [Google Scholar]
- 59.Mialhe A, Levacher G, Champelovier P, Martel V, Serres M, Knudsen K, Seigneurin D. 2000. Expression of E-, P-, N-cadherins and catenins in human bladder carcinoma cell lines. J. Urol. 164, 826–835. ( 10.1016/S0022-5347(05)67322-3) [DOI] [PubMed] [Google Scholar]
- 60.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. 2008. Cadherin switching. J. Cell Sci. 121, 727–735. ( 10.1242/jcs.000455) [DOI] [PubMed] [Google Scholar]
- 61.Nollet F, Kools P, van RF. 2000. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 299, 551–572. ( 10.1006/jmbi.2000.3777) [DOI] [PubMed] [Google Scholar]
- 62.Wheelock MJ, Knudsen KA. 1991. Cadherins and associated proteins. In vivo 5, 505–513. [PubMed] [Google Scholar]
- 63.Brasch J, Harrison OJ, Honig B, Shapiro L. 2012. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol. 22, 299–310. ( 10.1016/j.tcb.2012.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fichtner D, Lorenz B, Engin S, Deichmann C, Oelkers M, Janshoff A, Menke A, Wedlich D, Franz CM. 2014. Covalent and density-controlled surface immobilization of E-cadherin for adhesion force spectroscopy. PLoS ONE 9, e93123 ( 10.1371/journal.pone.0093123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ismail AF, Dasgupta P, Khan MS, Jiang W, Martin T, Wells CM. 2014. hP21-activated kinase 5 (PAK5) and epithelial mesenchymal transition in bladder cancer. Eur. Urol. Suppl. 13, e239 ( 10.1016/S1569-9056(14)60236-0) [DOI] [Google Scholar]
- 66.Zaidel-Bar R. 2013. Cadherin adhesome at a glance. J. Cell Sci. 126, 373–378. ( 10.1242/jcs.111559) [DOI] [PubMed] [Google Scholar]
- 67.Harrison OJ, et al. 2011. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure 19, 244–256. ( 10.1016/j.str.2010.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rieger-Christ KM, Cain JW, Braasch JW, Dugan JM, Silverman ML, Bouyounes B, Libertino JA, Summerhayes IC. 2001. Expression of classic cadherins type I in urothelial neoplastic progression. Hum. Pathol. 32, 18–23. ( 10.1053/hupa.2001.21140) [DOI] [PubMed] [Google Scholar]
- 69.Takeichi M. 1991. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251, 1451–1455. ( 10.1126/science.2006419) [DOI] [PubMed] [Google Scholar]
- 70.Shimoyama Y, Hirohashi S, Hirano S, Noguchi M, Shimosato Y, Takeichi M, Abe O. 1989. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 49, 2128–2133. [PubMed] [Google Scholar]
- 71.Shiozaki H, et al. 1991. Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am. J. Pathol. 139, 17–23. [PMC free article] [PubMed] [Google Scholar]
- 72.Takeichi M. 1988. The cadherins: cell–cell adhesion molecules controlling animal morphogenesis. Development 102, 639–655. [DOI] [PubMed] [Google Scholar]
- 73.Paredes J, et al. 2012. Epithelial E- and P-cadherins: role and clinical significance in cancer. Biochim. Biophys. Acta 1826, 297–311. [DOI] [PubMed] [Google Scholar]
- 74.van Roy F. 2014. Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat. Rev. Cancer 14, 121–134. ( 10.1038/nrc3647) [DOI] [PubMed] [Google Scholar]
- 75.Usui A, Ko SY, Barengo N, Naora H. 2014. P-cadherin promotes ovarian cancer dissemination through tumor cell aggregation and tumor-peritoneum interactions. Mol. Cancer Res. 12, 504–513. ( 10.1158/1541-7786.MCR-13-0489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De WO, Pauwels P, De CB, Sabbah M, Emami S, Redeuilh G, Gespach C, Bracke M, Berx G. 2008. Molecular and pathological signatures of epithelial–mesenchymal transitions at the cancer invasion front. Histochem. Cell Biol. 130, 481–494. ( 10.1007/s00418-008-0464-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Friedl P, Gilmour D. 2009. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457. ( 10.1038/nrm2720) [DOI] [PubMed] [Google Scholar]
- 78.Chiang AC, Massague J. 2008. Molecular basis of metastasis. N. Engl. J. Med. 359, 2814–2823. ( 10.1056/NEJMra0805239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tselepis C, Perry I, Jankowski J. 2000. Barrett's esophagus: disregulation of cell cycling and intercellular adhesion in the metaplasia-dysplasia-carcinoma sequence. Digestion 61, 1–5. ( 10.1159/000007729) [DOI] [PubMed] [Google Scholar]
- 80.Maeda M, Johnson KR, Wheelock MJ. 2005. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J. Cell Sci. 118, 873–887. ( 10.1242/jcs.01634) [DOI] [PubMed] [Google Scholar]
- 81.Farahani E, Patra HK, Jangamreddy JR, Rashedi I, Kawalec M, Rao Pariti RK, Batakis P, Wiechec E. 2014. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis 35, 747–759. ( 10.1093/carcin/bgu045) [DOI] [PubMed] [Google Scholar]
- 82.Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y. 2005. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 65, 3092–3099. [DOI] [PubMed] [Google Scholar]
- 83.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. 2007. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin. Cancer Res. 13, 7003–7011. ( 10.1158/1078-0432.CCR-07-1263) [DOI] [PubMed] [Google Scholar]
- 84.Jaggi M, Nazemi T, Abrahams NA, Baker JJ, Galich A, Smith LM, Balaji KC. 2006. N-cadherin switching occurs in high Gleason grade prostate cancer. Prostate 66, 193–199. ( 10.1002/pros.20334) [DOI] [PubMed] [Google Scholar]
- 85.Patel IS, Madan P, Getsios S, Bertrand MA, MacCalman CD. 2003. Cadherin switching in ovarian cancer progression. Int. J. Cancer 106, 172–177. ( 10.1002/ijc.11086) [DOI] [PubMed] [Google Scholar]
- 86.Li G, Satyamoorthy K, Herlyn M. 2001. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 61, 3819–3825. [PubMed] [Google Scholar]
- 87.Wang P, et al. 2014. The prognostic value of P-cadherin in non-muscle-invasive bladder cancer. Eur. J. Surg. Oncol. 40, 255–259. ( 10.1016/j.ejso.2013.12.018) [DOI] [PubMed] [Google Scholar]
- 88.Wu K, Zeng J, Zhou J, Fan J, Chen Y, Wang Z, Zhang T, Wang X, He D. 2013. Slug contributes to cadherin switch and malignant progression in muscle-invasive bladder cancer development. Urol. Oncol. 31, 1751–1760. ( 10.1016/j.urolonc.2012.02.001) [DOI] [PubMed] [Google Scholar]
- 89.Lascombe I, Clairotte A, Fauconnet S, Bernardini S, Wallerand H, Kantelip B, Bittard H. 2006. N-cadherin as a novel prognostic marker of progression in superficial urothelial tumors. Clin. Cancer Res. 12, 2780–2787. ( 10.1158/1078-0432.CCR-05-2387) [DOI] [PubMed] [Google Scholar]
- 90.Mandeville JA, et al. 2008. P-cadherin as a prognostic indicator and a modulator of migratory behaviour in bladder carcinoma cells. BJU Int. 102, 1707–1714. ( 10.1111/j.1464-410X.2008.08115.x) [DOI] [PubMed] [Google Scholar]
- 91.Van Batavia J, et al. 2014. Bladder cancers arise from distinct urothelial sub-populations. Nat. Cell Biol. 16, 982–991. ( 10.1038/ncb3038) [DOI] [PubMed] [Google Scholar]
- 92.Sarrio D, Palacios J, Hergueta-Redondo M, Gomez-Lopez G, Cano A, Moreno-Bueno G. 2009. Functional characterization of E- and P-cadherin in invasive breast cancer cells. BMC Cancer 9, 74 ( 10.1186/1471-2407-9-74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ribeiro AS, et al. 2013. P-cadherin functional role is dependent on E-cadherin cellular context: a proof of concept using the breast cancer model. J. Pathol. 229, 705–718. ( 10.1002/path.4143) [DOI] [PubMed] [Google Scholar]
- 94.Paredes J, Albergaria A, Oliveira JT, Jeronimo C, Milanezi F, Schmitt FC. 2005. P-cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clin. Cancer Res. 11, 5869–5877. ( 10.1158/1078-0432.CCR-05-0059) [DOI] [PubMed] [Google Scholar]
- 95.Sato N, Fukushima N, Maitra A, Matsubayashi H, Yeo CJ, Cameron JL, Hruban RH, Goggins M. 2003. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 63, 3735–3742. [PubMed] [Google Scholar]
- 96.Milicic A, et al. 2008. Ectopic expression of P-cadherin correlates with promoter hypomethylation early in colorectal carcinogenesis and enhanced intestinal crypt fission in vivo. Cancer Res. 68, 7760–7768. ( 10.1158/0008-5472.CAN-08-0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ribeiro AS, Albergaria A, Sousa B, Correia AL, Bracke M, Seruca R, Schmitt FC, Paredes J. 2010. Extracellular cleavage and shedding of P-cadherin: a mechanism underlying the invasive behaviour of breast cancer cells. Oncogene 29, 392–402. ( 10.1038/onc.2009.338) [DOI] [PubMed] [Google Scholar]
- 98.Paredes J, Correia AL, Ribeiro AS, Milanezi F, Cameselle-Teijeiro J, Schmitt FC. 2008. Breast carcinomas that co-express E- and P-cadherin are associated with p120-catenin cytoplasmic localisation and poor patient survival. J. Clin. Pathol. 61, 856–862. ( 10.1136/jcp.2007.052704) [DOI] [PubMed] [Google Scholar]
- 99.Taniuchi K, et al. 2005. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 65, 3092–3099. [DOI] [PubMed] [Google Scholar]
- 100.Cheung LW, Leung PC, Wong AS. 2010. Cadherin switching and activation of p120 catenin signaling are mediators of gonadotropin-releasing hormone to promote tumor cell migration and invasion in ovarian cancer. Oncogene 29, 2427–2440. ( 10.1038/onc.2009.523) [DOI] [PubMed] [Google Scholar]
- 101.Cheung LW, Mak AS, Cheung AN, Ngan HY, Leung PC, Wong AS. 2011. P-cadherin cooperates with insulin-like growth factor-1 receptor to promote metastatic signaling of gonadotropin-releasing hormone in ovarian cancer via p120 catenin. Oncogene 30, 2964–2974. ( 10.1038/onc.2011.7) [DOI] [PubMed] [Google Scholar]
- 102.Zhang CC, et al. 2010. PF-03732010: a fully human monoclonal antibody against P-cadherin with antitumor and antimetastatic activity. Clin. Cancer Res. 16, 5177–5188. ( 10.1158/1078-0432.CCR-10-1343) [DOI] [PubMed] [Google Scholar]
- 103.Moltzahn FR, Volkmer JP, Rottke D, Ackermann R. 2008. ‘Cancer stem cells’: lessons from Hercules to fight the hydra. Urol. Oncol. 26, 581–589. ( 10.1016/j.urolonc.2008.07.009) [DOI] [PubMed] [Google Scholar]
- 104.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. 2008. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 40, 499–507. ( 10.1038/ng.127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoglund M. 2007. On the origin of syn- and metachronous urothelial carcinomas. Eur. Urol. 51, 1185–1193. ( 10.1016/j.eururo.2006.11.025) [DOI] [PubMed] [Google Scholar]
- 106.Chan KS, et al. 2009. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl Acad. Sci. USA 106, 14 016–14 021. ( 10.1073/pnas.0906549106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kolle G, Ho M, Zhou Q, Chy HS, Krishnan K, Cloonan N, Bertoncello I, Laslett AL, Grimmond SM. 2009. Identification of human embryonic stem cell surface markers by combined membrane-polysome translation state array analysis and immunotranscriptional profiling. Stem Cells 27, 2446–2456. ( 10.1002/stem.182) [DOI] [PubMed] [Google Scholar]
- 108.Vieira AF, et al. 2012. P-cadherin is coexpressed with CD44 and CD49f and mediates stem cell properties in basal-like breast cancer. Stem Cells 30, 854–864. ( 10.1002/stem.1075) [DOI] [PubMed] [Google Scholar]
- 109.Bryan RT, Zeegers MP, James ND, Wallace DM, Cheng KK. 2009. Biomarkers in bladder cancer. BJU Int. 105, 608–613. ( 10.1111/j.1464-410X.2009.08880.x) [DOI] [PubMed] [Google Scholar]
- 110.He K, Xu T, Xu Y, Ring A, Kahn M, Goldkorn A. 2014. Cancer cells acquire a drug resistant, highly tumorigenic, cancer stem-like phenotype through modulation of the PI3K/Akt/beta-catenin/CBP pathway. Int. J. Cancer 134, 43–54. ( 10.1002/ijc.28341) [DOI] [PubMed] [Google Scholar]
- 111.Rosen JM, Jordan CT. 2009. The increasing complexity of the cancer stem cell paradigm. Science 324, 1670–1673. ( 10.1126/science.1171837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Szala S, Froehlich M, Scollon M, Kasai Y, Steplewski Z, Koprowski H, Linnenbach AJ. 1990. Molecular cloning of cDNA for the carcinoma-associated antigen GA733–2. Proc. Natl Acad. Sci. USA 87, 3542–3546. ( 10.1073/pnas.87.9.3542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patriarca C, et al. 2009. Cell discohesion and multifocality of carcinoma in situ of the bladder: new insight from the adhesion molecule profile (E-cadherin, Ep-CAM, and MUC1). Int. J. Surg. Pathol. 17, 99–106. ( 10.1177/1066896908326918) [DOI] [PubMed] [Google Scholar]
- 114.Brunner A, Prelog M, Verdorfer I, Tzankov A, Mikuz G, Ensinger C. 2008. EpCAM is predominantly expressed in high grade and advanced stage urothelial carcinoma of the bladder. J. Clin. Pathol. 61, 307–310. ( 10.1136/jcp.2007.049460) [DOI] [PubMed] [Google Scholar]
- 115.Okegawa T, Hayashi K, Hara H, Nutahara K, Higashihara E. 2010. Immunomagnetic quantification of circulating tumor cells in patients with urothelial cancer. Int. J. Urol. 17, 254–258. ( 10.1111/j.1442-2042.2010.02454.x) [DOI] [PubMed] [Google Scholar]
- 116.Kowalski M, Entwistle J, Cizeau J, Niforos D, Loewen S, Chapman W, MacDonald GC. 2010. A phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCG-refractory and BCG-intolerant patients. Drug Des. Dev. Ther. 4, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maetzel D, et al. 2009. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 11, 162–171. ( 10.1038/ncb1824) [DOI] [PubMed] [Google Scholar]
- 118.Chaves-Perez A, Mack B, Maetzel D, Kremling H, Eggert C, Harreus U, Gires O. 2013. EpCAM regulates cell cycle progression via control of cyclin D1 expression. Oncogene 32, 641–650. ( 10.1038/onc.2012.75) [DOI] [PubMed] [Google Scholar]
- 119.Warneke VS, Behrens HM, Haag J, Kruger S, Simon E, Mathiak M, Ebert MP, Rocken C. 2013. Members of the EpCAM signalling pathway are expressed in gastric cancer tissue and are correlated with patient prognosis. Br. J. Cancer 109, 2217–2227. ( 10.1038/bjc.2013.536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kleiber K, Strebhardt K, Martin BT. 2007. The biological relevance of FHL2 in tumour cells and its role as a putative cancer target. Anticancer Res. 27, 55–61. [PubMed] [Google Scholar]
- 121.Coglievina M, Guarnaccia C, Zlatev V, Pongor S, Pintar A. 2013. Jagged-1 juxtamembrane region: biochemical characterization and cleavage by ADAM17 (TACE) catalytic domain. Biochem. Biophys. Res. Commun. 432, 666–671. ( 10.1016/j.bbrc.2013.02.022) [DOI] [PubMed] [Google Scholar]
- 122.Zeegers MP, et al. 2010. The West Midlands Bladder Cancer Prognosis Programme: rationale and design. BJU Int. 105, 784–788. ( 10.1111/j.1464-410X.2009.08849.x) [DOI] [PubMed] [Google Scholar]
- 123.Ralhan R, et al. 2010. Nuclear and cytoplasmic accumulation of Ep-ICD is frequently detected in human epithelial cancers. PLoS ONE 5, e14130 ( 10.1371/journal.pone.0014130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van der Gun BT, Melchers LJ, Ruiters MH, de Leij LF, McLaughlin PM, Rots MG. 2010. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis 31, 1913–1921. ( 10.1093/carcin/bgq187) [DOI] [PubMed] [Google Scholar]
- 125.Litvinov SV, Balzar M, Winter MJ, Bakker HA, Briaire-de Bruijn IH, Prins F, Fleuren GJ, Warnaar SO. 1997. Epithelial cell adhesion molecule (Ep-CAM) modulates cell–cell interactions mediated by classic cadherins. J. Cell Biol. 139, 1337–1348. ( 10.1083/jcb.139.5.1337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Winter MJ, Nagelkerken B, Mertens AE, Rees-Bakker HA, Briaire-de Bruijn IH, Litvinov SV. 2003. Expression of Ep-CAM shifts the state of cadherin-mediated adhesions from strong to weak. Exp. Cell Res. 285, 50–58. ( 10.1016/S0014-4827(02)00045-9) [DOI] [PubMed] [Google Scholar]
- 127.Winter MJ, Cirulli V, Briaire-de Bruijn IH, Litvinov SV. 2007. Cadherins are regulated by Ep-CAM via phosphaditylinositol-3 kinase. Mol. Cell Biochem. 302, 19–26. ( 10.1007/s11010-007-9420-y) [DOI] [PubMed] [Google Scholar]
- 128.Martowicz A, Spizzo G, Gastl G, Untergasser G. 2012. Phenotype-dependent effects of EpCAM expression on growth and invasion of human breast cancer cell lines. BMC Cancer 12, 501 ( 10.1186/1471-2407-12-501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martowicz A, Rainer J, Lelong J, Spizzo G, Gastl G, Untergasser G. 2013. EpCAM overexpression prolongs proliferative capacity of primary human breast epithelial cells and supports hyperplastic growth. Mol. Cancer 12, 56 ( 10.1186/1476-4598-12-56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slanchev K, Carney TJ, Stemmler MP, Koschorz B, Amsterdam A, Schwarz H, Hammerschmidt M. 2009. The epithelial cell adhesion molecule EpCAM is required for epithelial morphogenesis and integrity during zebrafish epiboly and skin development. PLoS Genet. 5, e1000563 ( 10.1371/journal.pgen.1000563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guerra E, Lattanzio R, La SR, Dini F, Tiboni GM, Piantelli M, Alberti S. 2012. mTrop1/Epcam knockout mice develop congenital tufting enteropathy through dysregulation of intestinal E-cadherin/beta-catenin. PLoS ONE 7, e49302 ( 10.1371/journal.pone.0049302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maghzal N, Kayali HA, Rohani N, Kajava AV, Fagotto F. 2013. EpCAM controls actomyosin contractility and cell adhesion by direct inhibition of PKC. Dev. Cell 27, 263–277. ( 10.1016/j.devcel.2013.10.003) [DOI] [PubMed] [Google Scholar]
- 133.Vannier C, Mock K, Brabletz T, Driever W. 2013. Zeb1 regulates E-cadherin and Epcam (epithelial cell adhesion molecule) expression to control cell behavior in early zebrafish development. J. Biol. Chem. 288, 18 643–18 659. ( 10.1074/jbc.M113.467787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang HP, et al. 2011. Epithelial cell adhesion molecule (EpCAM) complex proteins promote transcription factor-mediated pluripotency reprogramming. J. Biol. Chem. 286, 33 520–33 532. ( 10.1074/jbc.M111.256164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen HF, Chuang CY, Lee WC, Huang HP, Wu HC, Ho HN, Chen YJ, Kuo HC. 2011. Surface marker epithelial cell adhesion molecule and E-cadherin facilitate the identification and selection of induced pluripotent stem cells. Stem Cell Rev. 7, 722–735. ( 10.1007/s12015-011-9233-y) [DOI] [PubMed] [Google Scholar]
- 136.Ravindran G, Sawant SS, Hague A, Kingsley K, Devaraj H. In press Association of differential β-catenin expression with Oct-4 and Nanog in oral squamous cell carcinoma and their correlation with clinicopathological factors and prognosis. Head Neck ( 10.1002/hed.23699) [DOI] [PubMed] [Google Scholar]
- 137.Luo Y, Lan L, Jiang YG, Zhao JH, Li MC, Wei NB, Lin YH. 2013. Epithelial-mesenchymal transition and migration of prostate cancer stem cells is driven by cancer-associated fibroblasts in an HIF-1alpha/beta-catenin-dependent pathway. Mol. Cells 36, 138–144. ( 10.1007/s10059-013-0096-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Macaulay IC, Voet T. 2014. Single cell genomics: advances and future perspectives. PLoS Genet. 10, e1004126 ( 10.1371/journal.pgen.1004126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Van Loo P, Voet T. 2014. Single cell analysis of cancer genomes. Curr. Opin. Genet. Dev. 24C, 82–91. ( 10.1016/j.gde.2013.12.004) [DOI] [PubMed] [Google Scholar]
- 140.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. 2013. Cancer genome landscapes. Science 339, 1546–1558. ( 10.1126/science.1235122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Blaschuk OW. 2012. Discovery and development of N-cadherin antagonists. Cell Tissue Res. 348, 309–313. ( 10.1007/s00441-011-1320-5) [DOI] [PubMed] [Google Scholar]
- 142.Devemy E, Blaschuk OW. 2008. Identification of a novel N-cadherin antagonist. Peptides 29, 1853–1861. ( 10.1016/j.peptides.2008.06.025) [DOI] [PubMed] [Google Scholar]
- 143.Blaschuk OW, Devemy E. 2009. Cadherins as novel targets for anti-cancer therapy. Eur. J. Pharmacol. 625, 195–198. ( 10.1016/j.ejphar.2009.05.033) [DOI] [PubMed] [Google Scholar]