Abstract
Bio-nano interactions can be defined as the study of interactions between nanoscale entities and biological systems such as, but not limited to, peptides, proteins, lipids, DNA and other biomolecules, cells and cellular receptors and organisms including humans. Studying bio-nano interactions is particularly useful for understanding engineered materials that have at least one dimension in the nanoscale. Such materials may consist of discrete particles or nanostructured surfaces. Much of biology functions at the nanoscale; therefore, our ability to manipulate materials such that they are taken up at the nanoscale, and engage biological machinery in a designed and purposeful manner, opens new vistas for more efficient diagnostics, therapeutics (treatments) and tissue regeneration, so-called nanomedicine. Additionally, this ability of nanomaterials to interact with and be taken up by cells allows nanomaterials to be used as probes and tools to advance our understanding of cellular functioning. Yet, as a new technology, assessment of the safety of nanomaterials, and the applicability of existing regulatory frameworks for nanomaterials must be investigated in parallel with development of novel applications. The Royal Society meeting ‘Bio-nano interactions: new tools, insights and impacts' provided an important platform for open dialogue on the current state of knowledge on these issues, bringing together scientists, industry, regulatory and legal experts to concretize existing discourse in science law and policy. This paper summarizes these discussions and the insights that emerged.
Keywords: nanomedicine, nanosafety, nanoregulation, bio-nano interface, biomolecule corona, advanced materials
1. Background to the meeting
Throughout the 21st century, reports about dangers and benefits of using well-established toxins, as well as substances that were previously considered innocuous, have been diligently examined by the Royal Society. The Royal Society has proactively engaged in discussions regarding nanotechnologies since the early 2000s. It was the first major international scientific community to publish its opinion regarding the opportunities and challenges posed by nanotechnologies and nanomaterials for society [1]. As noted in the Royal Society's report in 2004, nanotechnology offers a fresh perspective for scientific understanding of the physical properties of matter. Subsequently, Royal Society journals have published pioneering articles concerning this emerging field, starting with inhalation toxicology and exploring scientific concerns that ultrafine airborne particles might cause harmful effects (figure 1), and the implications of that possible harm when applying nanotechnology in a diverse range of consumer applications [2]. Those findings prompted a pause to reconsider the overwhelmingly positive attitudes regarding the ‘nanotechnology revolution’. Thus, although potential dangers from substances used in nanomedicine are unquantified as yet, both the potential benefits of applications and any possible negative implications following their use have consistently been the subject of lively discourse at the Royal Society.
Figure 1.
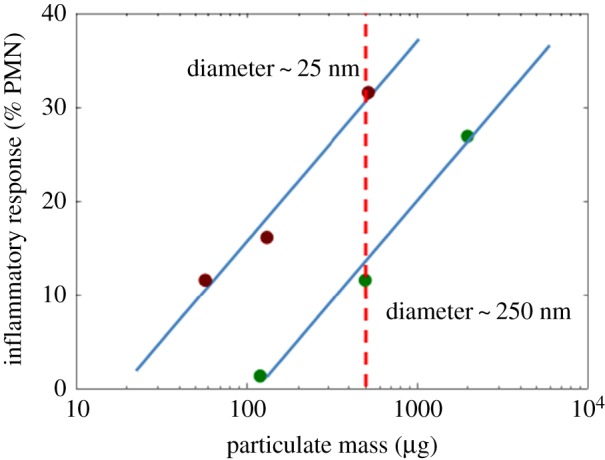
Impact of particle size on the inflammatory response induced in rats following instillation of TiO2 particles at different doses. Reproduced with permission from Oberdürster [2]. (Online version in colour.)
On 30 April and 1 May 2014, The Royal Society of London, therefore, sponsored a meeting1 entitled ‘Bio-nano interactions: new tools, insights and impacts' at Chicheley Hall.2 This 2-day session on bio-nano interactions tackled key scientific issues regarding nanotechnology applications in medicine and diagnostics as well as considering aspects of safety, governance and regulation of new materials, devices and technologies, and the capacity of existing regulatory frameworks to balance benefits, unquantified risks and public health. The 54 participants in the meeting included UK and international academics, practicing clinicians, industry and industry organizations, an international legal scholar and an official representative of the Council of Europe. Presentations and discussions on the first day addressed recent and prospective developments in nanomedicine that operationalize new, detailed understanding of bio-nano interactions. The second day focused on nanosafety and regulation, asking whether enhanced understanding of bio-nano interactions requires new regulatory approaches and drafting of new laws for nanomaterials, or do regulatory frameworks already in place have sufficient flexibility to incorporate these advanced materials.
2. Scope of the meeting
‘Bio-nano interactions: new tools, insights and impacts' gave concrete evidence that the newest phase is dawning in the nanotechnology era—the so-called fourth generation has arrived, illustrated by state-of-the-art examples, including self-assembly and bioelectronics for medicines as discussed below. As predicted by the Royal Society over a decade before [1], the nanotechnology revolution offers new biomaterials, and better understanding and manipulation of bio-nano interactions of materials and cellular activity at the nanoscale, with fascinating opportunities for business and human health. An added-value from study of nanotechnologies and nanomaterials is that these tiny materials allow scientists to probe cellular and tissue functioning and spotlight gaps in human knowledge. Indeed, several speakers highlighted the fact that getting very new ideas to a level of robust maturity in an emergent field can take years, especially when new knowledge and untested know-how challenge established norms in the medical field. For example, the validity of in vitro models and approaches, which have traditionally used 10% calf serum protein to sustain cell cultures, was questioned, because the introduction of nanomaterials results in binding of these proteins to the nanoparticle surfaces with the potential for presentation of a non-native biological identity due to unfolding of the proteins at the nanoparticle surface. Across the wide range of topics discussed, the key underpinning concept (whether for discrete nanoparticles or nanostructured surfaces) was the role of nanomaterials as scaffolds for protein/biomolecule binding (as illustrated in figure 2) and the utilization of this for targeted therapeutic delivery, electrochemical sensing, control of stem-cell differentiation, as well as potentially as a basis for predicting nanoparticle fate, behaviour and safety.
Figure 2.
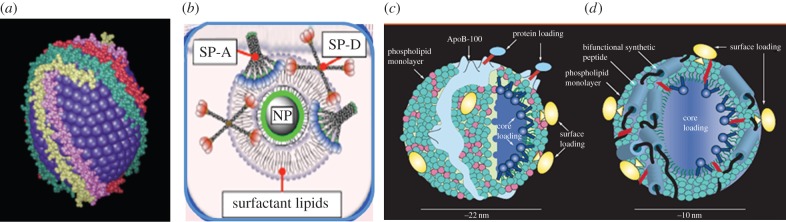
Nanomaterials as scaffolds for protein (biomolecule) binding: nanomaterials have the right size and shape for interacting with transport proteins such as apolipoproteins, offering them access to all cells via the low-density lipoprotein receptor (a). (b) Schematic illustration of the nanoparticle corona formed from lung surfactant proteins. Structure of native low-density lipoprotein (c) and synthetic nano low-density lipoprotein (d) designed for nanomedicine. Various compartments for loading exogenous agents are shown including protein loading via covalent attachment of compounds to lysine amino acid residues in apolipoprotein B-100 or surface loading: intercalation of amphiphilic compounds into the phospholipid monolayer. Core loading can also be achieved via reconstitution of hydrophobic compounds into the nanocarrier apolar core. Adapted from Corbin & Zheng [3]. (Online version in colour.)
The meeting's central topic thus concerned bio-nano interactions, involving a new view of the fate and behaviour of nanomaterials or nanosurfaces at the interface with the biological environment. This ‘binding’ or biointeraction tendency of nanoscale surfaces results from their need to reduce their surface energy by binding available biomolecules [4], and can be used for a range of applications at the interface between medicine and engineering, including diagnostic devices, nano-enabled therapies such as targeted drug delivery, or for tissue engineering, as summarized in figure 3 [5]. Indeed, biointeractions have long been understood to determine the fate of implant materials such as stent coatings, where integration of the device into the body requires the adsorption of a specific set of proteins, while binding of the wrong proteins leads to immune recognition and subsequent rejection of the implant [6–8]. Similarly, bio-nano interactions and protein adsorption at nanoscale surfaces play key roles in cellular adhesion, mobility and phenotype development on material surfaces [9,10].
Figure 3.
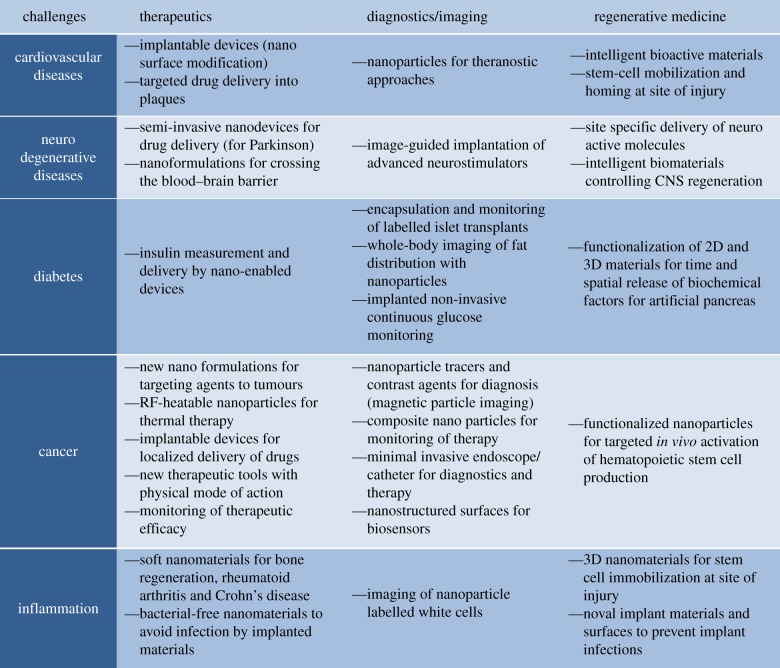
A snapshot of some application areas where nanotechnology has already had an impact in terms of treatment, diagnosis or imaging of some major diseases. From the European Technology Platform on Nanomedicine (ETPN) White Paper 2013 ‘Contribution of Nanomedicine to Horizon 2020’ [5]. (Online version in colour.)
The meeting included 12 invited lectures, 12 poster spotlights, extended discussions and two rapporteur summaries, one integrating the scientific perspectives and one summarizing policy outcomes from advanced understanding of bio-nano interactions.
3. Technical highlights from the meeting
(a). Advances in drug delivery and mimicking biological processes
Utilization of bio-nano interactions as the basis for advanced drug delivery was addressed in two talks: (i) Matthias Epple (University of Duisburg-Essen) presented his recent work on applications of biodegradable multishell calcium phosphate (CaP) nanoparticles for delivery of DNA, proteins and siRNA and (ii) Rein Ulijn (University of Strathclyde) presented some new directions using self-assembly of biomolecules such as peptides to drive charge transfer, an essential step in many biological processes such as photosynthesis and respiration.
The CaP nanoparticles were functionalized with the model antigen Hen Egg Lysozyme (HEL) to take advantage of a HEL-specific B-cell receptor transgenic mouse model. Epple and his team demonstrated that functionalized nanoparticles were able to effectively cross-link B-cell receptors at the surface of antigen-matched B cells, with the result that they were 100-fold more efficient in the activation of B-cells than soluble HEL. This suggests that these are promising vaccine candidates for inducing humoral immunity, i.e. immunity induced by antibodies [11]. In another potential application, functionalization of CaP nanoparticles with serum proteins fetuin-A and albumin (greater than or equal to 1 µM) reduced intracellular Ca2+ elevation and cell death in human vascular smooth muscle cells (VSMC) in response to CaP particles. Fetuin-A, a key protein whose levels are lowered in patients with kidney disease, also reduced dissolution of CaP particles under acidic conditions, which may contribute to its cytoprotective effects after CaP particle exposure to VSMCs [12]. Thus, Epple's work demonstrated the vital role played by bio-nano interactions in determining nanoparticle fate, behaviour and induced responses, which are all key components of effective design of drug delivery carriers and nanotherapeutics.
Adaptive nanotechnology is the development of new molecular technologies using the fundamental principles that biology uses for adaptation (the evolutionary process whereby an organism becomes better able to live in its habitat) but with simpler components, such as peptides. Ulijn's team has made significant progress towards understanding how catalysis and molecular self-assembly can be linked to produce adaptive and selective systems capable of electron transfer between macromolecules. For example, coupling the reversible in situ formation of a self-assembly building block by enzymatic condensation with biocatalytic condensation (assembly) gives rise to nanostructures with optimized supramolecular interactions, as evidenced by substantial aggregation-induced emission upon assembly. These supramolecular assemblies are capable of forming efficient charge-transfer complexes in the presence of suitable donors [13]. In another example, small-molecule-based bioactive hydrogels were used as three-dimensional platforms for culturing human dermal fibroblasts, whereby the cells self-organized the fibrous extracellular matrix and contracted the gel without differentiating into myofibroblasts, in a 14-day culture [14]. Using dynamic nanostructures and templating or self-assembly thus provides enormous opportunities for developing new applications of nanotechnology, because their formation and function are driven by free-energy minimization, making them inherently self-healing and thus adaptive to changes in their surroundings. While the current supramolecular assemblies are a long way from the clinic, one can envision a future whereby changes in protein or peptide content of blood plasma might trigger self-assembly of a selected peptide as the initiating step in an adaptive therapeutic response.
(b). Nanopatterned surfaces for tissue regeneration
Utilization of bio-nano interactions with nanopatterned surfaces to drive cellular behaviour was addressed in several presentations and from a number of different approaches including a presentation on control of stem-cell differentiation from Matthew Dalby (University of Glasgow) and a talk on the development of synthetic biomaterials for skeletal tissue regeneration by Pamela Habibovic (University of Twente).
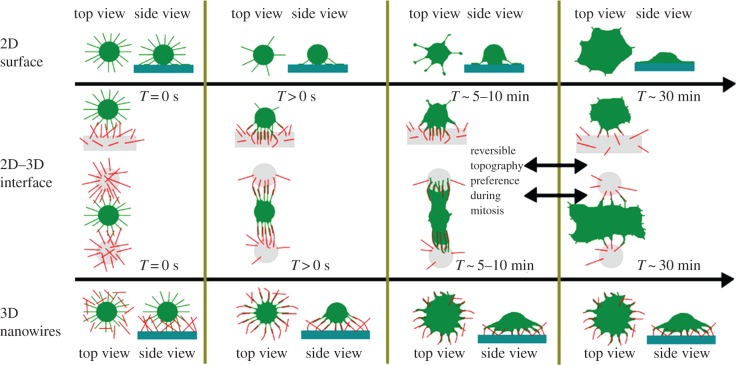
Design of nanopatterned surfaces to control growth and differentiation of mesenchymal stem cells (MSC) is an important emerging area. The interaction of nanotopographical features with integrin receptors in the cells’ focal adhesions alters how cells adhere to material surfaces and defines cellular fate through changes in both cell biochemistry and cell morphology. Depending on the scale of patterning, Dalby and his team found that the stem cells have quite different adhesion and cellular tension, with 10 nm islands resulting in cellular fillopodia, whereas 8 nm patterning resulted in loss of fillopodia and reduced adhesion [15]. Knowledge gained from the study of cell–nanotopography interactions is expected to accelerate the development of next-generation stem cell culture materials and implant interfaces, and to fuel discovery of stem cell therapeutics to support regenerative therapies [16]. Figure 4 provides a schematic of some of the sorts of bio-nano interactions that can occur between nanostructured surfaces and cells and their consequences for cell shape, polarity and spreading.
Figure 4.
Summary of how differences in cell shape and polarity as well as the spreading dynamics are controlled by filopodia-nanowire (NW) interactions. The first row describes for flat glass (2D) how fibroblasts undergo a rapid phase transition from filopodia-rich initial state towards a lamellipodia-mediated spreading. The second row shows how cells sitting at interfaces between flat surface and NWs contact the NWs, quickly adhere, align and spread towards NW adhesions, while filopodia peel off from the adjacent flat surfaces. After approximately 30 min, delayed lamellae/ruffle formation leads to migration towards the flat surface (data not shown). This dynamic change in topography preference is reversible during mitotic rounding of fibroblasts. The bottom row shows how fibroblasts on purely NW-decorated surfaces quickly spread via aligned filopodia-NW adhesions into dendritic shapes, without formation of lamellipodia. Adapted from Albuschies & Vogel [17]. (Online version in colour.)
Consistent with these views on the significance of surface nanotopography, Habibovic spoke about recent approaches in her laboratory to improve the clinical performance of synthetic biomaterials in skeletal tissue regeneration. Specifically, she described the use of nanotechnology methods to distinguish between impacts from chemical composition and those resulting from nanotopography. Habibovic presented a longitudinal study in which three types of biofunctionalizing surface treatments were compared in terms of apatite (bone) forming ability, cell attachment, cell proliferation, gene expression from bone tissue, bone regeneration, biomechanical stability and bone–biomaterial contact. The results suggest that the beneficial effects of cell–nanotopography modulation (topography) surpassed the benefits of improved bone-forming ability (chemistry) [18].
(c). Bio-nano interactions to probe or manipulate cellular function
Another aspect of manipulation of bio-nano interactions at surfaces that emerged from the meeting was the use of nanotechnologies for enhanced understanding of cellular functioning, as exemplified by the talks of Jeff Karp (Harvard Medical School) who used targeting approaches to influence the behaviour of stem cells; and Frankie Rawson (University of Nottingham) who offered a clear example of how nanoscale tools can call into question established biological knowledge with his electrochemical sensing research that has shed new light on redox processes in cells and at cellular membranes.
Emerging methods to induce a robust ‘rolling’ response by engineering of the cell surface in order to enhance engraftment (the process of transplanted stem cells reproducing new cells) of systemically infused stem cells were presented. Using a platform approach that preserves the MSC phenotype and does not require genetic manipulation, Karp showed how modification of the surface of MSCs with a nanometer-scale polymer construct (found on the surface of leucocytes) mediates cell rolling within inflamed tissue, thereby overcoming the problem of inadequate expression of cell surface adhesion receptors. A shortage of cell-surface adhesion receptors has been considered a major limitation of existing MSC-based therapeutic approaches [19]. Karp also discussed recent research using aptamers, short nucleic acid molecules with binding properties and biochemical characteristics that may make them suitable for use as targeting molecules, which can be conjugated with nanoparticles for targeted delivery and to probe cell surfaces and the cell secretome following transplantation. Karp presented an aptamer-based cell-surface sensor that his team developed to study cellular microenvironments [20].
Rawson discussed the potential use of carbon nanowires to probe charge-transfer events between cells and their external environment. Electrochemical sensing research from Rawson's post-doctoral work used mouse macrophages, and his most recent work involves translating this knowledge to better understand electron shedding in yeast cells. Rawson showed that vertically aligned single-walled carbon nanotubes can enter cells naturally without applying external force, and then sense the intracellular presence of a redox active moiety, methylene blue [21]. Further work demonstrated the potential for the electrochemical sensing platform to monitor and determine the precise location of redox activities occurring within the cell [22], and for elucidating, for example, the pathways via which yeast cells shed electrons with their external environment across the cell wall, suggesting that there is a need to redefine the function of the yeast cell wall (work in progress).
(d). Interactions of nanomaterials with the human body: nanosafety aspects
Assessing the safety aspects of nanomedicine was the topic of two talks: (i) Howard Clark (University of Southampton), addressed nanoparticles entering the lung and (ii) Jonathan Powell (University of Cambridge) addressed uptake of nanoparticles from the gastrointestinal tract.
Clark's group has investigated the interaction between nanoparticles and one of the major proteins associated with the lung surfactant layer that allows lungs to expand during breathing, namely surfactant protein D (SP-D), and the subsequent uptake of the protein-coated nanoparticles by cells involved in lung immunity, using a native human SP-D and a recombinant fragment SP-D [23]. The lung–air barrier maintains lung homeostasis (constant internal environment, via gas exchange and pH regulation) and is a critical part of human biological defence mechanisms, with SP-D modulating pro- and anti-inflammatory processes when threatened by toxic challenges. In vivo studies investigating nanoparticle uptake by alveolar macrophages and lung dendritic cells from control and SP-D deficient mice showed decreased uptake of nanoparticles in the SP-D deficient mice compared with wild-type mice, and confirmed an interaction between SP-D and nanoparticles, and subsequent enhanced nanoparticle uptake [23]. Thus, nanoparticles can subvert the natural defence mechanisms of the lung, and may persist in the respiratory tract and translocate into the circulatory system, as indeed has been observed for fine and ultrafine particles over the last few decades.
Similarly, there are purposeful pathways for nanomineral uptake via the gastrointestinal tract, which can be hijacked by nanoscale additions to human food, toothpaste and cosmetics. Powell and his team investigated the toxic and inflammatory potential of two types of particles that might become increasingly relevant to the food industry, namely SiO2 and ZnO. Particle pre-treatment under simulated gastric and intestinal pH conditions resulted in reduced acellular reactive oxygen species formation but did not influence cytotoxicity (WST-1 assay) or IL-8 expression from human intestinal Caco-2 cells [24], again demonstrating a critical role of bio-nano interactions. Interestingly, the differentiation status of the cells markedly determined the cytotoxic potency of the particles [24]. The naturally occurring uptake process revolves around mineralized calcium. Powell showed data from recent studies demonstrating that nano-Fe3+ crosses the apical surface of Caco-2 cells via an endocytic pathway before being dissolved in endosomes or lysosomes inside the cell (figure 5), and that its subsequent homeostasis is under the normal regulatory control of dietary iron absorption, namely via ferroportin-dependent efflux from enterocytes. This suggests that nano-Fe3+ thus offers potential as a novel oral iron supplement with enhanced uptake in the nano form followed by release and processing in the body via the normal soluble iron pathway [25].
Figure 5.
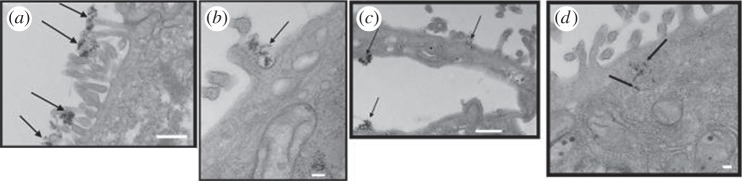
Cellular uptake of nanoparticulate ligand-modified (LM) Fe(III) poly oxo-hydroxide by Caco-2 cells. Transmission electron microscopy images showing differentiated Caco-2 cells incubated with LM Fe(III) poly oxo-hydroxide. (a) Arrows show particle clusters adhering to the cell membrane microvilli (scale bar, 500 nm). (b) Arrows indicate invagination on the cell membrane (scale bar, 100 nm). (c,d) Arrows indicate Fe accumulation inside the cell (scale bars: panel c, 500 nm and panel d, 100 nm). Reproduced with permission from Pereira et al. [25].
Also approaching the topic from a clinical perspective, Andre Nel (University of California, Los Angeles) discussed existing data that supports predictive modelling and alternative testing strategies for regulatory decision making regarding nanomaterials. This rapidly advancing new field requires novel test strategies that allow multiple toxicants to be screened in robust, mechanism-based assays. The notion that most investigation can be carried out at the cellular and biomolecular level without much animal use is based on understanding the contribution of toxicological pathways to the pathophysiology of disease [26]. Nel presented the UC Centre for Environmental Implications of Nanotechnologies approach of selecting screening pathways on the basis of known physiological effects and gave examples of assessing the pulmonary hazard potential of carbon nanotubes [27] and metal oxides [28] using high-content or high-throughput testing, and how the data can be used for hazard ranking, risk assessment, regulatory decision-making and ‘safer-by-design’ strategies [26].
Nel offered a three-tiered approach for prioritizing responses, using oxidative stress as his example: tier 1 ranking the steepness of cellular dose–response curves; tier 2 using intratracheal installation to assess th possibility of predicting acute inflammation from oxidative stress, and tier 3 using inhalation studies about selected nanomaterials as the final verification. As more data emerges, fewer materials should require progress through tiers 2 and 3, enabling regulatory decisions to be justified on the basis of alternative test strategies data [26].
(e). Policy dilemma: small particles, unknown risks, do we need more law?
As summarized above, this meeting gave an excellent overview of the state of the art for bio-nano interfacial interactions in advanced materials. Yet, perennial questions remain as to whether these materials represent a new threat, requiring new rules of law?
Researchers are rushing to fill the regulatory void and, therefore, associated nanosafety studies of new materials have developed at a dramatically rapid pace. The number of studies in the reported nanosafety literature has increased exponentially from 1000 articles a year to 12 000 in the period 2000–2012 [29], with the concomitant effect that no scientist is prepared to state conclusively that their study represents the mainstream or majority view for these questions, even when their studies have robust methods with powerful empirical support. With this important limit upon understanding from the standpoint of managing data as well as understanding risk, the meeting did accept the conclusion that yes, it is possible that in a very small number of cases, these interactions may indeed be different in character, but only a few consumer items would cause such a concern. New research regarding data management efforts will try to group and classify nanomaterials in order to support the identification of those that raise cause for concern [30,31]. Control banding and techniques for categorizing groups of nanomaterials makes good sense from a scientific standpoint, but does not bring decision-makers closure on the underlying questions targeted with prescience by the Royal Commission on Environmental Pollution in 2008 [32,33].
Nanotechnology has also given a shot of adrenaline to an ailing global economy, with its promises of new medicines, lighter packaging and cheaper transport of goods, with the economic importance of nanotechnology estimated to be 3.5 trillion dollars by 2015 [34]. Consequently, every nation and many regional organizations have created draft nanotechnology regulations to support research funding, build strategic platforms and establish performance-based regulatory goals with subsequent regulation of business to protect health and life at work in civil society [35,36]. The final session of the conference therefore made a path-breaking effort to place research advances in the broader policy context, and adeptly changed the focus from the laboratory bench to the judicial bar. This session discussed whether society can allow some nanomaterials to remain unregulated, whether law already exists to deal with nanotechnology applications (despite unquantified risk), or whether it is a matter of simply modifying existing laws/regulatory frameworks within the context of civil society's demand for safe nanoproducts and a healthy environment.
A key area of debate for over a decade in regulatory and industry circles is whether or not ‘new’ regulation and thus legislation is required for nanomaterials. The potential for nanotechnologies to impact on human rights, such as health and a safe environment, has led to the Council of Europe assessing whether there should be guidelines or a charter on nanomaterials [37]. Tanja Kleinsorge, Head of the Secretariat of the Committee on Social Affairs, Health and Sustainable Development, Parliamentary Assembly of the Council of Europe, offered an analysis of possible avenues for a pan-European treaty or a series of reciprocal agreements, as recommended in the Parliamentary Assembly report ‘Nanotechnology: balancing benefits and risks to public health and the environment’ [37].
Steffi Friedricks from the Nanotechnologies Industry Association suggested that there are no health endpoints that have been reported for nanomaterials that have not previously been observed with chemicals. Citing the recent paper of Prof. Ken Donaldson that called into question nano-specific effects (although confirming health impacts from particulate matter per se) [38], her argument was that there are no ‘new’ cytokines released in response to nanomaterials, and that while nanomaterials might alter the localization and distribution of chemicals, the final impacts are well known, i.e. oxidative stress, etc. This led to challenges from the audience, including a listing by Nel of some features of nanomaterials that are not present for chemicals or micrometre-scale particles, such as (i) band-gap effects which for several nanomaterials have been shown to overlap with the conductance bands present in cells [28]; (ii) aspect ratio, as well as the fact that (iii) chemicals do not acquire a ‘corona’ of biomolecules or environmental macromolecules whose composition depends on the surroundings [4], and indeed this corona can evolve as the nanomaterials' surroundings themselves evolve [39]. Indeed, it is worth highlighting that even knowing all that we do about asbestos, there is still no single toxicology assay that would predict mesothelioma.
Andrew Maynard (University of Michigan) outlined innovative approaches to emergent risks where he discussed the opportunities provided by nanomaterials to also develop novel frameworks for responsible research and innovation. He discussed the ideas of acceptable risk and acceptable safety, which need to be at the forefront of discussions, and indeed linked to monetization of risk and who will ultimately bear the cost of risk (i.e. insurance). Maynard highlighted that while nanotechnologies have been a pioneer of responsible innovation, some mis-steps have been made along the way, and we are now a decade on in this debate [40]. He suggested that risk management strategies and regulation must be grounded in plausibility and based on concepts of ‘likely to effect’ rather than ‘might effect’, which he illustrated as shown in figure 6. Thus, while the range of novel physico-chemical parameters is broad, not all of these will lead to novel mechanisms of interaction, and of those not all will lead to novel health impacts, not all of which will be harmful. Thus, we need to ensure that our regulatory (legal) frameworks allow for innovation and exploitation of the space for nanomedicine and other approaches, while ensuring that the small minority of cases that are harmful are captured and managed appropriately.
Figure 6.
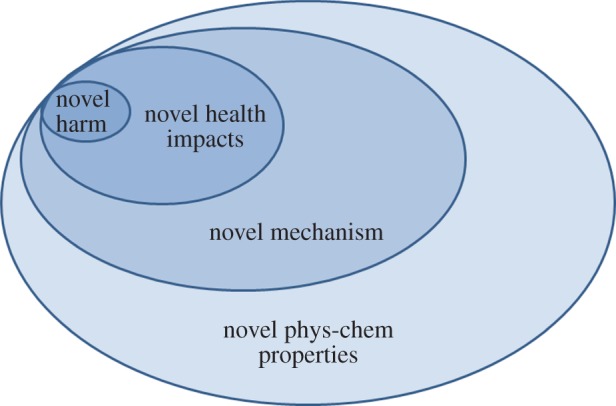
Illustration of the relevant parameter space for nanoregulation and nanorisk management. Thus, despite the relatively large parameter space of new materials, it is likely that truly novel harm will occur only in very few cases. Focusing on identifying these is critical. (Online version in colour.)
4. Analysis and integration: how can the benefits of nanotechnology applications in industry be realized while minimizing the unquantified risks?
A regulatory history of dealing with the unknown or unquantified risks associated with new technologies is consistent with the remarks by Steffi Friedrichs, representative of the Nanotechnology Industries Association (NIA). As Friedrichs noted when discussing nanosafety by design, only some of the regulatory challenges are nano-specific: nano-enabled innovations have become examples of both the new vistas and the limits of existing frameworks. Andre Nel (UCLA) suggested that there are several new alternative approaches to nanotechnology regulation, already in use in California. Nel offered his approach as a potential model for regulation by the US Environmental Protection Agency (EPA) and, consistent with the USA's ability to create new regulatory paradigms, as a possible model for global assessments.
Ilise Feitshans (University of Lausanne), rapporteur on policy outcomes of the meeting, outlined in her sum-up session the reasons why the answer is yes to all three of the regulatory questions posed at the meeting, i.e. are there gaps in the law, is there existing law governing nanomaterials and will we have new law?
First, many administrative agencies have attempted to stretch regulatory jurisdiction to grasp nanotechnology, in many places. The question of whether REACH (Registration, Evaluation and Authorisation of Chemicals: EU Chemical legislation) covers nanomaterials has been answered by the European Union itself by modifying the law to include previously considered safe materials such as carbon, in the form of carbon nanotubes and graphene [41]. So too, the US EPA has hotly debated regulations for nanosilver [42] and the US Food and Drug Administration (FDA) has attempted to regulate nanomaterials as a medical ‘device’ without regard to the possible changes in chemical properties of nanoparticles due to size, and without a clear definition of nanomaterials involved. Second, while there are aspects of nanomaterials that may require special or new regulation, there are more areas that may prove to be safe and therefore additional regulations would be surplus, merely clogging the administrative system [43]. Third, the reality that risks are unquantified has not stemmed the tide of nanoregulation: every nation and many international organizations have established or are drafting nanotechnology administrative schemes often for no other reason than the popularity of the term nanotechnology and the need to be seen by their populous to be taking some action.
Feitshans observed that this policy judgement to regulate may be unfair to the fledgling nanotechnology applications in commerce that may later prove to have low risks, or to the consumers who will feel a new comfort zone because they believe that nanomaterials are regulated. Although the implications for bioaccumulation and nanotoxicity remain unknown, the state of the art of legislative drafting and litigation has progressed in the past decade, especially in the USA [44]. Now, the real regulatory problem is the potential conflict of rapidly written laws. Noting that governments from the size of the municipality of Cambridge, MA, USA to the Kingdom of Morocco see fit to regulate applications of nanotechnology, Feitshans emphasized that it is not the functionality or context of nanomaterials themselves that will be the driver for these policies. Furthermore, it is not a matter of excellence and forethought in legislative drafting because even the best written laws may not overcome a challenge based on conflicts of law. Harmonization of these laws is, therefore, key to good regulation.
The result of a challenge to jurisdiction will be an admixture of contractual obligations that offer a choice of law to agreeing parties, principles of conflicts of law that apply internationally and political will. Therefore, according to Feitshans, the key issue from a regulatory standpoint has moved beyond the initial questions of whether to regulate, into the more complex realm of which system of laws will prevail in the context of globalization. At the time of the Royal Society meeting, therefore, the key law and policy question that is emerging in nanotechnology is that of how to achieve global harmonization. Pointing to the United Nations (UN) ‘global efforts to unify chemical safety labels, training and handling across every nation in a harmonized system’ (Global Harmonised System, GHS), Feitshans suggested that harmonization is one form of regulation that promotes commerce, as the alternative to a swamp of well-intended but possibly conflicting laws, in an era of increasing globalization [45]. She offered the notion that perhaps not immediately, but in the next 5–10 years, nanotechnology regulators will become enlightened to the need for harmonization in order to make workable the best parts of their own system [46]. Therefore, she views as inevitable an internationally integrated unified system for nanotechnology regulation, whether created or opposed by the UN [47].
An interesting aside is that the conclusions of a recent Royal Society report on an entirely different topic (that of Neuroscience and the law [48]) seem to be equally applicable to nanosafety and bio-nano interactions and its status relative to law: simply replacing ‘neuro’ with ‘nano’ in the concluding statements from that report also summarizes the current state of nanosafety, 10 years on from the original Royal Society report on nanotechnologies [1]. Thus:
— There is a big gap between research conducted by (nano)scientists and the realities of the day to day work of the justice system.
— There is currently no forum in (the UK) for bringing together (nano)scientists and legal professionals to explore areas of mutual interest.
— Professionals at all stages of the legal system who might encounter (nano)science should understand some of the key principles on which it is based; the limitations to what studies can tell us; and some of the generic challenges of its application.
— Lawyers and judges in England and Wales often have no training in scientific principles. Undergraduates in (nano)science are not necessarily taught about the societal implications of the discipline [48].
In both situations (neuro- and nanosciences), a plethora of new laws are emerging with no clear indicators to which of those laws will become the prevailing rule. Only a small fraction of nanotechnology's emerging risks, however, can be subjected to regulation. And, the few regulations that have begun to emerge are fraught with confusion, often exacerbated by litigation [42]. There is a small window of opportunity for preventive use of scientific principles under law to manage risk and protect public health [44]. It is therefore equally unclear what nanotechnology will mean for governance at the national and international level, as government and private resources are blended to create new methods of regulation once global harmonization occurs. ‘Nanotechnology is neither the first nor (hopefully!) the last such example in a long chain of precedents when humans have embraced new technologies mindful of risk, in the hope of furthering the progress of all humanity. Whether such goals can be achieved using old models for governance, or requires new approaches that synthesize many sets of ideals from various perspectives and from many nations, remains to be seen’ [32, p. 34].
5. Analysis and integration: how can the scientific community support/champion the use of scientific principles under law to manage risk and protect public health?
In her summary statement, Technical Rapporteur Dr Iseult Lynch (University of Birmingham), suggested that although concepts of the bio-nano interface as the mediator of nanoscale interactions with biology are now well established, there remains much to be done to link biomolecule/macromolecule coronas to health effects. A first step in this might be to codify it, i.e. to move beyond lists of identified proteins to identification of functionally modified domains and their (signalling) implications, and then, to identify key domains that can be screened as biomarkers of exposure and health impacts. Some significant progress towards this approach includes fingerprinting the corona of nanomaterials [49] and understanding the impact of adsorbed protein conformation on receptor activation [50]. Lynch, therefore, posed the question: can we go further towards combinatorial and personalized medicine approaches, such as bar codes of individual health based on protein signatures—e.g. detect changes in protein phosphorylation for example?
Lynch also highlighted important roadblocks to rapid progress, both for the scientific community and policymakers at the interface of science and policy: there is an urgent need for agreed terminology in this arena, especially concerning the protein corona, its evolution and consequent ageing of the corona and nanomaterials themselves. There is also a significant challenge in terms of data quality: with the increasing pace of publications, how can one discern the relevant from the plain wrong? Again, this profound dilemma is as equally relevant to scientists as policymakers, regulators and legal practitioners.
Another significant challenge is the parallel development of the fields of nanomedicine and nanosafety, with nanomedicine focusing primarily on the applications, and often not being fully cognisant of the significance of much of the emerging knowledge in the nanosafety or nanotoxicology arena, which has traditionally been seen as less exciting science. This has resulted in many of the key findings from nanosafety research, such as the importance of detailed characterization of nanomaterials in the exposure medium as the basis for realistic (including often nonlinear) dose-response profiles [51], the potential for dye to elute from (commercially and in-house) fluorescently labelled nanoparticles [52] and the impact of particle ageing on observed impacts, being slow to be adopted in nanomedicine research, further proliferating poor science and hampering the translation of scientific principles to policy.
One of the discussion highlights, alluded to already, relates to whether there are differences between nanomaterials and chemicals in terms of their impacts and thus regulatory requirements. From the scientific perspective, there are clear differences related to surface area (although this obviously also includes ambient combustion particulates) and related to manipulation of aspect ratio, band gap and surface strain. Overall, there is consensus in the scientific community that the main challenge with nanomaterials is the convergence of multiple complexities simultaneously, such as surface interactions (bio-nano interface), small size and thus enhanced accessibility to living organisms, and quantum effects [53], and as such understanding their behaviour and reducing it to simple quantitative structure–activity relationships which are the basis of regulation of chemicals and pharmaceuticals, is non-trivial.
Finally, and related to the convergence issue, Lynch alluded to the fact that as an enabling technology, the uses of nanotechnologies are less confined than those of chemicals: while for chemicals and ultrafine particles, exposure patterns and exposed cohorts can be mapped easily, the presence of nanomaterials is already ubiquitous in a vast number of products (1600+ manufacturer-identified nanotechnology-based consumer products were introduced to the market [54]). Since consumers are exposed via multiple routes, and even potentially regulated products are available to buy via the Internet, occupational exposure may be less of an issue than consumer exposure in the future. If so, consumer exposure has very significant consequences for decisions about how we manage and track exposure over consumer lifetimes. Here perhaps nanomedicine offers an opportunity, via tracking of nanomedical interventions in patients.
6. Conclusion: the challenges of nanotechnology's revolutionary change
The Royal Society conference ‘Bio-nano interactions: new tools, insights and impacts' provided a stimulating and timely look at new understanding regarding the role of bio-nano interactions and the interface between nanomaterials and living systems from two viewpoints: translating potential nanomedicine applications into the clinic, coupled with an assessment of their safety and science-based pathways for regulating the safety of such applications.
Political aspects of nanotechnology embrace questions of potential liability, informed consent for biomedical nanotechnology applications, end-user needs to understand risk and to have access to information, and marketing issues pleasing consumers with attractive and informative labelling.
Law and science have partnered together in the recent past to solve major public health issues. Historically, this partnership between law and science enables policymakers to write regulatory programmes that incubate new industries and advance human development using new technologies, in the face of unknown but great risks [55]. Together, law and science have created and implemented policies that serve the greater social good, ranging from preventing the threat of bankruptcy in the asbestos industry to averting the threat of nuclear holocaust. Lessons learned from the late twentieth century initiatives that funded ‘Big Science’ teach us that governments can use regulatory programmes to supervise and promote risky new technologies, without creating a new race of genetically engineered monsters or blowing up the whole world [3].
7. Recommendations for action: bringing order to regulatory chaos
A prescient approach to developing nanoregulation and nano-governance frameworks will look to the future to the methods for creating a flexible framework for regulation that will be transnational, embracing many types of nanotechnology applications across industries and applying to a vast variety of substances, and building scientific knowledge into the process.
Harmonizing law of nanotechnology is important because commerce will be paralysed by the conflict of law. The best approach will harmonize many laws from several nations, as well as across disciplines such as environmental sciences, toxicology, medicine and public health. To be effective, this approach will be transparent because effective systems require stakeholder participation in order to create workable laws that will address emerging risks without blocking the growth of invaluable new industries and commerce.
Categorization of scientific data regarding the positive and negative impacts of nanotechnologies in a range of applications must be prioritized, as a sensible nanoinformatics framework is a logical next step in the development of applications for nanotechnology to benefit civil society.
Acknowledgements
The Royal Society of London sponsored the meeting ‘Bio-nano interactions: new tools, insights and impacts,’ organized by Dr Michaela Kendall, Prof. Kevin Kendall FRS, Prof. Liam Grover and Dr Paula Mendes, from the University of Birmingham.
Endnotes
This was a satellite meeting to the Discussion Meeting on adhesion (Cell adhesion century: culture breakthrough).
Funding statement
I.L. is funded via the EU FP7 projects NanoMILE (NMP4-LA-2013–310451) and Marie Curie Career Integration grant EcofriendlyNano (PCIG14-GA-2013–631612). I.L.F. is funded under the auspices of the Work, Health and Survival Project, USA and Switzerland.
References
- 1.The Royal Society and Royal Academy of Engineering's report on nanotechnologies—nanoscience and nanotechnologies: opportunities and uncertainties, 29 July 2004 See http://www.raeng.org.uk/news/publications/list/reports/nanoscience_nanotechnologies.pdf.
- 2.Oberdürster G. 2000. Toxicology of ultrafine particles: in vivo studies. Phil. Trans. R. Soc. Lond. A 358, 2719–2740. ( 10.1098/rsta.2000.0680) [DOI] [Google Scholar]
- 3.Corbin IR, Zheng G. 2007. Mimicking nature's nanocarrier: synthetic low-density lipoprotein-like nanoparticles for cancer-drug delivery. Nanomedicine 2, 375–380. ( 10.2217/17435889.2.3.375) [DOI] [PubMed] [Google Scholar]
- 4.Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. 2010. What the cell ‘sees’ in bionanoscience. J. Am. Chem. Soc. 132, 5761–5768. ( 10.1021/ja910675v) [DOI] [PubMed] [Google Scholar]
- 5.European Technology Platform on Nanomedicine. 2013. Contribution of nanomedicine to Horizon 2020. See http://www.etp-nanomedicine.eu/public/press-documents/publications/etpn-publications/etpn-white-paper-H2020/at_download/file.
- 6.Anderson JM. 2001. Biological responses to materials. Annu. Rev. Mater. Res. 31, 81–110. ( 10.1146/annurev.matsci.31.1.81) [DOI] [Google Scholar]
- 7.Santin M, Mikhalovska L, Lloyd AW, Mikhalovsky S, Sigfrid L, Denyer SP, Field S, Teer D. 2004. In vitro host response assessment of biomaterials for cardiovascular stent manufacture. J. Mater. Sci. Mater. Med. 15, 473–477. ( 10.1023/B:JMSM.0000021123.51752.11) [DOI] [PubMed] [Google Scholar]
- 8.Vicario PP, Lu ZJ, Grigorian IA, Schottman T. 2009. A lubricious formulation exhibiting reduced thrombogenicity, cell proliferation, and protein adsorption. J. Biomed. Mater. Res. B 90, 452–560. ( 10.1002/jbm.b.31306) [DOI] [PubMed] [Google Scholar]
- 9.Miller IS, Lynch I, Dowling D, Dawson KA, Gallagher WM. 2010. Surface-induced cell signaling events control actin rearrangements and motility. J. Biomed. Mater. Res. A 93, 493–504. ( 10.1002/jbm.a.32530) [DOI] [PubMed] [Google Scholar]
- 10.Allen LT, et al. 2006. Surface-induced changes in protein adsorption and implications for cellular phenotypic responses to surface interaction. Biomaterials 27, 3096–3108. ( 10.1016/j.biomaterials.2006.01.019) [DOI] [PubMed] [Google Scholar]
- 11.Temchura VV, Kozlova D, Sokolova V, Uberla K, Epple M. 2014. Targeting and activation of antigen-specific B-cells by calcium phosphate nanoparticles loaded with protein antigen. Biomaterials 35, 6098–6105. ( 10.1016/j.biomaterials.2014.04.010) [DOI] [PubMed] [Google Scholar]
- 12.Dautova Y, Kozlova D, Skepper JN, Epple M, Bootman MD, Proudfoot D. 2014. Fetuin-a and albumin alter cytotoxic effects of calcium phosphate nanoparticles on human vascular smooth muscle cells. PLoS ONE 9, e97565 ( 10.1371/journal.pone.0097565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nalluri SK, Berdugo C, Javid N, Frederix PW, Ulijn RV. 2014. Biocatalytic self-assembly of supramolecular charge-transfer nanostructures based on n-type semiconductor-appended peptides. Angew. Chem. Int. Ed. 53, 5882–5887 ( 10.1002/anie.201311158) [DOI] [PubMed] [Google Scholar]
- 14.Zhou M, Ulijn RV, Gough JE. 2014. Extracellular matrix formation in self-assembled minimalistic bioactive hydrogels based on aromatic peptide amphiphiles. J. Tissue Eng. 5, 2041731414531593 ( 10.1177/2041731414531593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNamara LE, Sjöström T, Seunarine K, Meek RMD, Su B, Dalby MJ. 2014. Investigation of the limits of nanoscale filopodial interactions. J. Tissue Eng. 5, 2041731414536177 ( 10.1177/2041731414536177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalby MJ, Gadegaard N, Oreffo ROC. 2014. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 13, 558–569. ( 10.1038/nmat3980) [DOI] [PubMed] [Google Scholar]
- 17.Albuschies J, Vogel V. 2013. The role of filopodia in the recognition of nanotopographies. Sci. Rep. 3, 1658 ( 10.1038/srep01658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin Yavari S, et al. 2014. Bone regeneration performance of surface-treated porous titanium. Biomaterials 35, 6172–6181. ( 10.1016/j.biomaterials.2014.04.054) [DOI] [PubMed] [Google Scholar]
- 19.Sarkar D, et al. 2011. Engineered cell homing. Blood 118, e184 ( 10.1182/blood-2010-10-311464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sridharan R, Karp JM, Zhao W. 2014. Bioengineering tools to elucidate and control the fate of transplanted stem cells. Biochem. Soc. Trans. 42, 679–687. ( 10.1042/BST20130276) [DOI] [PubMed] [Google Scholar]
- 21.Rawson FJ, Yeung CL, Jackson SK, Mendes PM. 2013. Tailoring 3D single-walled carbon nanotubes anchored to indium tin oxide for natural cellular uptake and intracellular sensing. Nano Lett. 13, 1–8. ( 10.1021/nl203780d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawson FJ, Yeung CL, Jackson SK, Mendes PM. 2013. Intracellular ‘wiring’ for real-time cell communication. Nano Rev. 4 ( 10.3402/nano.v4i0.22429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendall M, Ding P, Mackay RM, Deb R, McKenzie Z, Kendall K, Madsen J, Clark H. 2013. Surfactant protein D (SP-D) alters cellular uptake of particles and nanoparticles. Nanotoxicology 7, 963–973. ( 10.3109/17435390.2012.689880) [DOI] [PubMed] [Google Scholar]
- 24.Gerloff K, Pereira DI, Faria N, Boots AW, Kolling J, Förster I, Albrecht C, Powell JJ, Schins RP. 2013. Influence of simulated gastrointestinal conditions on particle-induced cytotoxicity and interleukin-8 regulation in differentiated and undifferentiated Caco-2 cells. Nanotoxicology 7, 353–366. ( 10.3109/17435390.2012.662249). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira DI, et al. 2013. Caco-2 cell acquisition of dietary iron(III) invokes a nanoparticulate endocytic pathway. PLoS ONE 8, e81250 ( 10.1371/journal.pone.0081250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nel AE. 2013. Implementation of alternative test strategies for the safety assessment of engineered nanomaterials. J. Intern. Med. 274, 561–577. ( 10.1111/joim.12109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R, et al. 2013. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano 7, 2352–2368. ( 10.1021/nn305567s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, et al. 2012. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 6, 4349–4368. ( 10.1021/nn3010087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahru A, Ivask A. 2013. Mapping the dawn of nanoecotoxicological research. Acc. Chem. Res. 46, 823–833. ( 10.1021/ar3000212) [DOI] [PubMed] [Google Scholar]
- 30.Oomen AG, et al. 2014. Concern-driven integrated approaches to nanomaterial testing and assessment—report of the NanoSafety Cluster Working Group 10. Nanotoxicology 8, 334–348. ( 10.3109/17435390.2013.802387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horev-Azaria L, et al. 2013. Predictive toxicology of cobalt ferrite nanoparticles: comparative in-vitro study of different cellular models using methods of knowledge discovery from data. Part. Fibre Toxicol. 10, 32 ( 10.1186/1743-8977-10-32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royal Commission on Environmental Pollution. 2008. Novel materials in the environment: the case of nanotechnology. 27th Report presented to Parliament, November 2008. Available at https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/228871/7468.pdf.
- 33.UK DEFRA. 2010. Research into the likelihood and possible pathways of human exposure via inhalation arising throughout the life cycle of a selection of commercially available articles containing carbon nanotubes. CB0423. See http://www.defra.gov.uk/.
- 34.NASA. 2008 Nanotechnology. See http://www.nasa.gov/centers/ames/research/technology-onepagers/nanotechnology-landing.html.
- 35.CDC/NIOSH. 2013. Controlling worker exposures to nanomaterials: NIOSH issues new research-based recommendations. See http://www.cdc.gov/niosh/updates/upd-11-08-13.html.
- 36.Federal Office of Public Health/Federal Office for the Environment. 2008. Guidelines on the precautionary matrix for synthetic nanomaterials, version 1.0, Berne. See http://www.bag.admin.ch/themen/chemikalien/00228/00510/05626/index.html?lang=en.
- 37.Parliamentary Assembly of the Council of Europe (PACE). 2013. Nanotechnology: balancing benefits and risks to public health and the environment Recommendation 2017. See http://assembly.coe.int/ASP/Doc/XrefViewPDF.asp?FileID=19730&Language=EN.
- 38.Donaldson K, Poland CA. 2013. Nanotoxicity: challenging the myth of nano-specific toxicity. Curr. Opin. Biotechnol. 24, 724–734. ( 10.1016/j.copbio.2013.05.003) [DOI] [PubMed] [Google Scholar]
- 39.Albanese A, Walkey CD, Olsen JB, Guo H, Emili A, Chan WC. 2014. Secreted biomolecules alter the biological identity and cellular interactions of nanoparticles. ACS Nano. 8, 5515–5526. ( 10.1021/nn4061012) [DOI] [PubMed] [Google Scholar]
- 40.Maynard AD. 2014. A decade of uncertainty. Nat. Nanotechnol. 9, 159–160. ( 10.1038/nnano.2014.43) [DOI] [PubMed] [Google Scholar]
- 41.Monica JC. 2008. Registration of carbon nanoscale materials required under REACH. Nanotechnology Law Report, posted on 29 October 2008 See http://www.nanolawreport.com/admin/trackback/93636.
- 42.US Government. 2013. Natural Resources Defense Council v. U.S. Environmental Protection Agency, case number 12–70268, in the U.S. Court of Appeals for the Ninth Circuit. Washington, DC: US Government Printing Office.
- 43.Feitshans I. 2012. Forecasting nano law: defining nanotechnology. Nanotechnol. Percept. 8, 17–34. ( 10.4024/N15FE11A.ntp.08.01) [DOI] [Google Scholar]
- 44.Feitshans I. 2011. Legal basis and justification: NIOSH recommendations preventing risk from carbon nanotubes and nanofibers. Public testimony before NIOSH re Current Intelligence Bulletin: occupational exposure to carbon nanotubes and nanofibers. Docket No. NIOSH-161 2011. Available at www.cdc.gov.
- 45.Feitshans I. 2013. Designing an effective OSHA compliance program. Vol. 1, Corporate Compliance Series. Available from www.Westlaw.com.
- 46.Ambassade de France aux Etas-Unis/ADIT. 2013. Gestion des risques liés aux nanotechnologies. Une coopération Europe–Etas Unis. BE Etas-Unis 348. See http://www.bulletins-electroniques.com/actualites/74320.htm.
- 47.Feitshans I. 2013. The international need for harmonization of nanotechnology regulations. Webinar 8 Nov. 2013 for the USA-EU COR See https://us-eu.org/2013-u-s-eu-nanoehs-workshop/.
- 48.The Royal Society. 2011. Brain Waves 4: neuroscience and the law, 13 December 2011 See https://royalsociety.org/policy/projects/brain-waves/responsibility-law/.
- 49.Walkey CD, Olsen JB, Song F, Liu R, Guo H, Olsen DW, Cohen Y, Emili A, Chan WC. 2014. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 8, 2439–2455. ( 10.1021/nn406018q) [DOI] [PubMed] [Google Scholar]
- 50.Fleischer CC, Payne CK. 2014. Secondary structure of corona proteins determines the cell surface receptors used by nanoparticles. J. Phys. Chem. B [Epub ahead of print] PMID: 24779411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tenuta T, Monopoli MP, Kim J, Salvati A, Dawson KA, Sandin P, Lynch I. 2011. Elution of labile fluorescent dye from nanoparticles during biological use. PLoS ONE 6, e25556 ( 10.1371/journal.pone.0025556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fubini B, Ghiazza M, Fenoglio I. 2010. Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology 4, 347–363. ( 10.3109/17435390.2010.509519) [DOI] [PubMed] [Google Scholar]
- 53.Malkiewicz K, Pettitt M, Dawson KA, Toikka A, Hansson SO, Hukkinen J, Lynch I, Lead J. 2011. Nanomaterials in REACH. Project Report 15 August See http://www.steptoe.com/assets/htmldocuments/SKEPP%202011%20Nanomaterials_in_REACH_report_15082011.pdf.
- 54.Woodrow Wilson Centre. 2014 Consumer Products Inventory. An inventory of nanotechnology-based consumer products introduced on the market. See http://www.nanotechproject.org/cpi/.
- 55.Feitshans I. 2014. Forecasting nano law: risk management protecting public health under international law. Unpublished PhD thesis, Geneva School of Diplomacy, Switzerland.