Abstract
A procedure has been devised to isolate 3T3 mouse fibroblasts transformed by simian virus 40 (SV40) that express their transformed phenotype at low (32°C) but not at high (39°C) temperature. Three parameters typical of malignant growth in vitro: (a) high saturation density in culture, (b) ability to form colonies on monolayers of normal 3T3 cells, and (c) lack of contact inhibition of DNA synthesis, are temperature sensitive. These phenotypic changes are fully reversible. The serum requirement for growth appears to be largely unchanged by temperature. These cells seem to owe their behavior to a cellular, rather than to a viral, alteration since after fusion of the temperature-sensitive transformed cells with permissive monkey cells, a procedure that leads to rescue (i.e., multiplication of the virus), wild-type SV40 virus is produced.
Keywords: SV40, 3T3 mouse cells, growth control
Full text
PDF

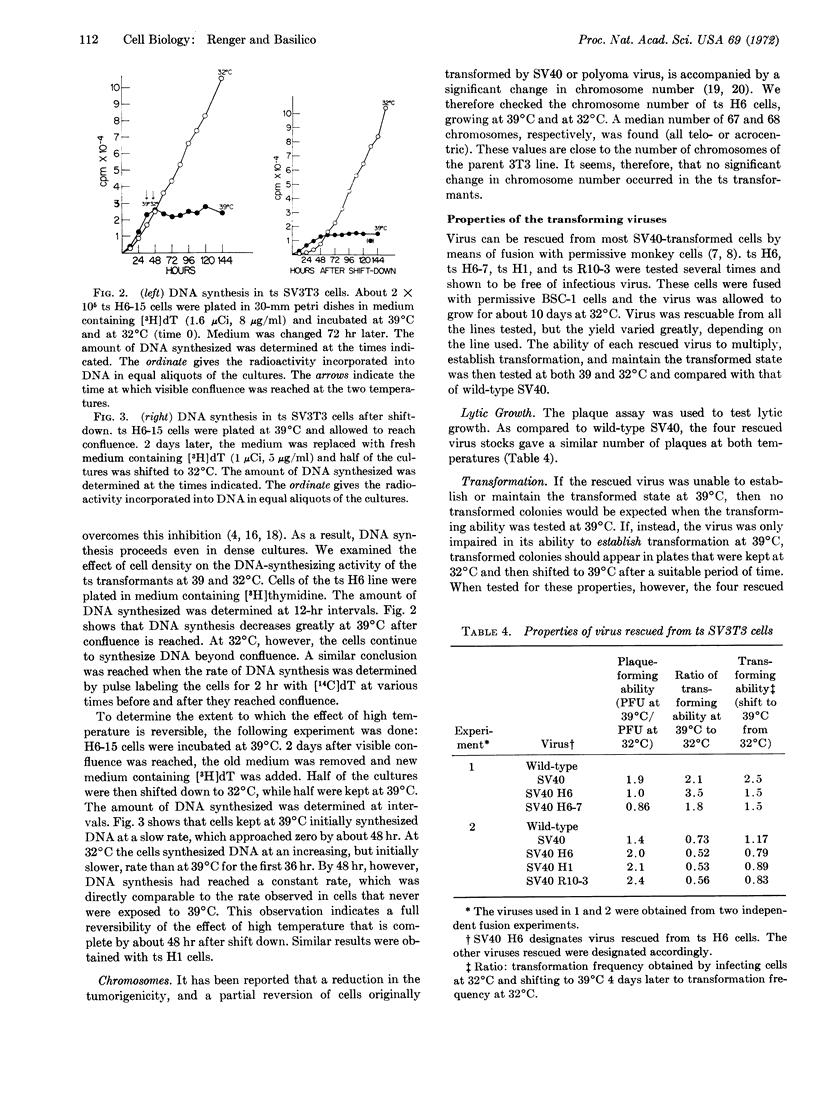


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Freeman A. E. Human sarcoma cells in culture. Identification by colony-forming ability on monolayers of normal cells. Exp Cell Res. 1970 Jul;61(1):1–5. doi: 10.1016/0014-4827(70)90250-8. [DOI] [PubMed] [Google Scholar]
- Basilico C., Matsuya Y., Green H. Origin of the thymidine kinase induced by polyoma virus in productively infected cells. J Virol. 1969 Feb;3(2):140–145. doi: 10.1128/jvi.3.2.140-145.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., Matsuya Y., Green H. The interaction of polyoma virus with mouse-hamster somatic hybrid cells. Virology. 1970 Jun;41(2):295–305. doi: 10.1016/0042-6822(70)90082-6. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Eckhart W. Temperature-dependent properties of cells transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1775–1781. doi: 10.1073/pnas.67.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Dulbecco R., Burger M. M. Temperature-dependent surface changes in cells infected or transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1971 Feb;68(2):283–286. doi: 10.1073/pnas.68.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIED M. CELL-TRANSFORMING ABILITY OF A TEMPERATURE-SENSITIVE MUTANT OF POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Mar;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Habel K. Virus-induced tumor antigens. Curr Top Microbiol Immunol. 1967;41:85–99. doi: 10.1007/978-3-642-46062-3_4. [DOI] [PubMed] [Google Scholar]
- Heidelberger C. Fluorinated pyrimidines. Prog Nucleic Acid Res Mol Biol. 1965;4:1–50. doi: 10.1016/s0079-6603(08)60783-7. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Todaro G. J. Stimulation of cell growth in vitro by serum with and without growth factor. Relation to contact inhibition and viral transformation. Exp Cell Res. 1970 Jan;59(1):137–146. doi: 10.1016/0014-4827(70)90632-4. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Burger M. M. Surface-specific characteristics of a contact-inhibited cell line containing the SV40 viral genome. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1074–1076. doi: 10.1073/pnas.62.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Wolman S., Vogel A. Reversion of virus-transformed cell lines: hyperploidy accompanies retention of viral genes. Nature. 1970 Dec 5;228(5275):938–passim. doi: 10.1038/228938a0. [DOI] [PubMed] [Google Scholar]
- Rabinowitz Z., Sachs L. Control of the reversion of properties in transformed cells. Nature. 1970 Jan 10;225(5228):136–139. doi: 10.1038/225136a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordaro G. J., Green H. An assay for cellular transformation by SV40. Virology. 1964 May;23(1):117–119. doi: 10.1016/s0042-6822(64)80018-0. [DOI] [PubMed] [Google Scholar]
- Watkins J. F., Dulbecco R. Production of SV40 virus in heterokaryons of transformed and susceptible cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1396–1403. doi: 10.1073/pnas.58.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]


