Abstract
Energy metabolism and immunity are characterized as abnormal in schizophrenia. Because these two systems are highly coordinated, we measured expression of prototypic obesogenic and immunogenic genes in freshly harvested PBMC from controls and participants with schizophrenia. We report significant increases in PPARγ, SREBP1, IL-6 and TNFα, and decreases in PPARα and C/EPBα mRNA levels from patients with schizophrenia, with additional BMI interactions, characterizing dysregulation of genes relating to metabolic-inflammation in schizophrenia.
Keywords: Schizophrenia, obesity, inflammation
1. Introduction
Energy metabolism and immunity are both reported to be abnormally regulated in schizophrenia. Abnormal energy metabolism, manifested as obesity and insulin resistance is an accepted major public health concern (McEvoy et al., 2005). More subtly, signatures of a chronic subclinical inflammatory state in schizophrenia are increasingly supported and published findings include increases in pro-inflammatory cytokines (Xiu et al., 2012); and a decrease in anti-inflammatory cytokines (Kim et al., 2009; Potvin et al., 2008).
Obesity can be categorized as a pro-inflammatory phenotype, with adipose tissue sitting at the crossroad of metabolism and immunity. Approximately 40% of the cell population in engorged adipose tissue consists of macrophages, which are activated by the abundance of necrotic adipocytes (Meijer et al., 2011; Shapiro et al., 2011; Weisberg et al., 2006). In parallel, adipocytes can release pro-inflammatory cytokines (Meijer et al., 2011) At the signaling level, both obesogenic and immunogenic tissues share common pathways and co-expressed molecules (Chase and Sharma, 2013), which may serve to coordinate their messages. Taken together, obesity and inflammation can induce a composite state titled metabolic-inflammation (Lumeng and Saltiel, 2011; Miller et al., 2011).
In this study, we examine the transcription of both obesogenic and immunogenic genes for the following reasons. Firstly, there is a dearth of studies examining combined obesogenic and immunogenic molecular gene expression in schizophrenia patients (Mansur et al., 2012; Miller et al., 2011; Na et al., 2014; Song et al., 2011). Secondly, due to the multiple sources of cytokine and adipose molecules found in the serum (originating from adipose, lymphoid, liver and muscle tissue (Ferno et al., 2009; Koutnikova et al., 2003; Raschke and Eckel, 2013), we utilized mRNA from a single, identifiable source: the peripheral blood mononuclear cell (PBMC). Our selection of obesity-related genes is based on known developmental and regulatory properties of PPARγ2, PPARα, C/EBPα and SREBP1. The pro-inflammatory cytokines IL-6 and TNFα, both demonstrated in the literature to be were selected as they are up-regulated in obesity, inflammation and schizophrenia.
2. Methods
2.1 Patient information and clinical measures
Subjects (n=62) were recruited from the University of Illinois at Chicago Medical Center after receiving approval from the IRB, and provided written informed consent. General inclusion criteria for all subjects were: good physical health (with no reported infections), no history of neurological disease or head trauma, no lifetime history of substance/alcohol dependence or recent (2 months) substance abuse, and not pregnant. Healthy individuals (n=31) had no major Axis I disorder (as assessed by SCID interview). Patients with schizophrenia (n=31) were diagnosed by clinical consensus using DSM-IV-TR criteria. At the time of sampling, 71% (n=22) of the patients were inpatients, with the remainder outpatients. All antipsychotic use was converted to both Chlorpromazine (CPZ) units and the Defined Daily Dose (DDD), with two patients unmediated at time of sampling (Gardner et al., 2010; Nose et al., 2008; Rijcken et al., 2003; Wertheimer, 1986). Both BMI and waist circumference were collected on all participants. Tobacco consumption was collected and was categorically coded as yes/no. Patient demographics are presented in Supplemental Table 1.
2.2 PBMC collection
Blood was collected in the morning prior to breakfast. Fresh PBMC were isolated using the Ficoll gradient method (Chase et al., 2013; Gavin et al., 2009; Jayaraman et al., 1999).
2.3 mRNA extraction
Total RNA was isolated using TRIzol reagent (Life Technologies). Samples were treated with DNase (Amersham 27-0514-03) after extraction (Mannhalter et al., 2000).
2.4 Real Time RT-PCR quantification
Total RNA was used to prepare cDNA using the Applied Biosystems High Capacity Archive Kit (#4368813). For detection and measurement of expression, Fermentas Maxima SYBR Green/ROX qPCR Master Mix (#K0222) was used. PCR mixtures were run in triplicate on a Thermo Scientific PikoReal System. Cycle threshold value was used for relative quantification, and all values were normalized to a geometric mean of three housekeeping genes: GAPDH, TFRC and β-Actin (Chase and Sharma, 2012; Vandesompele et al., 2002) Primer sequences are listed in the Supplemental Table 2.
2.5 Data Analysis
We performed a linear regression with each gene of interest as the dependent variable and diagnosis, medication use (in both CPZ and DDD units), age, BMI, sex, diabetes, age of onset of psychotic symptoms and tobacco consumption as the explanatory variables. To measure any interactions with weight “risk”, BMI was separated out into two groups: “normal” weight (BMI = 18.5-24.9) and “risk” (BMI ≥ 25) (World Health Organisation, 1995; Rajkovic et al., 2014; Salazar et al., 2014). This categorization is the most widely accepted predictor of morbidity in the metabolic literature. All mRNA values were tested for normality in SPSS, and variables not exhibiting a normal distribution were natural log transformed.
3. Results
3.1 Diagnosis, demographics and morphometrics
Four obesogenic genes were examined, PPARγ, PPARα, SREBP1 and C/EBPα. Both a diagnosis of schizophrenia (β = 0.30; p = 0.013; Fig. 1A) and a “risk” BMI of 25 or above (β = −0.31; p = 0.007; Fig. 1G) were significant predictors for increased levels of PPARγ mRNA (F2,59 = 8.42, p = 0.001). Further analysis revealed a significant interaction (F1,60 = 6.47, p = 0.014), with overweight patients with schizophrenia exhibiting the highest amounts of PPARγ mRNA.
Fig. 1.
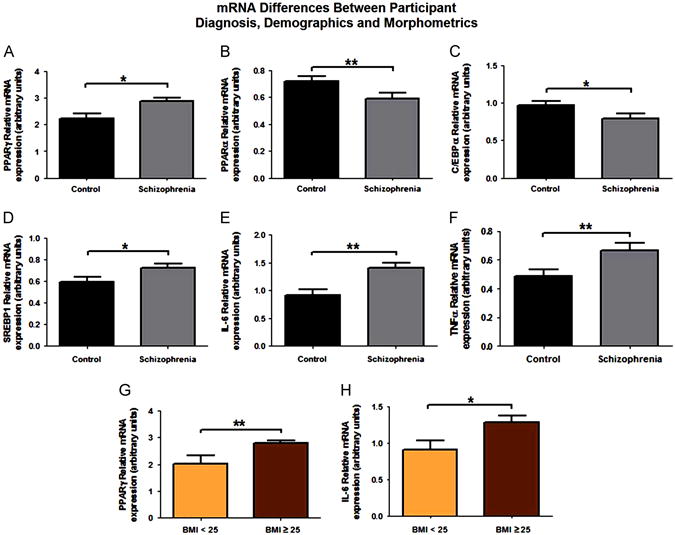
Diagnosis, demographics and morphometrics of obesogenic and immunogenic genes. Significant results are obtained from multiple regressions with each gene of interest as the dependent variable and diagnosis, medication use (in CPZ units), age, BMI, sex, a previous diagnosis of diabetes, age of onset of psychotic symptoms and tobacco consumption as explanatory variables. (A) Increased PPARγ mRNA levels were seen in patients with schizophrenia when compared to normal controls. Patients with schizophrenia had significantly decreased levels of both PPARα (B) and C/EBPα (C) mRNA when compared to normal controls. Increased SREBP1 (D), IL-6 (E) and TNFα (F) mRNA levels were seen in patients with schizophrenia when compared to their normal control counterparts. PPARγ and IL-6 mRNA were also significantly increased in overweight participants (BMI≥25), as determined by multiple linear regression. *p<0.05, **p<0.01.
A diagnosis of schizophrenia was the only predictor of decreases of both PPARα (F1,60=7.97, p=0.006; Fig. 1B) and C/EBPα mRNA levels (F1,60=4.9, p=0.032; Fig. 1C), while a diagnosis of schizophrenia was a significant predictor of increases of SREBP1 mRNA (F1,6 =4.8, p=0.033; Fig. 1D). We note here that SREBP1 mRNA was significantly increased in participants currently taking atypical antipsychotic medication. However, this effect cannot be separated from the previously noted diagnostic effect, given that only patients with schizophrenia were receiving antipsychotics. Additionally, this effect was lost if antipsychotic use was coded in CPZ equivalents.
Additionally, two immunogenic gene expression differences were examined: IL-6 and TNFα. Both a diagnosis of schizophrenia (β=0.38; p=0.002; Fig. 1E) and a “risk” BMI (β=−0.24; p=0.041; Fig. 1H) were significant predictors for increased levels of IL-6 mRNA (F2,59=8.63, p=0.001). Further analysis revealed no significant interactions between BMI and diagnosis. TNFα mRNA was significantly higher in men (t61=3.11; p=0.003). A diagnosis of schizophrenia (β=0.32; p=0.01; Fig. 1F) was also a significant predictor of differences in TNFα mRNA levels. A sex*diagnosis interaction was also significant in the multiple regression, but this interaction would require replication in a diagnostic sample more evenly distributed for the sex demographic.
4. Discussion
The primary findings of this study were the significant variations of both obesity and immune gene expression namely increases in PPARγ, SREBP1, IL-6 and TNFα mRNA and decreases in PPARα and C/EPBα levels, in freshly extracted peripheral blood cells from patients with schizophrenia.
PPARγ is expressed in a variety of tissues, but plays a well-defined role in adipose tissue, and is necessary for the initiation of adipogenesis. Coordinately, in tissues of hematopoietic origin, PPARγ can also decrease expression and activity of pro-inflammatory pathways promoted by the transcription factor NF-κB, and may thereby serve as a mechanism of immune tolerance (Wen et al., 2010). Martinez-Gras et al., found reduced levels of PPARγ protein in PBMC nuclear extracts as well as plasma levels of its endogenous ligand 15d-PGJ2, in patients with schizophrenia (Martinez-Gras et al., 2011).
Generally, C/EPBα is significantly anti-mitotic, and is inhibited by inflammatory stimuli (Ramji & Foka, 2002). In fact, C/EBPα can be down-regulated at the transcriptional level by recombinant cytokines such as IL-6, IL-1, and TNFα (Akira et al., 1990; Alam et al., 1992; Poli, 1998). Taken together, increased levels of IL-6 and TNFα and decreases in C/EBPα mRNA are suggestive of interactions between obesogenic and immunogenic function in patients with schizophrenia. Our results with IL-6 and TNFα mRNA levels as a proxy for a pro-inflammatory state are consistent with the substantial literature indicating that pro-inflammatory cytokines are elevated in both schizophrenia and obesity (Eder et al., 2009; Potvin et al., 2008; Song et al., 2009). However, we interpret the sex differences seen in TNFα mRNA levels with caution, as our cohort had significantly more males with schizophrenia; thus any statistical difference seen here could be a result of an unbalanced sex ratio.
Waist circumference and BMI, which was found to be related to increases in both PPARγ and IL-6 mRNA levels, has been shown to be the best indicator of the presence and intensity of an inflammatory response and a metabolic syndrome (Rogowski et al., 2010). IL-6 has a PPRE sequence within its promoter, indicating PPAR binding, dimerization and manipulation of IL-6 gene transcription (Denner et al., 2012; Ramji & Foka, 2002). The confluence of these two systems is also predicted by a common epigenetic mechanism involved in their interaction with the environment (Chase and Sharma, 2013).
The findings of this study have several limitations. First, our cohort consisted of significantly more male patients with schizophrenia. Additionally, in our naturalistic clinical study we did not control for dose, type or duration of antipsychotic. Data indicates a significant effect of atypical antipsychotics on SREBP1 mRNA that would be useful to confirm using a more controlled paradigm to separate a diagnostic from pharmacological effect in PBMCs, as the literature has indicated the ability of atypical antipsychotics to induce both inflammatory and obesogenic gene expression (Klemettila et al., 2014; Sarvari et al., 2014). Finally, many diseases and medical conditions can directly affect metabolism and inflammation, including, but not limited to autoimmune diseases, lipid disorders, the use of statins or even the duration of a diabetes diagnosis. This data was not collected systematically, and could not be controlled for in our data analysis.
Supplementary Material
Supplemental Table 1. Means and standard deviations of participant demographics comparing healthy controls and patients with schizophrenia in PBMC samples. Statistical differences between the various parameters were determined by a either a One-way ANOVA or a Student's t-test, and results were presented where performed.
Supplemental Table 2. Real-time RT-PCR mRNA primers and gene function.
Highlights.
Examined both obesogenic and immunogenic gene expression in the same sample.
Utilized mRNA from discrete tissue (peripheral lymphocytes) to avoid interpretation from multiple sources.
Discovered dysregulated expression of both obesogenic and immunogenic genes in schizophrenia when compared to normal controls.
Acknowledgments
This work was supported in part by PHS grant (NIH) R01MH094358 (RPS)
Abbreviations
- PBMC
fresh peripheral blood mononuclear cells
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, Edition IV-TR
- CPZ
Chlorpromazine
- DDD
defined daily dose
Footnotes
Contributors: Kayla A. Chase (KAC), Cherise Rosen (CR), Hannah Gin (HG), Olivia, Bjorkquist (OB), Benjamin Feiner (BF), Sean Conrin (SC), Robert Marvin (RM) and Rajiv P. Sharma (RPS).
Conception of study – KAC and RPS
Data collection, entry and analysis – KAC, CR, HG, OB, BF, SC, RM and RPS
Interpretation of results and manuscript drafting – KAC, CR, OB, RPS, Review of manuscript – KAC, OB, BF, RPS
Conflict of interest statement: There are no conflicts of interest.
Financial disclosure statement: There are no financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, et al. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. The EMBO Journal. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam T, An MR, Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. The Journal of Biological Chemistry. 1992;267:5021–5024. [PubMed] [Google Scholar]
- Chase KA, Sharma RP. Epigenetic developmental programs and adipogenesis: Implications for psychotropic induced obesity. Epigenetics: Official Journal of the DNA Methylation Society. 2013;8 doi: 10.4161/epi.26027. [DOI] [PubMed] [Google Scholar]
- Chase KA, Gavin DP, Guidotti A, Sharma RP. Histone methylation at H3K9: Evidence for a restrictive epigenome in schizophrenia. Schizophrenia Research. 2013;149:15–20. doi: 10.1016/j.schres.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase KA, Sharma RP. Nicotine induces chromatin remodelling through decreases in the methyltransferases GLP, G9a, Setdb1 and levels of H3K9me2. The International Journal of Neuropsychopharmacology. 2012:1–10. doi: 10.1017/S1461145712001101. [DOI] [PubMed] [Google Scholar]
- Denner LA, Rodriguez-Rivera J, Haidacher SJ, Jahrling JB, Carmical JR, Hernandez CM, et al. Cognitive enhancement with rosiglitazone links the hippocampal PPARgamma and ERK MAPK signaling pathways. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32:16725–35a. doi: 10.1523/JNEUROSCI.2153-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflammation Research: Official Journal of the European Histamine Research Society. 2009;58:727–736. doi: 10.1007/s00011-009-0060-4. [DOI] [PubMed] [Google Scholar]
- Ferno J, Vik-Mo AO, Jassim G, Havik B, Berge K, Skrede S, et al. Acute clozapine exposure in vivo induces lipid accumulation and marked sequential changes in the expression of SREBP, PPAR, and LXR target genes in rat liver. Psychopharmacology. 2009;203:73–84. doi: 10.1007/s00213-008-1370-x. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. The American Journal of Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Gavin DP, Kartan S, Chase KA, Jayaraman S, Sharma RP. Histone deacetylase inhibitors and candidate gene expression: An in vivo and in vitro approach to studying chromatin remodeling in a clinical population. Journal of Psychiatric Research. 2009;43:870–876. doi: 10.1016/j.jpsychires.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Castro M, O'Sullivan M, Bragdon MJ, Holtzman MJ. Resistance to fas-mediated T cell apoptosis in asthma. Journal of Immunology. 1999;162:1717–1722. [PubMed] [Google Scholar]
- Kim YK, Myint AM, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Cytokine changes and tryptophan metabolites in medication-naive and medication-free schizophrenic patients. Neuropsychobiology. 2009;59:123–129. doi: 10.1159/000213565. [DOI] [PubMed] [Google Scholar]
- Klemettila JP, Kampman O, Seppala N, Viikki M, Hamalainen M, Moilanen E, et al. Association study of the HTR2C, leptin and adiponectin genes and serum marker analyses in clozapine treated long-term patients with schizophrenia. European Psychiatry: The Journal of the Association of European Psychiatrists. 2014;14 doi: 10.1016/j.eurpsy.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Koutnikova H, Cock TA, Watanabe M, Houten SM, Champy MF, Dierich A, Auwerx J. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14457–14462. doi: 10.1073/pnas.2336090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of Clinical Investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhalter C, Koizar D, Mitterbauer G. Evaluation of RNA isolation methods and reference genes for RT-PCR analyses of rare target RNA. Clinical Chemistry and Laboratory Medicine. 2000;38:171–177. doi: 10.1515/CCLM.2000.026. [DOI] [PubMed] [Google Scholar]
- Mansur RB, Zugman A, Asevedo EM, da Cunha GR, Bressan RA, Brietzke E. Cytokines in schizophrenia: Possible role of anti-inflammatory medications in clinical and preclinical stages. Psychiatry and Clinical Neurosciences. 2012;66:247–260. doi: 10.1111/j.1440-1819.2012.02354.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Gras I, Perez-Nievas BG, Garcia-Bueno B, Madrigal JL, Andres-Esteban E, Rodriguez-Jimenez R, et al. The anti-inflammatory prostaglandin 15d-PGJ2 and its nuclear receptor PPARgamma are decreased in schizophrenia. Schizophrenia Research. 2011;128:15–22. doi: 10.1016/j.schres.2011.01.018. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the clinical antipsychotic trials of intervention effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophrenia Research. 2005;80:19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M, et al. Human primary adipocytes exhibit immune cell function: Adipocytes prime inflammation independent of macrophages. PloS One. 2011;6 doi: 10.1371/journal.pone.0017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biological Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2014;48:277–286. doi: 10.1016/j.pnpbp.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Nose M, Tansella M, Thornicroft G, Schene A, Becker T, Veronese A, et al. Is the defined daily dose system a reliable tool for standardizing antipsychotic dosages? International Clinical Psychopharmacology. 2008;23:287–290. doi: 10.1097/YIC.0b013e328303ac75. [DOI] [PubMed] [Google Scholar]
- Physical status: The use and interpretation of anthropometry Report of a WHO expert committee. Vol. 854. World Health Organization; 1995. pp. 1–452. (Technical Report Series). [PubMed] [Google Scholar]
- Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. The Journal of Biological Chemistry. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: A systematic quantitative review. Biological Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Rajkovic N, Zamaklar M, Lalic K, Jotic A, Lukic L, Milicic T, et al. Relationship between obesity, adipocytokines and inflammatory markers in type 2 diabetes: Relevance for cardiovascular risk prevention. International Journal of Environmental Research and Public Health. 2014;11:4049–4065. doi: 10.3390/ijerph110404049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramji DP, Foka P. CCAAT/enhancer-binding proteins: Structure, function and regulation. The Biochemical Journal. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke S, Eckel J. Adipo-myokines: Two sides of the same coin-mediators of inflammation and mediators of exercise. Mediators of Inflammation. 2013:320724. doi: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijcken CA, Monster TB, Brouwers JR, de Jong-van den Berg LT. Chlorpromazine equivalents versus defined daily doses: How to compare antipsychotic drug doses? Journal of Clinical Psychopharmacology. 2003;23:657–659. doi: 10.1097/01.jcp.0000096247.29231.3a. [DOI] [PubMed] [Google Scholar]
- Rogowski O, Shapira I, Bassat OK, Chundadze T, Finn T, Berliner S, Steinvil A. Waist circumference as the predominant contributor to the micro-inflammatory response in the metabolic syndrome: A cross sectional study. Journal of Inflammation. 2010;7 doi: 10.1186/1476-9255-7-35. 9255-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar MR, Carbajal HA, Espeche WG, Balbin E, Aizpurua M, Marillet AG, Reaven GM. Do differences in waist circumference modify the relationships among body mass index, insulin resistance, and related cardiometabolic risk factors in apparently healthy women? Journal of the American College of Nutrition. 2014;33:32–38. doi: 10.1080/07315724.2014.869982. [DOI] [PubMed] [Google Scholar]
- Sarvari AK, Vereb Z, Uray IP, Fesus L, Balajthy Z. Atypical antipsychotics induce both proinflammatory and adipogenic gene expression in human adipocytes in vitro. Biochemical and Biophysical Research Communications. 2014;450:1383–1389. doi: 10.1016/j.bbrc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Shapiro H, Lutaty A, Ariel A. Macrophages, meta-inflammation, and immuno-metabolism. The Scientific World Journal. 2011;11:2509–2529. doi: 10.1100/2011/397971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biological Psychiatry. 2009;65:481–488. doi: 10.1016/j.biopsych.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Song YY, Kim KR, Park JY, Lee SY, Kang JI, Lee E, et al. Associated factors of quality of life in first-episode schizophrenia patients. Psychiatry Investigation. 2011;8:201–206. doi: 10.4306/pi.2011.8.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of Clinical Investigation. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Li Y, Liu Y. Opposite action of peroxisome proliferator-activated receptor-gamma in regulating renal inflammation: Functional switch by its ligand. The Journal of Biological Chemistry. 2010;285:29981–29988. doi: 10.1074/jbc.M110.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheimer AI. The defined daily dose system (DDD) for drug utilization review. Hospital Pharmacy. 1986;21:233–4. 239–41, 258. [PubMed] [Google Scholar]
- Xiu MH, Chen da C, Wang D, Zhang K, Dong A, Tang W, et al. Elevated interleukin-18 serum levels in chronic schizophrenia: Association with psychopathology. Journal of Psychiatric Research. 2012;46:1093–1098. doi: 10.1016/j.jpsychires.2012.04.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Means and standard deviations of participant demographics comparing healthy controls and patients with schizophrenia in PBMC samples. Statistical differences between the various parameters were determined by a either a One-way ANOVA or a Student's t-test, and results were presented where performed.
Supplemental Table 2. Real-time RT-PCR mRNA primers and gene function.


