Abstract
Periodontitis is a dysbiotic inflammatory disease with an adverse impact on systemic health. Recent studies have provided insights into the emergence and persistence of dysbiotic oral microbial communities, which can mediate inflammatory pathology at local as well as distant sites. This Review discusses mechanisms of microbial immune subversion that tip the balance from homeostasis to disease in oral or extraoral sites.
Periodontitis is a chronic inflammatory disease that compromises the integrity of the tooth-supporting tissues, that is gingiva, periodontal ligament and alveolar bone, collectively known as the periodontium1(BOX 1). Known since antiquity, periodontitis became prevalent after the domestication of plants and animals in Neolithic societies (≈10,000 years ago) when the oral microbiota underwent a distinct compositional shift — with increased frequency of Porphyromonas gingivalis and other periodontitis-associated species — compared with earlier hunter-gatherer societies2. In its severe form, which afflicts 8.5% of U.S. adults3, periodontitis may not only cause tooth loss, but can also affect systemic health by increasing the patients’ risk for atherosclerosis, adverse pregnancy outcomes, rheumatoid arthritis, aspiration pneumonia and cancer4-9.
Box 1. Periodontitis and susceptibility factors.
Periodontitis has a complex etiology acting at multiple levels: at the microbial level, based on the presence of dysbiotic microbial communities with potential for destructive inflammation; at the host level, based on genetic factors that may predispose to or protect from disease; and at the level of environmental factors and systemic health status that modify the host response in either protective or destructive direction151. Accordingly, dysbiosis by itself may not necessarily precipitate periodontitis, but it could initiate disease in the context of other risk factors associated with host genotype, stress, diet or risk-related behaviour such as smoking92,152-156. For instance, there might be individuals who can tolerate dysbiosis by virtue of their intrinsic immuno-inflammatory status; hyporesponsive or lack-of-function polymorphisms in immune response genes could attenuate inflammation and prevent development of overt disease20. Bacterial dysbiosis will only lead to disease in susceptible hosts as there are individuals who remain periodontally healthy despite massive tooth-associated biofilm formation, whereas others with less biofilm accumulation are extremely susceptible to periodontitis154. Although a genetic basis for periodontitis is supported by twin studies and familial aggregation of severe forms of the disease, the implication of identified specific genes — such as IL1, IL6, TNF, FCGR2A, C5, CD14, WNT5A — is debatable153-155. This uncertainty is probably attributed to the fact that chronic periodontitis is a polygenic disease, in which multiple genes contribute cumulatively to the overall disease risk (or protection) by influencing the host immune response and the microbiota composition and structure155. This notion stands in stark contrast to monogenic forms of the disease, such as aggressive periodontitis in young patients with leukocyte adhesion deficiency, where a single gene (ITGB2, encoding integrin β-2) invariably precipitates periodontal disease57.
A triadic group of oral anaerobic bacteria that comprises P. gingivalis, Treponema denticola and Tannerella forsythia have traditionally been considered as causative agents of periodontitis, based on their virulence properties and strong association with diseased sites10. However, recent advances from metagenomic, metatranscriptomic and mechanistic studies11-16 are consistent with a new model of periodontal disease pathogenesis, which suggests that a more diverse periodontitis-associated microbiota than previously thought is involved in disease. In this model, disease results not from individual pathogens but rather from polymicrobial synergy and dysbiosis, which perturbs the ecologically balanced biofilm associated with periodontal tissue homeostasis17-19(FIG.1). The dysbiosis of the periodontal microbiota signifies an imbalance in the relative abundance or influence of microbial species, which mediate distinct roles that synergize to shape a pathogenic entity that can cause disease in oral or extraoral tissues of susceptible individuals6,8,11,20. In this new context of pathogenesis, the roles of individual bacteria and their interactions with host need to be re-evaluated.
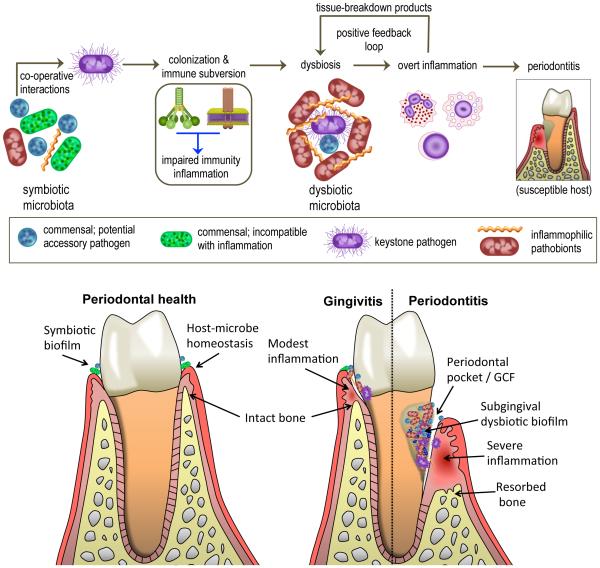
Figure 1. Polymicrobial synergy and dysbiosis in periodontitis.
Periodontitis is induced in susceptible hosts by a polymicrobial community, in which different members fulfil distinct roles that converge synergistically to cause destructive inflammation. Keystone pathogens, the colonization of which is facilitated by accessory pathogens, initially subvert the host response leading to a dysbiotic microbiota, in which pathobionts over-activate the inflammatory response and cause periodontal tissue destruction, including resorption of the supporting alveolar bone. Inflammation and dysbiosis positively reinforce each other because inflammatory tissue breakdown products are used as nutrients by the dysbiotic microbiota. The lower panel depicts the progression from periodontal health (swallow gingival crevice; ≤2 mm) to gingivitis (periodontal inflammation without bone loss; gingival crevice ≤3 mm) to periodontitis (formation of periodontal pockets ≥4 mm and inflammatory bone loss). Inflammation-induced collagenolytic enzymes can contribute to loss of tissue attachment to the teeth and the deepening and ulceration of the pockets (up to 10-12 mm covering a surface area of 8-20 cm2), which serve as a niche that can harbour 108 to 1010 bacteria feeding on the inflammatory spoils (for example collagen peptides, haem-containing compounds) carried with the gingival crevicular fluid (GCF) that bathes the pocket.
Central to the new model of pathogenesis, and constituting the main theme of this Review, is the active bacterial subversion of the host immune response in ways that enables pathogen persistence in the local inflammatory environment of periodontitis and induction of pathology or complications at systemic sites. We discuss evidence and mechanisms whereby periodontal organisms disseminate from their oral habitat to distant sites, including to atherosclerotic plaques, the lungs and placenta where they can disrupt immune surveillance and homeostasis to promote or accelerate pathogenic processes9,21-29. Moreover, P. gingivalis in particular is examined as a potential cause for the generation of autoantibodies in rheumatoid arthritis5,30-33. Understanding how oral pathogens misdirect the host immune response can provide novel mechanistic insights into the pathogenesis of periodontitis and associated systemic conditions as well as reveal new therapeutic targets.
Microbial synergy and dysbiosis
The transition from periodontal health to disease is associated with a dramatic shift from a symbiotic microbial community — composed mostly of facultative bacterial genera such as Actinomyces and Streptococci — to a dysbiotic microbial community structure composed mainly of anaerobic genera from the phyla Firmicutes, Proteobacteria, Spirochaetes, Bacteroidetes and Synergistetes. The dysbiotic oral microbiota is enriched in virulence factors and adapted to thrive in an inflammatory environment11-14,34. The predominant habitat of periodontitis-associated bacteria is the subgingival crevice, where the bacteria are found in distinct microenvironments; the tooth-associated biofilm, the gingival crevicular fluid and the epithelium lining the crevice19 (FIG. 1). The subgingival environment is rich in immune and inflammatory mediators and provides unique challenges and opportunities for the bacteria35-37. Periodontal health requires a controlled immuno-inflammatory state that can maintain host–microbe homeostasis in the periodontium38. However, in periodontitis, the host immune response is dysregulated — either because it is subverted by the microbial community or because of host immunoregulatory defects — and is therefore ineffective to restrain bacterial outgrowth and overt pathogenicity19. A poorly controlled host immune response, in turn, can generate a self-perpetuating pathogenic cycle where dysbiosis and inflammation reinforce each other by forming a postive feedback loop (FIG. 1).
Most studies investigating microbial subversion of the periodontal host immune responses have primarily used P. gingivalis as a model pathogen, which is necessarily reflected in this Review. Decades of research have identified a plethora of documented or putative virulence factors of P. gingivalis that may contribute to its persistence in the subgingival region (for review see REFs.37,39). However, only now have we begun to understand how this Gram-negative asaccharolytic bacterium integrates its virulence attributes to enhance the pathogenicity of a polymicrobial community. Although P. gingivalis was thought to be capable of directly causing periodontitis in animal models40, it is now established that P. gingivalis-induced periodontitis requires the presence of the commensal microbiota as P. gingivalis is unable to cause periodontitis in germ-free mice despite colinizing this host15. In this context, P. gingivalis — while present at a low frequency — is pathogenic owing to its ability to induce dysbiotic microbial communities, and thereby act as a keystone pathogen16,41. Manipulation of the host immune response is fundamental to the capacity of P. gingivalis to instigate quantitative and qualitative alterations in the oral microbiota, which can thereby trigger inflammatory periodontal bone loss largely mediated by pathobionts42-44. Although non-pathogenic by themselves in the oral environment, certain commensals such as Streptococcus gordonii promote P. gingivalis colonization and, as such, are implicated as accessory pathogens19,45.
It should be noted that the presence of P. gingivalis does not necessarily precipitate disease. Indeed, P. gingivalis is detectable, albeit with decreased frequency, in periodontally healthy individuals11,46. This might be explained by the considerable strain diversity within the population structure of P. gingivalis. Moreover, key virulence factors of this pathogen (such as gingipains and lipid A phosphatases) are regulated by local environmental conditions, which may differ among individuals41. In a related context, there might be individuals who can resist the conversion of a symbiotic microbiota into a dysbiotic one by virtue of their intrinsic immune status, for example they might have alterations in signalling pathways required for immune subversion by P. gingivalis or other keystone-like pathogens.
The concept that P. gingivalis co-operates with other periodontal organisms is supported by findings in animal models of periodontitis where combined inoculation of P. gingivalis with accessory pathogens or other keystone-like pathogens such as S. gordonii or T. forsythia, respectively, leads to enhanced alveolar bone loss compared with P. gingivalis alone47-50. This synergism may not be restricted to manipulation of the host response, but may also entail co-operative interspecies communication that further promotes bacterial fitness and hence dysbiosis. At least in vitro, communication among periodontal bacteria causes reciprocal transcriptomic and proteomic responses that regulate nutrient acquisition, metabolic processes and production of virulence factors51-53.
In summary, the emerging polymicrobial synergy and dysbiosis model suggests that the host immune response is initially subverted by keystone pathogens aided by accessory pathogens and is subsequently over-activated by pathobionts, thereby linking homeostasis breakdown and destructive inflammation in susceptible individuals (FIG. 1). Specific molecular mechanisms by which periodontal bacteria manipulate the host response to cause dysbiotic inflammation are discussed below.
Subversion of host immune responses
The dysbiotic periodontal community is faced with a survival conundrum: on the one hand, these bacteria need to evade immune-mediated killing; on the other hand, they require inflammation to procure nutrients from tissue breakdown such as degraded collagen peptides and haem-containing compounds20. Hence, immunosuppression, though a common evasion strategy of many pathogens54, is not a viable option for inflammophilic bacteria55. Periodontal bacteria can manipulate the interaction with host immune responses — such as neutrophils and complement — to enhance bacterial fitness.
The role of neutrophils in periodontitis
Neutrophils are the most common leukocyte recruited to the subgingival crevice or periodontal pockets35,36. Individuals with congenital deficiencies in neutrophil numbers or recruitment develop severe periodontitis, suggesting that neutrophils are required for periodontal tissue homeostasis17,56,57. However, hyperactive, supernumerary or dysregulated neutrophils can cause collateral tissue damage through the release of inflammatory and toxic substances or tissue-degrading enzymes35,58-60. Indeed, ample clinical evidence show that neutrophils mediate a significant portion of periodontal tissue destruction61,62 and their local numbers positively correlate with the severity of chronic periodontitis63. The chronic recruitment of excessive numbers of neutrophils to diseased periodontal pockets possibly arises from their inability to control the microbial challenge, despite being viable and capable of eliciting immune responses20,58. This suggests that the microorganisms can evade neutrophil-mediated killing while promoting inflammation, thereby contributing to dysbiosis. Indeed, as discussed below, periodontal bacteria can subvert complement function in ways that interfere with neutrophil-mediated killing in a persisting inflammatory environment42.
Complement subversion
Complement is a cascade system of a network of proteins and receptors that are centrally involved in immunity and inflammation64. Complement activation is initiated by distinct mechanisms, which converge at the third complement component (C3) and lead to the generation of effector molecules. These effector molecules mediate microbial opsonization and phagocytosis, for example the opsonin C3b interacts with complement receptor-1; recruitment and activation of inflammatory cells, such as anaphylatoxin C5a, which interacts with the C5a receptor (C5aR); and direct lysis of targeted microbes, mediated by the C5b-C9 membrane attack complex64. P. gingivalis, T. forsythia and Prevotella intermedia can protect themselves and bystander bacteria from human complement-mediated opsonophagocytosis and killing by blocking complement activation through degradation of C3 or key upstream components such as the mannose-binding lectin65-67. Importantly, the bacterial proteases involved — namely, gingipains, karilysin and interpain A, expressed by P. gingivalis, T. forsythia and P. intermedia, respectively — act synergistically to inhibit complement, which may lead to enhanced protection of complement-susceptible bystander species65-67. P. intermedia and T. denticola can also evade human complement-mediated killing by capturing complement factor H, which is a physiological inhibitor of complement68,69.
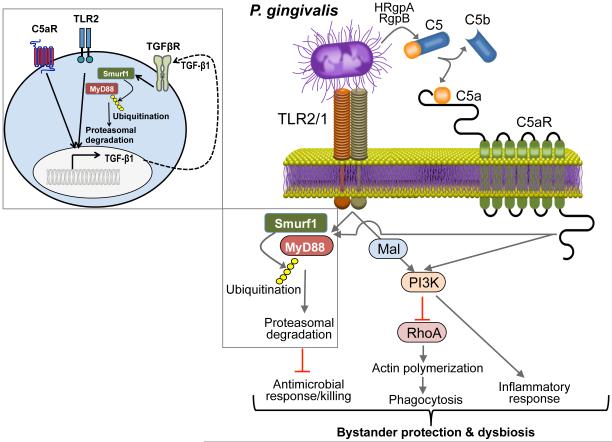
Intriguingly, the arginine-specific gingipains of P. gingivalis can cleave C5 to generate high local concentrations of C5a independently of canonical complement activation65,70. Hence, in neutrophils, which recognize P. gingivalis through Toll-like receptor 2 (TLR2)71, this pathogen can co-activate C5aR and TLR2, which in turn leads to signalling crosstalk42. In both human and mouse neutrophils, this C5aR–TLR2 crosstalk leads to ubiquitination and proteasomal degradation of the TLR2 signalling adaptor MYD88, and thereby suppress its antimicrobial effects42(FIG. 2). Moreover, the C5aR–TLR2 crosstalk activates an alternative pathway in which the TLR2 MYD88 adaptor-like protein (Mal) induces phosphoinositide 3-kinase (PI3K) signalling, which in turn inhibits GTPase RhoA-dependent actin polymerization and hence the phagocytosis of P. gingivalis and bystander bacteria42. The PI3K pathway also stimulates a robust inflammatory response42(FIG. 2). Importantly, the local inhibition of C5aR, TLR2 or PI3K in the periodontium of P. gingivalis-colonized mice leads to elimination of P. gingivalis, reverses the increase in total microbiota numbers induced by P. gingivalis colonization and blocks periodontal inflammation42. In summary, P. gingivalis manipulates neutrophils through distinct mechanisms that together ensure the survival of the microbial community and the perpetuation of inflammation.
Figure 2. Porphyromonas gingivalis subversion of neutrophils leads to dysbiotic inflammation.
Porphyromonas gingivalis expresses ligands that activate the Toll-like receptor 2 (TLR1)–TLR2 complex and enzymes (HRgpA and RgpB gingipains) with C5 convertase-like activity that generate high local concentrations of C5a ligand. The organism can co-activate C5aR and TLR2 in neutrophils and the resulting crosstalk leads to ubiquitination and proteasomal degradation of the TLR2 adaptor MYD88, thereby inhibiting a host-protective antimicrobial response. This proteolytic event requires C5aR–TLR2-dependent release of transforming growth factor-β (TGF-β1), which mediates MYD88 ubiquitination via the E3 ubiquitin ligase Smurf1 (enlarged inset). Moreover, the C5aR–TLR2 crosstalk activates phosphoinositide 3-kinase (PI3K), which prevents phagocytosis through inhibition of RhoA GTPase and actin polymerization, while stimulating the production of inflammatory cytokines. In contrast to MyD88, another TLR2 adaptor, Mal, contributes to immune subversion by acting upstream of PI3K. These functionally integrated pathways, as manipulated by P. gingivalis, provide ‘bystander’ protection to otherwise susceptible bacterial species and promote polymicrobial dysbiotic inflammation in vivo. C5aR, complement C5a receptor; HRgpA, high molecular mass arginine-specific gingipain A; Mal, MyD88 adaptor-like; MyD88, myeloid differentiation primary response protein 88; RgpB, arginine-specific gingipain B; Smurf1, Smad ubiquitin regulatory factor 1. Reproduced with permission from REF.42.
Interestingly, the karilysin of T. forsythia was recently shown to also cleave C5 to release C5a66. Since T. forsythia activates TLR272, it would be important to determine whether T. forsythia can instigate a C5aR–TLR2 subversive crosstalk similar to that of P. gingivalis or whether the two organisms can synergize in that regard. Intriguingly, both gingipains and karilysin readily degrade the C5b component of C5, and thereby prevent formation of the membrane attack complex65,66.
Additional immune subversive mechanisms
Major immune-subversive organisms probably use additional strategies to protect bystander bacteria and elevate the virulence of the entire microbial community, although most of these putative mechanisms have not been confirmed in vivo. For instance, the capacity of P. gingivalis to degrade and inactivate antimicrobial peptides might confer in vivo protection to bystander bacteria36,67. In a related context, T. denticola blocks the production of human β-defensins by gingival epithelial cells in response to Fusobacterium nucleatum, which promotes the colonization of periodontitis-associated bacteria73. Mechanistically, T. denticola blocks the fusion of internalized F. nucleatum with lysosomes and suppresses induction of intracellular reactive oxygen species, and thereby inhibits TLR responses that control human β-defensin expression73. Macrophages can engulf periodontal bacteria that invade the gingival connective tissue. However, P. gingivalis can induce cAMP-dependent activation of protein kinase A, which inhibits the expression of inducible nitric oxide synthase, thereby suppressing nitric oxide-dependent intracellular killing in macrophages74. Moreover, P. gingivalis suppresses human and mouse macrophage endocytosis of F. nucleatum, an event required for NLRP3 inflammasome activation in response to this bacterium75. This mechanism may promote the fitness of the periodontal community since inflammasome activation induces pyroptosis, a proinflammatory mode of lytic cell death protecting the host against infection76. P. gingivalis is also thought to manipulate adaptive immune responses by favouring the differentiation and recruitment of CD4+ T helper 17 (Th17) cells at the expense of the Th1 lineage77-79. Although it has been argued that Th17 cells contribute to destructive periodontal inflammation whereas Th1 cells are involved in protective cell-mediated immunity80,81, more research is warranted to elucidate the role of various T cell subsets in periodontal disease pathogenesis.
When the periodontium is chronically exposed to a dysbiotic microbial community — which apparently evolved to evade the host immune response while promoting its inflammatory aspects — it probably has an adverse impact on systemic health. Below, we examine established and emerging mechanisms whereby periodontal bacteria can subvert host-signalling pathways to instigate chronic inflammation in extraoral sites.
Periodontitis and cardiovascular disease
Numerous cross-sectional, case-control and cohort epidemiological studies suggest that periodontitis is associated with atherosclerotic cardiovascular disease, independently of confounding factors such as smoking and obesity6,82,83. Moreover, clinical interventional studies indicate that treatment of periodontitis reduces systemic inflammation and has favourable effects on subclinical markers of atherosclerosis, including improved endothelial function as determined by flow-mediated dilatation83-85. Moreover, a recent study has shown that longitudinal improvement in periodontal health is related to a decreased progression of carotid atherosclerosis in humans86.
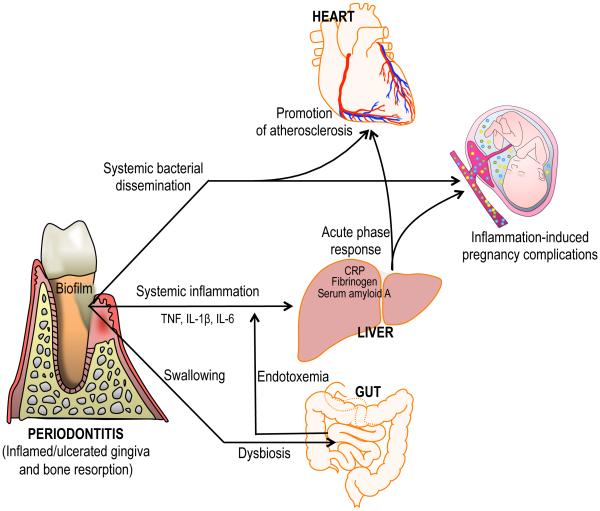
At least two biologically plausible mechanisms may account for a causative link between periodontitis and atherosclerosis4,83 (FIG. 3). First, gingival ulceration in periodontal pockets (FIG. 1) enables the translocation of bacteria into the systemic circulation, that is, causing bacteraemia that are well documented in patients with periodontitis and may provide an atherogenic stimulus4,83. This notion is supported by findings that recurrent experimental bacteraemia (using P. gingivalis as a model pathogen) promotes coronary and aortic atherogenesis in pigs with or without hypercholesterolemia87. Additionally, in a subset of patients with severe periodontitis and ulcerated gingival epithelium, locally produced proinflammatory cytokines, such as tumour necrosis factor (TNF), interleukin-1β (IL-1β) and IL-6, can enter the systemic circulation and induce an acute-phase response in the liver (including elevated C-reactive protein, fibrinogen and serum amyloid A), and thereby promote atherogenesis4,88. In support of this mechanism, patients with severe periodontitis have increased systemic inflammation — determined by elevated cytokines and acute-phase markers such as IL-6 and C-reactive protein, respectively — compared with healthy controls, whereas treatment of periodontitis reduces systemic inflammation in patients with or without history of cardiovascular disease83,85. An alternative mechanism was suggested by a recent study in mice, which showed that P. gingivalis can cause alterations to the gut microbiota, leading to indirect induction of systemic inflammation89(FIG. 3). Specifically, mice orally infected with P. gingivalis showed increased proportion of Bacteroidetes and decreased proportion of Firmicutes relative to sham-infected controls, which correlated with decreased expression of tight-junction proteins in the ileum, endotoxemia and systemic inflammation. Although large quantities of oral bacteria are constantly swallowed via the saliva in humans and animals, P. gingivalis was not detected in the gut of infected mice; therefore, the mechanism by which it caused compositional changes to the gut microbiota remains uncertain. The mechanistic basis of the association between periodontitis and atherosclerosis is substantiated by studies in animal models based on oral infection with P. gingivalis, which has been detected in human atherosclerotic tissue, and by clinical or in vitro studies attesting to the atherogenic potential of periodontal bacteria6,25-29,90-94.
Figure 3. Biologically plausible mechanisms linking periodontitis to systemic inflammation and disease.
In periodontitis, locally produced pro-inflammatory cytokines can enter the systemic circulation and induce an acute-phase response in the liver — which is characterized by increased levels of C reactive protein, fibrinogen and serum amyloid A — in turn contributing to atherosclerosis or exacerbating intra-uterine inflammation. Moreover, gingival ulceration in periodontal pockets enables the egress and systemic dissemination of periodontal bacteria. Certain bacteria including Porphyromonas gingivalis have been detected in circulating leukocytes and in atherosclerotic lesions, where they may act as pro-atherogenic stimuli. Other periodontal bacteria such as Fusobacterium nucleatum have been detected in the placenta where they can cause adverse pregnancy outcomes. Large quantities of oral bacteria are constantly swallowed on a daily basis via the saliva into the gut. In this context, an alternative, or additional, mechanism linking periodontitis to systemic inflammation was recently proposed: Swallowed P. gingivalis causes alterations to the gut microbiota, thereby leading to increased gut epithelial permeability and endotoxemia, which causes systemic inflammation. Although independent, the depicted events are not mutually exclusive but could in principle occur simultaneously. CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factor.
Animal model-based evidence
Oral infection with P. gingivalis of rabbits fed a high-fat diet or of atherosclerosis-prone (hyperlipidemic) apolipoprotein E-deficient (ApoE−/−) mice on a standard chow diet causes not only local bone loss but also systemic inflammation and atherosclerotic lesions28,90,91. The ApoE−/− mouse model of combined periodontitis and atherosclerosis has been extensively used and provided insights into the role of TLR signalling in the disease process. Whereas TLR2 has an important role in mediating P. gingivalis-induced inflammatory atherosclerosis95, the disease phenotype requires P. gingivalis to evade TLR4-mediated detection29.
In this regard, the organism can enzymatically modify the lipid A moiety of its lipopolysaccharide to either evade or antagonize TLR4 activation in macrophages17. The shifting of lipid A activity from TLR4-evasive to TLR4-antagonistic depends on endogenous lipid A phosphatase activity96, which is regulated by growth phase or environmental factors including temperature and haemin availability97,98. Differential activities of lipid A 1’- and 4′-phosphatases are associated with the synthesis of different lipid A structures, comprising non-phosphorylated tetra-acylated lipid A (which is inert for TLR4 activation), mono-phosphorylated penta-acylated lipid A (which is a weak TLR4 agonist) and mono-phosphorylated tetra-acylated lipid A (a TLR4 antagonist). Genetic ablation of 4′-phosphatase activity (or bacterial growth at ≥39°C) leads to the synthesis of TLR4 agonist lipid A, whereas ablation of 1-phosphatase activity — or growth in haemin-replete conditions expected to be present in an inflammatory environment — leads to TLR4 antagonist lipid A96,99. Remarkably, in addition to preventing TLR4 activation, the production of inert or antagonistic lipid A also increases the resistance of P. gingivalis to cationic antimicrobial peptides, owing to changes in the outer surface charge of the bacteria that affect the binding of cationic peptides29,96,97,99. Importantly, the TLR4-antagonist lipid A of P. gingivalis inhibits TLR4 activation in response to other bacteria that express TLR4-agonist lipid A species100,101.
In ApoE−/− mice orally infected with wild-type P. gingivalis or isogenic phosphatase mutants with a ‘locked’ lipid A profile, the expression of inert or antagonistic lipid A was associated with elevated vascular inflammation, macrophage infiltration and progression of atherosclerosis29. In macrophages infected with the same set of wild-type or mutant strains, the expression of lipid A structures that prevent or block TLR4 activation was associated with evasion of non-canonical inflammasome activation and increased bacterial survival, as compared to the expression of TLR4 agonist lipid A29. Taken together with an earlier study by the same group95, these findings suggest that the capacity of P. gingivalis to prevent TLR4 signalling while activating TLR2 leads to atherogenic inflammation at sites distant from initial infection. Using a similar ApoE−/− model, an independent study confirmed the causal link between periodontitis and atherosclerosis and, moreover, detected P. gingivalis in aortic tissues, including vascular endothelial cells, using fluorescent in situ hybridization28.
Immune subversion and atherogenic potential
Although periodontal bacteria have been identified in atheromas25-27,92-94, the mode of their relocation is uncertain and could involve mechanisms alternative or additional to the bacteraemic route. In principle, bacteria might exploit recirculating leukocytes — such as macrophages and/or dendritic cells (DCs) — as ‘Trojan horses’ for dissemination to systemic tissues. In this regard, the ability of P. gingivalis to survive intracellularly in macrophages29,102 and DCs27 is intriguing. To persist intracellularly in macrophages, P. gingivalis needs to enter the macrophage via complement receptor-3 in cholesterol-rich lipid rafts102,103, which is consistent with observations that pathogens which invade through lipid rafts are not readily directed to late endosomes and lysosomes where they would be killed104. To survive within DCs, P. gingivalis needs to enter via the C-type lectin DC-specific ICAM-3 grabbing non-integrin (DC-SIGN)105,106(FIG. 4). Both processes are mediated by fimbrial proteins of P. gingivalis: FimA fimbriae directly interact with complement receptor-3 on macrophages, whereas Mfa1 fimbriae interact with DC-SIGN on DCs102,106.
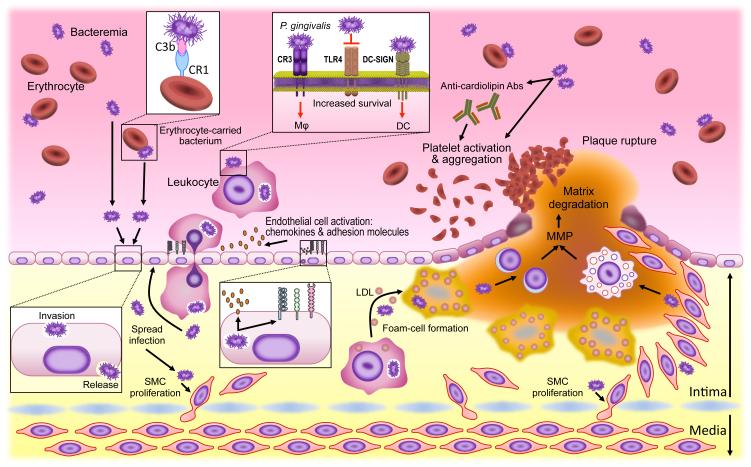
Figure 4. Microbial immune subversion in atherogenesis.
In addition to a bacteraemic route, periodontal bacteria may hijack leukocytes or erythrocytes (to which they attach via a C3b–CR1 interaction) to disseminate from the oral mucosa to aortic tissues. Bacteria not only invade but also activate endothelial cells (upregulation of cell adhesion molecules and chemokines) in ways that promote the transmigration of leukocytes that may harbour viable intracellular bacteria. The bacteria can spread to deeper tissues where they can induce smooth-muscle-cell proliferation in the intima. The uptake of low-density lipoprotein (LDL) by transmigrated macrophages is enhanced in the presence of bacteria leading to accelerated foam cell formation and atherogenesis. At later stages, atherosclerotic plaque rupture can be facilitated by bacterially induced production of matrix metalloproteinases (MMPs) by lymphocytes or myeloid cells. Bacteria-induced platelet aggregation (directly or through the induction of prothrombotic autoantibodies) may contribute to thrombotic vessel occlusion. Most of these studies supporting the above-discussed model utilized Porphyromonas gingivalis as model pathogen, the survival of which within leukocytes depends in part upon Toll-like receptor 4 (TLR4) evasion as well as on its capacity to exploit CR3 (in macrophages) or DC-SIGN (in dendritic cells) for safe intracellular entry. Abs, antibodies; CR1, complement receptor-1; CR3, complement receptor-3; DC, dendritic cell; DC-SIGN, DC-specific ICAM-3 grabbing nonintegrin; SMC, smooth muscle cell; TLR4, Toll-like receptor 4.
The exploitation of DCs as transport vehicles for P. gingivalis is supported by a recent clinical study that identified P. gingivalis (using 16S rDNA sequencing) within blood myeloid DC from chronic periodontitis patients27. The rate and frequency of DC carriage of P. gingivalis was increased after bacteraemia elicited by debridement, which is a routine clinical procedure involving removal of dental plaque biofilm and calculus from the teeth and gingiva. Moreover, immunofluorescence analysis revealed co-localization of P. gingivalis with myeloid DCs that are infiltrating either the oral mucosa or atherosclerotic plaques of patients with chronic periodontitis27.
Mechanistic underpinnings for these clinical observations were provided by in vitro studies showing that P. gingivalis subverts human DC function27,105,106. Specifically, following binding to DC-SIGN, P. gingivalis not only enters and survives within myeloid DCs, but also promotes an atherogenic phenotype in the cells, as indicated by upregulation of matrix metalloproteinase-9 and complement C1q which are indicators of plaque rupture risk27. Furthermore, P. gingivalis selectively upregulates the expression of chemokine receptor CXCR4, but not that of CC-chemokine receptor 7 (CCR7), and thereby disrupt DC migration toward CC-chemokine ligand 19 (CCL19) and promote migration toward CXC-chemokine ligand 12 (CXCL12)107. Consistently, peripheral blood myeloid DCs from patients with chronic periodontitis are characterized by high CXCR4 and low CCR7 expression compared with DCs from healthy individuals107. CCR7 mediates homing to secondary lymphoid organs, whereas CXCR4 mediates homing to sites of neovascularization such as atherosclerotic plaques108,109; therefore, the authors suggested that P. gingivalis hijacks and directs DC migration to inflammatory vascular sites, where the pathogen can exacerbate inflammatory pathology107.
An alternative transport vehicle for P. gingivalis in the blood might be erythrocytes, which bind C3b-opsonized P. gingivalis in a complement receptor-1-dependent manner110(FIG. 4). Although leukocytes fail to bind and internalize erythrocyte-attached P. gingivalis, this may not necessarily be an evasion strategy since erythrocyte-associated bacteria can eventually be cleared by liver macrophages (Kupffer cells). However, because the interaction of P. gingivalis with erythrocytes gradually declines over time — which is attributed to bacterial degradation of bound C3b — resulting in the release of viable bacteria in vitro, the authors speculated that erythrocyte-carried P. gingivalis can potentially infect endothelial cells at extraoral sites, and thereby contribute to vascular inflammation110(FIG. 4). This intriguing hypothesis remains to be tested in animal models.
P. gingivalis can indeed invade human aortic endothelial cells by means of its FimA fimbriae and subsequently suppresses the levels of key intracellular molecules involved in cell death and host defence (such as NLRP3 and RIPK1) leading to a permissive intracellular environment6,94,111,112. FimA fimbriae also induce TLR2-dependent expression of endothelial adhesion molecules (ICAM-1, VCAM-1 and E-selectin) and chemokines (MCP-1 and CXCL8) involved in leukocyte recruitment, as well as TLR2 inside-out signalling that activates leukocyte integrins that mediate transendothelial migration6,113,114. However, additional TLR2 ligands of P. gingivalis, including serine lipids and lipoproteins115,116, probably also participate in these pro-atherogenic activities.
Other studies using P. gingivalis as a pro-atherogenic model bacterium showed that it can accelerate atherothrombosis via the recruitment and activation of neutrophils117 and can induce platelet aggregation — an activity facilitated by interactions of P. gingivalis hemagglutinins with the platelet glycoprotein IIb–IIIa (also known as αIIbβ3 integrin) on platelets via a fibrinogen bridge118. P. gingivalis may contribute to cardiovascular disease pathology also via molecular mimicry. Specifically, compared with healthy individuals, a substantial subset of patients with periodontitis have elevated gingival crevicular fluid and serum concentrations of cardiolipin-specific antibodies, that is, prothrombotic autoantibodies associated with atherosclerosis and also adverse pregnancy outcomes (see discussion below). Such autoantibodies can be induced in response to bacterial epitopes — such as those found in P. gingivalis arginine-specific gingipains and T. denticola phosphoglycerate kinase — that bear homology to the TLRVYK peptide of the phospholipid-binding serum protein β2-glycoprotein-I119,120. Furthermore, P. gingivalis enhances low-density lipoprotein-uptake and foam cell formation by upregulating CD36 (a scavenger receptor mediating lipid uptake) and downregulating ATP-binding cassette transporter A1 (which mediates the efflux of cholesterol from macrophages)121,122. In a similar context, the pathogen induces vascular smooth-muscle-cell proliferation leading to neo-intima formation, and stimulates production of matrix metalloproteinases that contribute to plaque rupture and thrombotic vessel occlusion6(FIG. 4).
In summary, periodontal pathogens evade the immune system to induce atherogenic inflammation, although their impact beyond the oral cavity is not restricted to the cardiovascular system but includes additional tissues (see below).
Periodontitis and adverse pregnancy outcomes
Epidemiological and clinical studies suggest that maternal periodontitis may be associated with increased risk of adverse pregnancy outcomes, such as low birthweight, pre-term birth, miscarriage and/or stillbirth9,21,123. Similar to atherosclerosis, two major plausible biological mechanisms have been proposed: firstly, periodontal pathogens that disseminate systemically may cross the placenta into the fetal circulation and amniotic fluid, and secondly, inflammatory mediators produced locally in the periodontium could enter the systemic circulation and stimulate an acute-phase response and thereby adversely affect the placenta and fetus (FIG. 3). The notion that periodontal bacteria can cause pregnancy complications is supported by mechanistic studies in animal models.
F. nucleatum — a potential accessory pathogen that facilitates the colonization of periodontitis-associated bacteria — becomes an overt pathogen if it translocates to extraoral sites8. Indeed, the bacterium can be isolated from abscesses in several internal organs and is implicated in adverse pregnancy outcomes and colorectal cancer (BOX 2). Clinical studies have linked F. nucleatum to several pregnancy complications, including premature birth, stillbirth and neonatal sepsis8. Moreover, the identification of identical F. nucleatum clones in the subgingival biofilm of a mother with pregnancy-associated gingivitis and her stillborn infant suggest that the bacterium can disseminate from the mother’s gingiva to her uterus21. Experiments in pregnant mice have provided insights into how F. nucleatum can cause intra-uterine infection and inflammation. Specifically, intravenously administered F. nucleatum (to mimick bacteraemia) uses its E-cadherin-binding FadA adhesin to cross the endothelium and colonize the fetal–placental compartment, where it induces TLR4-dependent necroinflammatory responses124. In contrast to F. nucleatum, E. coli fails to induce fetal loss in the same model125. Whereas F. nucleatum can colonize the placenta of TLR4-deficient mice, these mice display substantially decreased fetal death rate compared with wild-type mice, which indicates that fetal loss is caused by inflammation rather than bacterial colonization per se124.
Box 2. Oral bacteria and cancer.
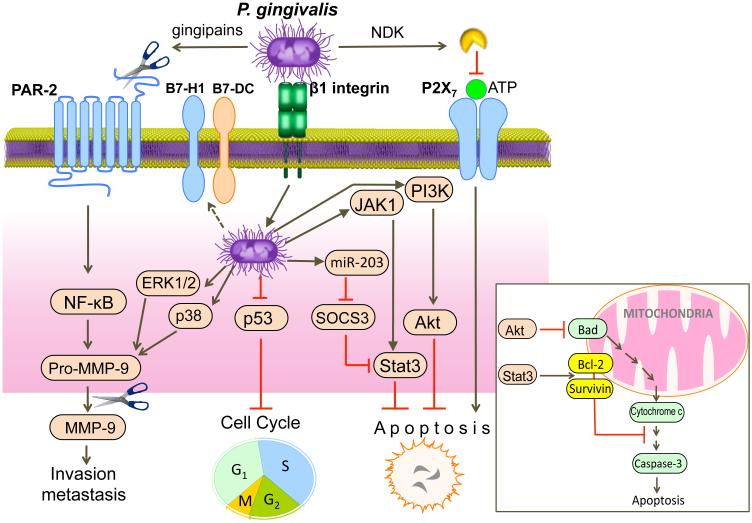
Accumulating evidence indicates a temporal and spatial association between oral bacteria and cancer7,157. Fusobacterium nucleatum is associated with colorectal cancer158, which has been attributed to its ability to stimulate the growth of colorectal cancer cells159. This activity depends upon interactions between E-cadherin and the F. nucleatum adhesin FadA, the expression of which (FadA) is elevated in the cancerous colon tissue and is correlated with the expression of oncogenic and inflammatory genes159. Porphyromonas gingivalis is associated with oral squamous cell carcinoma (OSCC)160,161, orodigestive cancer (independently of periodontitis)162 and pancreatic cancer163. Certain immune subversive mechanisms of P. gingivalis are consistent with a role in cancer development (see figure). In OSCC cells, P. gingivalis induces the expression of pro-matrix metalloproteinase-9 (MMP-9) by triggering proteinase activated receptor-2 (PAR-2)-mediated NF-κB activation (extracellular mechanism involving gingipain secretion) or by activating ERK1/2 and p38 MAPK pathways (intracellular mechanism requiring β1-integrin-dependent invasion)164. In addition, the gingipains additionally cleave the secreted proenzyme into mature MMP-9 (gelatinase), which promotes carcinoma cell migration164. P. gingivalis invasion of epithelial cells suppresses apoptosis and stimulates cell proliferation by inhibiting the p53 tumor suppressor165. The ability of P. gingivalis to activate Janus kinase 1 (JAK1) and signal transducer and activator of transcription 3 (STAT3) pathway as well as the phosphoinositide 3-kinase (PI3K)-AKT signalling causes inhibition of intrinsic mitochondrial apoptosis pathways149,166. Specifically, STAT3, which upregulates the anti-apoptotic molecules survivin and BCL-2, and AKT, which inhibits the pro-apoptotic molecule BCL-2-associated agonist of cell death (BAD), lead to caspase-3 inhibition148,149(inset). Moreover, extracellular release of nucleoside diphosphate kinase (NDK) by P. gingivalis cleaves ATP and prevents induction of apoptosis via the purinergic receptor P2X7167. Although suppressor of cytokine signalling 3 (SOCS3) can induce apoptosis by targeting STAT3168, P. gingivalis upregulates miR-203 which directly inhibits SOCS3169. P. gingivalis can additionally induce B7 family ligands (B7-H1, B7-DC) that interact with the programmed death-1 receptor on T cells170 potentially leading to their immunosuppression171.
Certain other periodontal bacteria, including P. gingivalis, were also shown to colonize the placenta and fetal tissues of mice or rats and thereby causing inflammation and pregnancy complications (reviewed in REF.9). P. gingivalis may also induce adverse pregnancy outcomes via alternative mechanisms. Cardiolipin-specific antibodies are associated with certain disorders, including adverse pregnancy outcomes119, and a subset of periodontitis patients have increased concentrations of such autoantibodies126, which could be induced in response to cross-reactive bacterial epitopes such as the arginine-specific gingipains of P. gingivalis119. The possible connection involving P. gingivalis, cardiolipin-specific antibodies and pregnancy complications was strengthened recently. A study showed that antibodies raised against P. gingivalis — but not against an isogenic gingipain-deficient mutant — cause fetal loss when passively administered to pregnant female mice127. Importantly, this effect was substantially inhibited when cardiolipin-specific antibodies were removed from the antibody preparations127.
In summary, pregnancy complications can be caused by periodontal pathogen-induced inflammatory responses or autoantibodies, which have also been implicated in the association of periodontitis with rheumatoid arthritis.
P. gingivalis and rheumatoid arthritis
Several studies indicate an epidemiological association between periodontitis and rheumatoid arthritis, even after adjusting for common risk factors such as smoking128-130. In a distinct clinical phenotype of rheumatoid arthritis, anti-citrullinated protein antibodies (ACPA) serve as diagnostic markers as they are detected in the serum prior to the onset of the disease and their serum levels correlate strongly with disease severity131. A recent study showed that patients with rheumatoid arthritis — particularly those with ACPA-positive rheumatoid arthritis — have higher frequency of periodontitis than control patients with osteoarthritis. Furthermore, in these patients the detection of P. gingivalis in subgingival biofilm samples was associated with increased levels of ACPA irrespective of smoking status32. The molecular underpinnings of these associations are examined below.
Peptidyl-arginine deiminase
P. gingivalis is unique among other periodontal bacteria, and possibly among all prokaryotes, with regard to expression of a peptidyl-arginine deiminase, which is an enzyme that converts protein arginine residues to citrulline5,33. Protein citrullination has been implicated in several physiological processes132; however, because citrullination can dramatically alter protein structure and function, dysregulated host protein citrullination by microbial enzymes could interfere with normal host cell signalling and immune or other homeostatic functions. For instance, P. gingivalis peptidyl-arginine deiminase (PPAD) citrullinates the C-terminal arginine of epidermal growth factor (EGF), and thereby inhibits its biological activity as shown by impaired EGF-induced fibroblast proliferation and migration133. Interestingly, citrullination of two internal arginine residues of EGF by human peptidyl-arginine deiminase enzymes does not abrogate EGF function133. As EGF has an essential role in wound healing and tissue regeneration, its inactivation by P. gingivalis may interfere with periodontal tissue healing and thus delay the resolution of inflammation.
Link between P. gingivalis and rheumatoid arthritis
The unique ability of P. gingivalis to citrullinate proteins has attracted considerable interest in the field of rheumatoid arthritis given the importance of ACPA in its pathogenesis5,131. In this context, P. gingivalis was shown to citrullinate human fibrinogen and α-enolase, which, in their citrullinated form, are two major rheumatoid arthritis autoantigens31. This activity requires concerted action between PPAD and arginine-specific gingipains, which co-localize with PPAD in the outer membrane of P. gingivalis31. Specifically, the cleavage of fibrinogen or α-enolase by gingipains exposes C-terminal arginine residues that are subsequently citrullinated by PPAD31. In principle, the unique mode of proteolytic processing and post-translational modification of host antigens by P. gingivalis could generate neoepitopes to which immunologic tolerance does not exist, leading to the generation of autoantibodies (FIG. 5). It should be noted that the breakdown of immune tolerance to citrullinated proteins requires susceptible individuals, such as carriers of HLA-DRB1 shared epitope alleles that bind selectively to citrullinated sequences and may influence antigen presentation in ways that lead to ACPA production134. Intriguingly, rheumatoid arthritis-specific autoantibodies to citrullinated α-enolase peptide 1 (the immunodominant B cell epitope of human α-enolase) were shown to cross-react with citrullinated enolase from P. gingivalis, suggesting that molecular mimicry can contribute to autoantibody generation135.
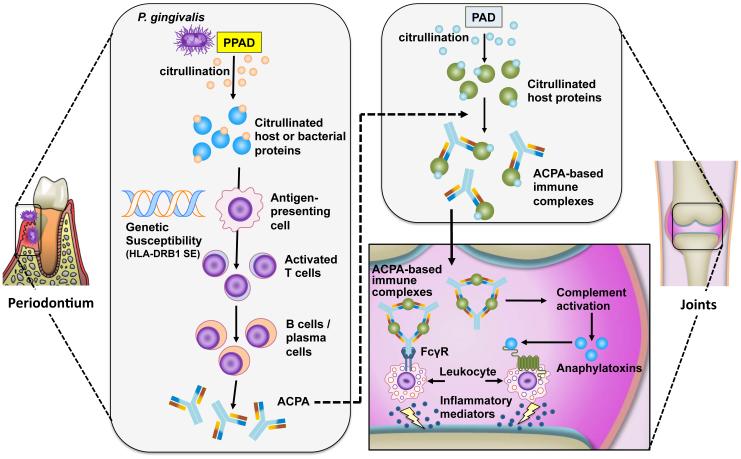
Figure 5. Porphyromonas gingivalis-mediated citrullination and induction of ACPA in rheumatoid arthritis.
P. gingivalis peptidylarginine deiminase (PPAD) citrullinates host-derived or bacterial proteins in the inflammatory environment of periodontitis. In susceptible individuals (carriers of HLA-DRB1 shared epitope (SE) alleles), distinct citrullinated peptides are presented in the context of HLA-DRB1 SE to activate T cells, which, in turn stimulate B-cell production of anti-citrullinated protein antibodies (ACPA). The induction of autoantibodies may be explained by mechanisms involving neoepitope formation or molecular mimicry. Citrullination of host proteins, such as α-enolase, fibrinogen and collagen type II, by human peptidylarginine deiminase (PAD) enzymes can occur in injured or inflamed joints. ACPA bind citrullinated proteins and form immune complexes that can mediate local synovial inflammation by activating complement or Fcγ receptors (FcγR).
A ‘two-hit’ model of rheumatoid arthritis pathogenesis was proposed involving initial breakdown of tolerance to citrullinated peptides generated by P. gingivalis in inflamed gingiva followed by epitope spreading to other host-citrullinated proteins in the inflamed joint5,31(FIG. 5). In this regard, citrullinated proteins have been detected in the gingiva of periodontitis patients5. Moreover, the notion that immunity to citrullinated proteins is initially triggered in inflamed mucosal surfaces distant from the joints is consistent with the presence of ACPA prior to signs of inflammation in the joints136. Mechanisms of epitope spreading or molecular mimicry could lead to cross-reactivity with citrullinated joint proteins and subsequent formation of immune complexes could exacerbate or perpetuate the inflammatory process in rheumatoid arthritis through several mechanisms, including activation of complement or Fcγ receptors5,31(FIG. 5). Epitope spreading precedes the development of rheumatoid arthritis and leads to maintenance and progression of inflammation as it sustains the generation of high-affinity ACPA to host citrullinated proteins5,137.
The ‘two-hit’ model is consistent with the results of two recent mechanistic studies by independent groups. Specifically, infection of mice with wild-type P. gingivalis exacerbates collagen- or collagen antibody-induced arthritis, as revealed by accelerated progression and enhanced severity of bone and cartilage destruction30,138. The ability of wild-type P. gingivalis strains to aggravate arthritis is strictly dependent on PPAD expression, since isogenic mutants lacking this enzyme fail to influence the disease outcome30,138. Furthermore, infection of mice with wild-type, but not with PPAD-deficient, P. gingivalis was associated with detection of citrullinated proteins at the site of infection and with production of antibodies to citrullinated proteins30,138. These findings suggest that, by virtue of its PPAD, P. gingivalis may constitute a mechanistic link between periodontitis and rheumatoid arthritis.
Periodontitis and respiratory diseases
Aspiration pneumonia
The tooth-associated bacterial biofilm is thought to be a reservoir for respiratory infections, and oral anaerobic bacteria are common isolates from aspiration pneumonia and lung abscesses22,139,140. Oropharyngeal aspiration of bacteria is a major cause of pneumonia in old or immunocompromised individuals139,140 and periodontitis is epidemiologically implicated as a mortality risk factor for aspiration pneumonia at least in the elderly141. Since patients probably aspirate fragments of biofilm composed of mixed bacterial species, the polymicrobial synergistic interactions seen in periodontitis might also occur in the lung tissue. Despite limited research in this area, mixed infection with P. gingivalis and T. denticola in a mouse model of aspiration pneumonia has been shown to cause considerably higher inflammatory responses, impaired bacterial clearance and more severe lung pathology compared with single infection with either bacterium142. Importantly, the control of the oral microbial burden substantially decreases the incidence of aspiration pneumonia in frail elderly people143,144, which suggests a direct association between oral bacteria and lung pathology in susceptible individuals. These results warrant more basic studies to understand the mechanisms involved.
Chronic obstructive pulmonary disease
Periodontitis is also associated with chronic obstructive pulmonary disease (COPD)23,145. Polymicrobial infections including the opportunistic pathogen Pseudomonas aeruginosa are associated with exacerbations of COPD increasing its morbidity and mortality23,146. P. gingivalis is readily detected with P. aeruginosa in tracheal aspirates of patients with acute COPD exacerbations24 and can enhance the pathogenicity of P. aeruginosa in the context of lower airway infection147,148. Indeed, P. gingivalis promotes the ability of P. aeruginosa to invade respiratory epithelial cells and modulates its apoptosis-inducing capacity147,148. Specifically, compared with P. aeruginosa invasion alone, co-invasion of respiratory epithelial cells with both bacteria leads to diminished apoptosis as a result of enhanced signal transducer and activator of transcription 3 (STAT3) signalling, which upregulates the expression of the anti-apoptotic molecules survivin and B cell lymphoma 2 (BCL-2), in turn leading to caspase 3 inhibition148. Moreover, co-invasion is followed by downregulation of the pro-apoptotic molecule BCL-2-associated agonist of cell death (BAD)148, possibly mediated by phosphoinositide 3-kinase (PI3K)–AKT signalling149(see Figure in BOX 2). However, the inhibition of apoptosis is transient since the signalling pathways involved start to decline 8 hours post-invasion of both bacteria, leading to dramatically increased caspase-3 activity by 12 hours post-invasion148. Rapid apoptosis of infected epithelial cells is thought to contribute to effective clearance of P. aeruginosa150. This notion suggests that inhibition of epithelial cell apoptosis for a substantial time following P. aeruginosa and P. gingivalis co-invasion may provide the bacteria with a safe intracellular niche and the opportunity to proliferate and establish infection.
Conclusions and perspective
Dysbiotic microbial communities in the periodontium resist immune elimination and create permissive conditions for growth in a nutritionally favourable inflammatory environment11,13,17-19,41,42(FIG. 1-2). The immune-subversive and pro-inflammatory strategies that promote the fitness of periodontal bacteria not only cause collateral damage to the periodontium but also have repercussions that link periodontitis to systemic afflictions (FIG. 3-5). The virulence of individual periodontal pathogens is maximized in the context of a polymicrobial infection17,19,47-51,53 and its impact on the host depends on genetic predispositions and environmental modifiers92,151-155(BOX 1). Hence, to better understand mechanisms of pathogenesis of periodontitis and associated systemic conditions, data from epidemiological and animal model studies need to be meaningfully integrated with those from metatranscriptomic and metaproteomic approaches as well as whole-genome transcriptomic and proteomic analyses of host tissue in health and different disease stages. This integration can offer insights into the dynamic nature of host–microbe interactions in disease development, and, moreover, can facilitate the formulation of novel hypotheses for further knowledge discovery. More importantly, key findings from basic research need to be translated into clinical applications for host-modulation therapies to counteract the immune-subversive mechanisms of periodontal bacteria, and thereby contribute to the treatment for periodontitis and associated systemic inflammatory disorders. Such host-modulation strategies are more likely to succeed than direct antimicrobial approaches — especially when targeting keystone pathogens as they can act at low abundance and will probably not be completely eradicated, partly because they can hide within permissive host cells.
Acknowledgements
The author’s research is supported by grants from the NIH (DE015254, DE017138, DE021685, and AI068730). The author regrets that several important studies could only be cited indirectly through comprehensive reviews, owing to space and reference number limitations.
Glossary terms
- Microbiota
A complex and diverse community of microorganisms living within a given anatomical niche, for example an environmentally exposed surface of a multicellular eukaryotic organism.
- Dysbiosis
A condition characterized by an imbalance in the relative abundance or influence of species within a microbial community associated with disease, for instance periodontitis or inflammatory bowel disease.
- Homeostasis
A condition of equilibrium or stability in a system maintained by adjusting physiological processes to counteract external changes, such as a balanced relationship between host tissues and the resident microbiota that prevents destructive inflammation or disease.
- Subgingival crevice
Narrow space between the tooth surface and the free gingiva.
- Gingival crevicular fluid
Serum exudate that originates in the gingival capillaries and flows into the gingival crevice carrying locally produced immune and inflammatory mediators such as complement, cytokines and antimicrobial peptides.
- Keystone pathogen
A pathogen with a disproportionately large effect on its environment relative to its abundance, for example low-abundance P. gingivalis remodels a commensal microbial community into a dysbiotic and disease-provoking microbiota.
- Pathobiont
Commensal with the potential to induce pathology under conditions of disrupted homeostasis.
- Accessory pathogen
A commensal bacterium that is not pathogenic by itself in a given niche, but which can enhance the virulence of keystone pathogens by, for example, facilitating their colonization or providing metabolic support.
- Gingipains
A family of trypsin-like cysteine proteinases which are secreted by P. gingivalis and contribute to its virulence and the pathogenesis of periodontitis. Members include the high molecular mass arginine-specific gingipain A (HRgpA), arginine-specific gingipain B (RgpB) and lysine-specific gingipain (Kgp).
- Inflammophilic
Refers to bacteria that thrive on inflammation as they feed off inflammatory tissue breakdown products; literally meaning attracted to inflammation, from the combined meaning of inflammation and the Greek suffix philic denoting fondness.
- Periodontal pocket
The pathologically deepened subgingival crevice in periodontitis, which is pathognomonic for the disease.
- Inflammasome
A cytosolic, multiprotein complex which responds to infection or tissue injury by activating pro-inflammatory caspases, mainly caspase-1, leading to the cleavage and release of pro-inflammatory cytokines such as IL-1β and IL-18 and — under certain conditions (myeloid cells infected with pathogenic bacteria) — to pyroptosis, a form of necrotic cell death.
- Non-canonical inflammasome
A caspase-11–dependent pathway of inflammasome activation which is critical for controlling infection by Gram-negative bacteria and can induce cell death (pyroptosis) independently of caspase-1.
- Atheroma
Accumulated fatty deposits in the inner lining (intima) of an artery, leading to restriction of blood flow and a risk of thrombosis.
Footnotes
Competing interests statement
The author declares no competing interests.
REFERENCES
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Adler CJ, et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nature Genet. 2013;45:450–455. doi: 10.1038/ng.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 4.Genco RJ, Van Dyke TE. Prevention: Reducing the risk of CVD in patients with periodontitis. Nature Rev. Cardiol. 2010;7:479–480. doi: 10.1038/nrcardio.2010.120. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg K, Wegner N, Yucel-Lindberg T, Venables PJ. Periodontitis in RA-the citrullinated enolase connection. Nature Rev. Rheumatol. 2010;6:727–730. doi: 10.1038/nrrheum.2010.139. [DOI] [PubMed] [Google Scholar]
- 6.Kebschull M, Demmer RT, Papapanou PN. "Gum bug leave my heart alone": Epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J. Dent. Res. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10:e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J. Dent. Res. 2013;92:485–491. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madianos PN, Bobetsis YA, Offenbacher S. Adverse pregnancy outcomes (APOs) and periodontal disease: pathogenic mechanisms. J. Clin. Periodontol. 2013;40(Suppl 14):S170–180. doi: 10.1111/jcpe.12082. [DOI] [PubMed] [Google Scholar]
- 10.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol. 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 11.Abusleme L, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–1025. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This metagenomic study characterized ecological events in subgingival microbial communities underpinning the development of periodontitis and elucidated the relationship between inflammation and the disease-associated microbiome.
- 12.Griffen AL, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176–1185. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorth P, et al. Metatranscriptomics of the human oral microbiome during health and disease. MBio. 2014;5:e01012–01014. doi: 10.1128/mBio.01012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewhirst FE, et al. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This study led to the Human Oral Microbiome Database, the first curated description of a human body site-specific microbiome.
- 15.Hajishengallis G, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This paper provided in vivo evidence that a single microbe acting as a keystone pathogen can cause quantitative and qualitative alterations to the commensal microbiota leading to dysbiosis.
- 16.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nature Rev. Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nature Rev. Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 18.Rosier BT, de Jager M, Zaura E, Krom BP. Historical and contemporary hypotheses on the development of oral diseases: are we there yet? Front Cell Infect. Microbiol. 2014;4 doi: 10.3389/fcimb.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: The Polymicrobial Synergy and Dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han YW, et al. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet. Gynecol. 2010;115:442–445. doi: 10.1097/AOG.0b013e3181cb9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heo SM, Haase EM, Lesse AJ, Gill SR, Scannapieco FA. Genetic relationships between respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from patients in the intensive care unit undergoing mechanical ventilation. Clin. Infect. Dis. 2008;47:1562–1570. doi: 10.1086/593193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scannapieco FA. Individuals with chronic obstructive pulmonary disease (COPD) may be more likely to have more severe periodontal disease than individuals without COPD. J. Evid. Based Dent. Pract. 2014;14:79–81. doi: 10.1016/j.jebdp.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Tan L, Wang H, Li C, Pan Y. 16S rDNA-based metagenomic analysis of dental plaque and lung bacteria in patients with severe acute exacerbations of chronic obstructive pulmonary disease. J. Periodont. Res. 2014 doi: 10.1111/jre.12159. [DOI] [PubMed] [Google Scholar]
- 25.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Jr., Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 2005;25:e17–e18. doi: 10.1161/01.ATV.0000155018.67835.1a. [DOI] [PubMed] [Google Scholar]
- 26.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 27.Carrion J, et al. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J. Immunol. 2012;189:3178–3187. doi: 10.4049/jimmunol.1201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This study combined clinical and mechanistic evidence implicating blood myeloid dendritic cells as transport vehicles for the systemic dissemination of P. gingivalis from its oral habitat.
- 28.Velsko IM, et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One. 2014;9:e97811. doi: 10.1371/journal.pone.0097811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slocum C, et al. Distinct lipid A moieties contribute to pathogen-induced site-specific vascular inflammation. PLoS Pathog. 2014;10:e1004215. doi: 10.1371/journal.ppat.1004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This study showed that the ability of P. gingivalis to enzymatically modify the lipid A moiety of its lipopolysaccharide allows it to escape TLR4-mediated immunity and persist while inducing atherogenic inflammation at sites distant from initial infection.
- 30.Maresz KJ, et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD) PLoS Pathog. 2013;9:e1003627. doi: 10.1371/journal.ppat.1003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First study to provide an in vivo mechanistic link between P. gingivalis peptidyl-arginine deiminase and rheumatoid arthritis associated with anti-citrullinated protein antibodies.
- 31.Wegner N, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheumatol. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikuls TR, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:1090–1100. doi: 10.1002/art.38348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr. Rheumatol. Rep. 2014;16:408. doi: 10.1007/s11926-014-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Chaparro PJ, et al. Newly identified pathogens associated with periodontitis: A systematic review. J. Dent. Res. 2014;93:846–858. doi: 10.1177/0022034514542468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sima C, Glogauer M. Neutrophil dysfunction and host susceptibility to periodontal inflammation: Current state of knowledge. Curr. Oral Health Rep. 2014;1:95–103. [Google Scholar]
- 36.Nussbaum G, Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J. Clin. Periodontol. 2011;38:49–59. doi: 10.1111/j.1600-051X.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz O. The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay. Microbiology. 2008;154:2897–2903. doi: 10.1099/mic.0.2008/021220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darveau RP. Porphyromonas gingivalis neutrophil manipulation: risk factor for periodontitis? Trends Microbiol. 2014 doi: 10.1016/j.tim.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett. 2012;333:1–9. doi: 10.1111/j.1574-6968.2012.02579.x. [DOI] [PubMed] [Google Scholar]
- 40.Holt SC, Ebersole J, Felton J, Brunsvold M, Kornman KS. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239:55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 41.Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 2012;91:816–820. doi: 10.1177/0022034512453589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maekawa T, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014;15:768–778. doi: 10.1016/j.chom.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This investigation mechanistically dissected how periodontal bacteria can interfere with immune-mediated killing while promoting a nutritionally favorable inflammatory response, thereby perpetuating dysbiosis.
- 43.Hajishengallis G, Lamont RJ. Breaking bad: Manipulation of the host response by Porphyromonas gingivalis. Eur. J. Immunol. 2014;44:328–338. doi: 10.1002/eji.201344202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiao Y, et al. Induction of bone loss by pathobiont-mediated nod1 signaling in the oral cavity. Cell Host Microbe. 2013;13:595–601. doi: 10.1016/j.chom.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This study demonstrated the concept of commensal-turned-pathobiont in periodontitis under conditions of disrupted tissue homeostasis.
- 45.Whitmore SE, Lamont RJ. The pathogenic persona of community-associated oral streptococci. Mol. Microbiol. 2011;81:305–314. doi: 10.1111/j.1365-2958.2011.07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haffajee AD, et al. Subgingival microbiota in healthy, well-maintained elder and periodontitis subjects. J. Clin. Periodontol. 1998;25:346–353. doi: 10.1111/j.1600-051x.1998.tb02454.x. [DOI] [PubMed] [Google Scholar]
- 47.Kesavalu L, et al. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect. Immun. 2007;75:1704–1712. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daep CA, Novak EA, Lamont RJ, Demuth DR. Structural dissection and in vivo effectiveness of a peptide inhibitor of Porphyromonas gingivalis adherence to Streptococcus gordonii. Infect. Immun. 2011;79:67–74. doi: 10.1128/IAI.00361-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orth RK, O'Brien-Simpson NM, Dashper SG, Reynolds EC. Synergistic virulence of Porphyromonas gingivalis and Treponema denticola in a murine periodontitis model. Mol. Oral Microbiol. 2011;26:229–240. doi: 10.1111/j.2041-1014.2011.00612.x. [DOI] [PubMed] [Google Scholar]
- 50.Polak D, et al. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J. Clin. Periodontol. 2009;36:406–410. doi: 10.1111/j.1600-051X.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- 51.Tan KH, et al. Porphyromonas gingivalis and Treponema denticola exhibit metabolic symbioses. PLoS Pathog. 2014;10:e1003955. doi: 10.1371/journal.ppat.1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amano A, et al. Genetic characteristics and pathogenic mechanisms of periodontal pathogens. Adv. Dent. Res. 2014;26:15–22. doi: 10.1177/0022034514526237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuboniwa M, et al. Insights into the virulence of oral biofilms: discoveries from proteomics. Expert Rev. Proteom. 2012;9:311–323. doi: 10.1586/epr.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cyktor JC, Turner J. Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect. Immun. 2011;79:2964–2973. doi: 10.1128/IAI.00047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol. Oral Microbiol. 2014 doi: 10.1111/omi.12065. DOI: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hajishengallis E, Hajishengallis G. Neutrophil homeostasis and periodontal health in children and adults. J. Dent. Res. 2014;93:231–237. doi: 10.1177/0022034513507956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moutsopoulos NM, et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17–driven inflammatory bone loss. Sci. Transl. Med. 2014;6:229ra240. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ryder MI. Comparison of neutrophil functions in aggressive and chronic periodontitis. Periodontol. 2000. 2010;53:124–137. doi: 10.1111/j.1600-0757.2009.00327.x. [DOI] [PubMed] [Google Scholar]
- 59.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 60.Eskan MA, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nature Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee W, Aitken S, Sodek J, McCulloch CA. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J. Periodon. Res. 1995;30:23–33. doi: 10.1111/j.1600-0765.1995.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 62.Hernandez M, et al. Associations between matrix metalloproteinase-8 and -14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: a longitudinal study. J. Periodontol. 2010;81:1644–1652. doi: 10.1902/jop.2010.100196. [DOI] [PubMed] [Google Scholar]
- 63.Landzberg M, Doering H, Aboodi GM, Tenenbaum HC, Glogauer M. Quantifying oral inflammatory load: oral neutrophil counts in periodontal health and disease. J. Periodont. Res. 2014 doi: 10.1111/jre.12211. DOI: 10.1111/jre.12211. [DOI] [PubMed] [Google Scholar]
- 64.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J. Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 66.Jusko M, et al. A metalloproteinase karilysin present in the majority of Tannerella forsythia isolates inhibits all pathways of the complement system. J. Immunol. 2012;188:2338–2349. doi: 10.4049/jimmunol.1101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Potempa M, et al. Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS Pathog. 2009;5:e1000316. doi: 10.1371/journal.ppat.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller DP, et al. Structure of factor H-binding protein B (FhbB) of the periopathogen, Treponema denticola: insights into progression of periodontal disease. J. Biol. Chem. 2012;287:12715–12722. doi: 10.1074/jbc.M112.339721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malm S, et al. Acquisition of complement inhibitor serine protease factor I and its cofactors C4b-binding protein and factor H by Prevotella intermedia. PLoS One. 2012;7:e34852. doi: 10.1371/journal.pone.0034852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang S, et al. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J. Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 72.Myneni SR, et al. TLR2 signaling and Th2 responses drive Tannerella forsythia-induced periodontal bone loss. J Immunol. 2011;187:501–509. doi: 10.4049/jimmunol.1100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin JE, Baek KJ, Choi YS, Choi Y. A periodontal pathogen Treponema denticola hijacks the Fusobacterium nucleatum-driven host response. Immunol. Cell Biol. 2013;91:503–510. doi: 10.1038/icb.2013.35. [DOI] [PubMed] [Google Scholar]
- 74.Wang M, et al. Microbial hijacking of complement-toll-like receptor crosstalk. Sci. Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taxman DJ, et al. Porphyromonas gingivalis mediates inflammasome repression in polymicrobial cultures through a novel mechanism involving reduced endocytosis. J. Biol. Chem. 2012;287:32791–32799. doi: 10.1074/jbc.M112.401737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Moutsopoulos NM, et al. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J. Autoimmun. 2012;39:294–303. doi: 10.1016/j.jaut.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jauregui CE, et al. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect. Immun. 2013;81:2288–2295. doi: 10.1128/IAI.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This study showed that the P. gingivalis-induced 'local chemokine paralysis', originally shown to affect innate immunity, also involves suppression of T-helper 1 chemokines.
- 79.Hayashi C, et al. Protective role for TLR4 signaling in atherosclerosis progression as revealed by infection with a common oral pathogen. J. Immunol. 2012;189:3681–3688. doi: 10.4049/jimmunol.1201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol. 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 81.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J. Dent. Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bahekar AA, Singh S, Saha S, Molnar J, Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am. Heart J. 2007;154:830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 83.Friedewald VE, et al. The American Journal of Cardiology and Journal of Periodontology Editors' Consensus: periodontitis and atherosclerotic cardiovascular disease. Am. J. Cardiol. 2009;104:59–68. doi: 10.1016/j.amjcard.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Tonetti MS, et al. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 85.Offenbacher S, et al. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J. Periodontol. 2009;80:190–201. doi: 10.1902/jop.2009.080007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Desvarieux M, et al. Changes in clinical and microbiological periodontal profiles relate to progression of carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology study. J. Am. Heart Assoc. 2013;2:e000254. doi: 10.1161/JAHA.113.000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brodala N, et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2005;25:1446–1451. doi: 10.1161/01.ATV.0000167525.69400.9c. [DOI] [PubMed] [Google Scholar]
- 88.Tonetti MS. Periodontitis and risk for atherosclerosis: an update on intervention trials. J. Clin. Periodontol. 2009;36(Suppl 10):15–19. doi: 10.1111/j.1600-051X.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- 89.Arimatsu K, et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014;4:4828. doi: 10.1038/srep04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gibson FC, 3rd, et al. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 91.Jain A, Batista EL, Jr., Serhan C, Stahl GL, Van Dyke TE. Role for periodontitis in the progression of lipid deposition in an animal model. Infect. Immun. 2003;71:6012–6018. doi: 10.1128/IAI.71.10.6012-6018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zelkha SA, Freilich RW, Amar S. Periodontal innate immune mechanisms relevant to atherosclerosis and obesity. Periodontol. 2000. 2010;54:207–221. doi: 10.1111/j.1600-0757.2010.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chiu B. Multiple infections in carotid atherosclerotic plaques. Am. Heart J. 1999;138:S534–536. doi: 10.1016/s0002-8703(99)70294-2. [DOI] [PubMed] [Google Scholar]
- 94.Reyes L, Herrera D, Kozarov E, Roldan S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J. Clin. Periodontol. 2013;40(Suppl 14):S30–50. doi: 10.1111/jcpe.12079. [DOI] [PubMed] [Google Scholar]
- 95.Hayashi C, et al. Pathogen-mediated inflammatory atherosclerosis is mediated in part via Toll-like receptor 2-induced inflammatory responses. J. Innate Immun. 2010;2:334–343. doi: 10.1159/000314686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coats SR, et al. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4'- phosphatase activities. Cell Microbiol. 2009;11:1587–1599. doi: 10.1111/j.1462-5822.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Curtis MA, et al. Temperature dependent modulation of Porphyromonas gingivalis lipid A structure and interaction with the innate host defences. Infect. Immun. 2011;79:1187–1193. doi: 10.1128/IAI.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Qutub MN, et al. Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect. Immun. 2006;74:4474–4485. doi: 10.1128/IAI.01924-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refs. 97 and 98 have shown at the molecular level how environmental factors can influence microbial virulence and host-microbe interactions.
- 99.Zenobia C, et al. Porphyromonas gingivalis lipid A phosphatase activity is critical for colonization and increasing the commensal load in the rabbit ligature model. Infect. Immun. 2014;82:650–659. doi: 10.1128/IAI.01136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Coats SR, Do CT, Karimi-Naser LM, Braham PH, Darveau RP. Antagonistic lipopolysaccharides block E. coli lipopolysaccharide function at human TLR4 via interaction with the human MD-2 lipopolysaccharide binding site. Cell Microbiol. 2007;9:1191–1202. doi: 10.1111/j.1462-5822.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 101.Bostanci N, et al. Porphyromonas gingivalis antagonises Campylobacter rectus induced cytokine production by human monocytes. Cytokine. 2007;39:147–156. doi: 10.1016/j.cyto.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 102.Wang M, et al. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J. Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 103.Wang M, Hajishengallis G. Lipid raft-dependent uptake, signalling and intracellular fate of Porphyromonas gingivalis in mouse macrophages. Cell Microbiol. 2008;10:2029–2042. doi: 10.1111/j.1462-5822.2008.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manes S, del Real G, Martinez AC. Pathogens: raft hijackers. Nat. Rev. Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- 105.Zeituni AE, McCaig W, Scisci E, Thanassi DG, Cutler CW. The native 67-kilodalton minor fimbria of Porphyromonas gingivalis is a novel glycoprotein with DC-SIGN-targeting motifs. J. Bacteriol. 2010;192:4103–4110. doi: 10.1128/JB.00275-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeituni AE, Jotwani R, Carrion J, Cutler CW. Targeting of DC-SIGN on human dendritic cells by minor fimbriated Porphyromonas gingivalis strains elicits a distinct effector T cell response. J. Immunol. 2009;183:5694–5704. doi: 10.4049/jimmunol.0901030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miles B, et al. Secondary lymphoid organ homing phenotype of human myeloid dendritic cells disrupted by an intracellular oral pathogen. Infect Immun. 2014;82:101–111. doi: 10.1128/IAI.01157-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liang Z, et al. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- 109.Sainz J, Sata M. CXCR4, a key modulator of vascular progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2007;27:263–265. doi: 10.1161/01.ATV.0000256727.34148.e2. [DOI] [PubMed] [Google Scholar]
- 110.Belstrom D, et al. The atherogenic bacterium Porphyromonas gingivalis evades circulating phagocytes by adhering to erythrocytes. Infect. Immun. 2011;79:1559–1565. doi: 10.1128/IAI.01036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Madrigal AG, Barth K, Papadopoulos G, Genco CA. Pathogen-mediated proteolysis of the cell death regulator RIPK1 and the host defense modulator RIPK2 in human aortic endothelial cells. PLoS Pathog. 2012;8:e1002723. doi: 10.1371/journal.ppat.1002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huck O, Elkaim R, Davideau JL, Tenenbaum H. Porphyromonas gingivalis-impaired innate immune response via NLRP3 proteolysis in endothelial cells. Innate Immun. 2014 doi: 10.1177/1753425914523459. doi: 10.1177/1753425914523459. [DOI] [PubMed] [Google Scholar]
- 113.Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J. Immunol. 2006;176:7645–7656. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 114.Takahashi Y, Davey M, Yumoto H, Gibson FC, 3rd, Genco CA. Fimbria-dependent activation of pro-inflammatory molecules in Porphyromonas gingivalis infected human aortic endothelial cells. Cell Microbiol. 2006;8:738–757. doi: 10.1111/j.1462-5822.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 115.Jain S, Coats SR, Chang AM, Darveau RP. A novel class of lipoprotein lipase-sensitive molecules mediates Toll-like receptor 2 activation by Porphyromonas gingivalis. Infect. Immun. 2013;81:1277–1286. doi: 10.1128/IAI.01036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clark RB, et al. Serine lipids of Porphyromonas gingivalis are human and mouse Toll-like receptor 2 ligands. Infect. Immun. 2013 doi: 10.1128/IAI.00803-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Delbosc S, et al. Porphyromonas gingivalis participates in pathogenesis of human abdominal aortic aneurysm by neutrophil activation. Proof of concept in rats. PLoS One. 2011;6:e18679. doi: 10.1371/journal.pone.0018679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakayama K. Porphyromonas gingivalis cell-induced hemagglutination and platelet aggregation. Periodontol. 2000. 2010;54:45–52. doi: 10.1111/j.1600-0757.2010.00351.x. [DOI] [PubMed] [Google Scholar]
- 119.Han YW, Houcken W, Loos BG, Schenkein HA, Tezal M. Periodontal disease, atherosclerosis, adverse pregnancy outcomes, and head-and-neck cancer. Adv. Dent. Res. 2014;26:47–55. doi: 10.1177/0022034514528334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen YW, et al. Association between periodontitis and anti-cardiolipin antibodies in Buerger disease. J. Clin. Periodontol. 2009;36:830–835. doi: 10.1111/j.1600-051X.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 121.Li XY, et al. Porphyromonas gingivalis lipopolysaccharide increases lipid accumulation by affecting CD36 and ATP-binding cassette transporter A1 in macrophages. Oncol. Rep. 2013;30:1329–1336. doi: 10.3892/or.2013.2600. [DOI] [PubMed] [Google Scholar]
- 122.Giacona MB, et al. Porphyromonas gingivalis induces its uptake by human macrophages and promotes foam cell formation in vitro. FEMS Microbiol. Lett. 2004;241:95–101. doi: 10.1016/j.femsle.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 123.Sanz M, Kornman K. Periodontitis and adverse pregnancy outcomes: consensus report of the Joint EFP/AAP workshop on periodontitis and systemic diseases. J. Clin. Periodontol. 2013;40(Suppl 14):S164–169. doi: 10.1111/jcpe.12083. [DOI] [PubMed] [Google Scholar]
- 124.Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J. Immunol. 2007;179:2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- First mechanistic study linking an oral pathogen to pregnancy complications in an animal model.
- 125.Han YW, et al. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect. Immun. 2004;72:2272–2279. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schenkein HA, et al. Anti-cardiolipin antibodies in sera from patients with periodontitis. J. Dent. Res. 2003;82:919–922. doi: 10.1177/154405910308201114. [DOI] [PubMed] [Google Scholar]
- 127.Schenkein HA, Bradley JL, Purkall DB. Anticardiolipin in Porphyromonas gingivalis antisera causes fetal loss in mice. J. Dent. Res. 2013;92:814–818. doi: 10.1177/0022034513497959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This report implicated molecular mimicry as a mechanism by which the host response to P. gingivalis can induce cardiolipin-specific autoantibodies that cause fetal loss in mice.
- 128.Scher JU, Bretz WA, Abramson SB. Periodontal disease and subgingival microbiota as contributors for rheumatoid arthritis pathogenesis: modifiable risk factors? Curr. Opin. Rheumatol. 2014;26:424–429. doi: 10.1097/BOR.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Joseph R, Rajappan S, Nath SG, Paul BJ. Association between chronic periodontitis and rheumatoid arthritis: a hospital-based case-control study. Rheumatol. Int. 2013;33:103–109. doi: 10.1007/s00296-011-2284-1. [DOI] [PubMed] [Google Scholar]
- 130.de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J. Rheumatol. 2008;35:70–76. [PubMed] [Google Scholar]
- 131.Suwannalai P, Trouw LA, Toes RE, Huizinga TW. Anti-citrullinated protein antibodies (ACPA) in early rheumatoid arthritis. Mod. Rheumatol. 2012;22:15–20. doi: 10.1007/s10165-011-0486-8. [DOI] [PubMed] [Google Scholar]
- 132.Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int. J. Biochem. Cell Biol. 2006;38:1662–1677. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 133.Pyrc K, et al. Inactivation of Epidermal Growth Factor by Porphyromonas gingivalis as a potential mechanism for periodontal tissue damage. Infect. Immun. 2013;81:55–64. doi: 10.1128/IAI.00830-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pratesi F, et al. HLA shared epitope and ACPA: just a marker or an active player? Autoimmun. Rev. 2013;12:1182–1187. doi: 10.1016/j.autrev.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 135.Lundberg K, et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008;58:3009–3019. doi: 10.1002/art.23936. [DOI] [PubMed] [Google Scholar]
- This study identified an immunodominant epitope in citrullinated α-enolase that is cross-reactive with citrullinated P. gingivalis enolase and is implicated in rheumatoid arthritis, hence consistent with a role for P. gingivalis and its cittrullinating enzyme in priming autoimmunity.
- 136.Catrina AI, Ytterberg AJ, Reynisdottir G, Malmstrom V, Klareskog L. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nature Rev. Rheumatol. 2014 doi: 10.1038/nrrheum.2014.115. [DOI] [PubMed] [Google Scholar]
- 137.Sokolove J, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gully N, et al. Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis. PLoS One. 2014;9:e100838. doi: 10.1371/journal.pone.0100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007;13:508–512. doi: 10.1111/j.1601-0825.2007.1410a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Finegold SM. Aspiration pneumonia. Rev Infect Dis. 1991;13(Suppl 9):S737–742. doi: 10.1093/clinids/13.supplement_9.s737. [DOI] [PubMed] [Google Scholar]
- 141.Awano S, et al. Oral health and mortality risk from pneumonia in the elderly. J. Dent. Res. 2008;87:334–339. doi: 10.1177/154405910808700418. [DOI] [PubMed] [Google Scholar]
- 142.Kimizuka R, Kato T, Ishihara K, Okuda K. Mixed infections with Porphyromonas gingivalis and Treponema denticola cause excessive inflammatory responses in a mouse pneumonia model compared with monoinfections. Microbes Infect. 2003;5:1357–1362. doi: 10.1016/j.micinf.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 143.Muller F. Oral hygiene reduces the mortality from aspiration pneumonia in frail elders. J. Dent. Res. 2014 doi: 10.1177/0022034514552494. doi: 10.1177/0022034514552494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iinuma T, et al. Denture wearing during sleep doubles the risk of pneumonia in the very elderly. J. Dent. Res. 2014 doi: 10.1177/0022034514552493. doi: 10.1177/0022034514552493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peter KP, et al. Association between periodontal disease and chronic obstructive pulmonary disease: a reality or just a dogma? J. Periodontol. 2013;84:1717–1723. doi: 10.1902/jop.2013.120347. [DOI] [PubMed] [Google Scholar]
- 146.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 147.Pan Y, Teng D, Burke AC, Haase EM, Scannapieco FA. Oral bacteria modulate invasion and induction of apoptosis in HEp-2 cells by Pseudomonas aeruginosa. Microb. Pathog. 2009;46:73–79. doi: 10.1016/j.micpath.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 148.Li Q, et al. Porphyromonas gingivalis modulates Pseudomonas aeruginosa-induced apoptosis of respiratory epithelial cells through the STAT3 signaling pathway. Microbes Infect. 2014;16:17–27. doi: 10.1016/j.micinf.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 149.Yao L, et al. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral Microbiol. 2010;25:89–101. doi: 10.1111/j.2041-1014.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cannon CL, Kowalski MP, Stopak KS, Pier GB. Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am. J. Respir. Cell Mol. Biol. 2003;29:188–197. doi: 10.1165/rcmb.4898. [DOI] [PubMed] [Google Scholar]
- 151.Papapanou PN. In: Oral Microbiology and Immunology. Lamont RJ, Hajishengallis G, Jenkinson H, editors. ASM Press; Washington DC: 2014. pp. 251–271. [Google Scholar]
- 152.Kumar PS. Smoking and the subgingival ecosystem: a pathogen-enriched community. Future Microbiol. 2012;7:917–919. doi: 10.2217/fmb.12.71. [DOI] [PubMed] [Google Scholar]
- 153.Stabholz A, Soskolne WA, Shapira L. Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontol 2000. 2010;53:138–153. doi: 10.1111/j.1600-0757.2010.00340.x. [DOI] [PubMed] [Google Scholar]
- 154.Laine ML, Crielaard W, Loos BG. Genetic susceptibility to periodontitis. Periodontol. 2000. 2012;58:37–68. doi: 10.1111/j.1600-0757.2011.00415.x. [DOI] [PubMed] [Google Scholar]
- 155.Divaris K, et al. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum. Mol. Genet. 2013;22:2312–2324. doi: 10.1093/hmg/ddt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bagaitkar J, et al. Tobacco smoke augments Porphyromonas gingivalis-Streptococcus gordonii biofilm formation. PLoS One. 2011;6:e27386. doi: 10.1371/journal.pone.0027386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Atanasova KR, Yilmaz O. Looking in the Porphyromonas gingivalis cabinet of curiosities: the microbium, the host and cancer association. Mol. Oral Microbiol. 2014;29:55–66. doi: 10.1111/omi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Castellarin M, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rubinstein MR, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This study generated both mechanistic and clinical evidence implicating the oral bacterium F. nucleatum in colorectal cancer.
- 160.Nagy KN, Sonkodi I, Szoke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34:304–308. [PubMed] [Google Scholar]
- 161.Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int. J. Oral Sci. 2011;3:209–215. doi: 10.4248/IJOS11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33:1055–1058. doi: 10.1093/carcin/bgs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Michaud DS, et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62:1764–1770. doi: 10.1136/gutjnl-2012-303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Inaba H, et al. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16:131–145. doi: 10.1111/cmi.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kuboniwa M, et al. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008;10:122–128. doi: 10.1016/j.micinf.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mao S, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yilmaz O, et al. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008;10:863–875. doi: 10.1111/j.1462-5822.2007.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Iwahori K, et al. Overexpression of SOCS3 exhibits preclinical antitumor activity against malignant pleural mesothelioma. Int. J. Cancer. 2011;129:993–1005. doi: 10.1002/ijc.25716. [DOI] [PubMed] [Google Scholar]
- 169.Moffatt CE, Lamont RJ. Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect. Immun. 2011;79:2632–2637. doi: 10.1128/IAI.00082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Groeger S, Domann E, Gonzales JR, Chakraborty T, Meyle J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology. 2011;216:1302–1310. doi: 10.1016/j.imbio.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 171.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]