Abstract
Background: In eutherian mammals and in humans, the female fetus may be masculinized while sharing the intra-uterine environment with a male fetus. Telomere length (TL), as expressed in leukocytes, is heritable and is longer in women than in men. The main determinant of leukocyte TL (LTL) is LTL at birth. However, LTL is modified by age-dependent attrition.
Methods: We studied LTL dynamics (LTL and its attrition) in adult same-sex (monozygotic, n = 268; dizygotic, n = 308) twins and opposite-sex (n = 144) twins. LTL was measured by Southern blots of the terminal restriction fragments.
Results: We observed that in same-sex (both monozygotic and dizygotic) twins, as reported in singletons, LTL was longer in females than in males [estimate ± standard error (SE):163 ± 63 bp, P < 0.01]. However, in opposite-sex twins, female LTL was indistinguishable from that of males (−31 ± 52 bp, P = 0.6), whereas male LTL was not affected. Findings were similar when the comparison was restricted to opposite-sex and same-sex dizygotic twins (females relative to males: same-sex: 188 ± 90 bp, P < 0.05; other-sex: −32 ± 64 bp, P = 0.6).
Conclusions: These findings are compatible with masculinization of the female fetus in opposite-sex twins. They suggest that the sex difference in LTL, seen in the general population, is largely determined in utero, perhaps by the intrauterine hormonal environment. Further studies in newborn twins are warranted to test this thesis.
Keywords: Telomeres, twins, sex, women, men
Key Messages.
Women have a longer LTL than men.
The sex effect on LTL is largely determined in utero.
The sex effect on LTL might be mediated in part by the intra-uterine milieu.
Introduction
Leukocyte telomere length (LTL) heritability is estimated at 60%1–3 and is affected by paternal age at conception4 and the environment.5 Notably, LTL is longer in women than in men.2,6–8 LTL is defined by two elements: LTL at birth, which is ostensibly the main determinant of LTL throughout the human life course; and its attrition thereafter. Thus, intra-uterine variables, some of which are genetic but others are not, are largely involved in fashioning human LTL. The question then is what mechanisms, independent of those controlled by the individual’s genetic make-up, might influence LTL at birth?
In the course of studying LTL dynamics (LTL and its attrition) in adult twins we found that LTL was equivalent between male and female co-twins, i.e. opposite-sex (OS) twins. These findings contrasted with those across same-sex (SS) pairs of male and female dizygotic (DZ) twins as well as monozygotic (MZ) twins. The equivalence in LTL between OS twins also contrasted with findings in singletons reported in previous studies.2,6–8 The potential ‘masculinization’ of female co-twins of OS twins has been observed before with respect to both behavioural and anatomical traits,9–11 although the underlying mechanisms are poorly understood. Here we present our findings on the equivalence in LTL between male and female OS co-twins and consider their potential biological meaning.
Methods
Subjects
Healthy subjects were originally recruited through the National Danish Twin Registry12,13 to participate in the longitudinal GEMINAKAR Study. At baseline, participants were without overt diabetes or cardiovascular disease. For this specific study we included only intact twin pairs. Twins were recruited in equal strata of age, sex and zygosity. The study was approved by the Regional Scientific Ethical Committees for Southern Denmark and the Danish Data Protection Agency. All participants provided written informed consent.
Leukocyte telomere length measurements
Measurement of the terminal restriction fragments was performed in duplicate on different gels by Southern blots as previously described.14 The inter-assay coefficient of variation for the duplicate measures was 1.3%.
Statistical analysis
Data were analysed with mixed general linear models. We included twin identity as a random effect because LTL measurements of co-twins are not statistically independent. Although there was no association of zygosity with LTL or LTL attrition, we analysed the data both including and excluding MZ twins. In light of small age differences between OS and SS twins in our sample, we adjusted for age throughout the analysis.
For longitudinal statistical analysis, age was split into two terms because between-individual effects may have a different slope when compared with within-individual effects. The two terms were firstly the average of the age at the two sampling points for each individual, and secondly the deviation of the age at each of the two points from the individual average (delta age). For example, for an individual whose LTL was measured at ages 20 (baseline examination) and 30 years (follow-up examination), the average age is 25 for both examinations, whereas delta age is −5 and +5 for the baseline examination and follow-up examination, respectively. This procedure yields an unbiased estimate of the within-individual effect of age on LTL.15
SS twins can be either MZ or DZ, but OS twins are always DZ. It can be argued therefore that comparing OS and SS twins is less likely to be confounded when restricting the analysis to DZ twins. We chose to first analyse the complete data set (i.e. combining MZ and DZ twins), and tested statistically whether the OS effect on LTL depends on zygosity. We subsequently verified the results for DZ twins only.
Results
General characteristics of the twins, including age, baseline LTL and follow-up LTL ∼ 11.5 years later, are displayed in Table 1.
Table 1.
General characteristics of the twin sample from the GEMINAKAR Study, means ± standard deviation
| Characteristic | Males | Females | Males | Females | Males | Females | |
|---|---|---|---|---|---|---|---|
| OS | OS | DZ | DZ | MZ | MZ | ||
| N | 72 | 72 | 132 | 176 | 172 | 196 | |
| AgeB (years) | 39.8 ± 11.2 | 39.8 ± 11.2 | 39.2 ± 9.9 | 37.6 ± 8.2 | 37.4 ± 9.7 | 36.0 ± 10.4** | |
| AgeFU (years) | 51.0 ± 11.2 | 51.0 ± 11.2 | 51.2 ± 9.7 | 50.0 ± 8.2 | 49.6 ± 9.6 | 48.2 ± 10.4* | |
| BMIB | 25.0 ± 3.2 | 24.2 ± 3.2 | 25.4 ± 3.6 | 23.4 ± 3.4### | 24.9 ± 2.8 | 24.1 ± 3.8 | |
| BMIFU | 26.4 ± 3.6 | 25.7 ± 4.2 | 26.5 ± 4.0 | 24.5 ± 4.1### | 26.3 ± 3.5 | 25.5 ± 4.8 | |
| LTLB (kb) | 6.78 ± 0.67 | 6.74 ± 0.60 | 6.83 ± 0.60 | 7.04 ± 0.71**## | 6.93 ± 0.65 | 7.09 ± 0.65***# | |
| LTLFU (kb) | 6.61 ± 0.64 | 6.59 ± 0.58 | 6.59 ± 0.55 | 6.80 ± 0.67*## | 6.70 ± 0.65 | 6.86 ± 0.65**# | |
| LTL attrition (bp/yr) | 13.2 ± 15.4 | 12.6 ± 14.1 | 20.1 ± 14.7** | 18.4 ± 13.9** | 19.5 ± 12.2** | 20.7 ± 15.3*** | |
| LTL attritionBA (bp/yr) | 14.4 ± 14.5 | 14.0 ± 13.9 | 20.7 ± 13.7** | 17.9 ± 13.0* | 19.6 ± 12.2* | 19.8 ± 14.8* | |
MZ, monozygotic; DZ, dizygotic same-sex; OS, opposite-sex; AgeB, age at baseline examination; AgeFU, age at follow-up examination; LTLB, LTL at baseline examination; LTLFU, LTL at follow-up examination; LTL attritionBA, LTL attrition adjusted for baseline LTL, calculated from residual values.
*P < 0.05, **P < 0.01, and ***P < 0.001 vs. same-sex OS twins; #P < 0.05, ##P < 0.01, and ###P < 0.001 for the sex difference in each twins' type, respectively.
Cross-sectional analyses
We first examined whether the sex association with LTL was dependent on the sex composition of the twin pair, OS vs SS twins, by estimating the interaction between sex and ‘opposite sex’ as a factor. We started by estimating this interaction in the complete data set, that is combining baseline and follow-up measurements, and DZ and MZ twins. In this analysis, each longitudinally sampled individual was represented by two sampling points that were not independent, and therefore individual identity was nested in twin identity and included as a random effect. The parameter estimate of the Sex * OS interaction was almost the exact opposite of the parameter estimate of ‘Sex’ (Table 2A), with the result that these cancelled each other out. Accordingly, there was a sex difference among SS twins, with males having shorter LTL, but not among OS twins, where female LTL was similar to male LTL in both SS and OS twins (Figure 1). Notably, MZ and DZ twins did not differ in LTL (P = 0.38 when added to the model in Table 2A), and zygosity did not interact with sex (P = 0.7). We subsequently repeated this analysis for DZ twins only, for the baseline and follow-up measurement combined (Table 2B), and also for each of these measurements in isolation (Table 2C, D; Figure S1, available as Supplementary data at IJE online). These analyses confirmed the initial results, in that parameter estimates in all these analyses were very similar. Lastly, we repeated the analyses above, but now including body mass index (BMI) as an additional covariate, which resulted in very little change in the parameter estimates (Table S1, available as Supplementary data at IJE online).
Table 2.
Cross sectional analysis of LTL in relation to age, sex and twin sex composition (opposite sex vs same sex)
| A. Baseline and follow-up combined, DZ and MZ combined, n=1534, R2=0.98 | ||||
|---|---|---|---|---|
| Parameter | Estimate (SE) | F | DF denominator | P |
| Intercept | 7.752 (0.0466) | |||
| Age | −0.0186 (0.0005) | 1309.10 | 787.3 | <0.0001 |
| Sex | −0.1643 (0.0615) | 7.14 | 402.0 | 0.008 |
| Opposite sex (OS) | −0.2378 (0.0818) | 8.46 | 492.9 | 0.004 |
| Sex * OS | 0.1952 (0.0808) | 5.84 | 775.3 | 0.016 |
| B. Baseline and follow-up combined, DZ twins only, n=845, R2=0.98 | ||||
|---|---|---|---|---|
| Parameter | Estimate (SE) | F | DF denominator | P |
| Intercept | 7.685 (0.0651) | |||
| Age | −0.0175 (0.0007) | 608.12 | 433.4 | <0.0001 |
| Sex | −0.1911 (0.0876) | 3.58 | 276.0 | <0.06 |
| Opposite sex (OS) | −0.2229 (0.0909) | 2.57 | 222.5 | 0.11 |
| Sex * OS | 0.2230 (0.1082) | 4.25 | 402.1 | 0.04 |
| C. Baseline, DZ twins only, n=433, R2=0.76 | ||||
|---|---|---|---|---|
| Parameter | Estimate (SE) | F | DF denominator | P |
| Intercept | 7.800 (0.156) | |||
| Age | −0.0203 (0.0038) | 28.09 | 219.8 | <0.0001 |
| Sex | −0.1649 (0.0916) | 3.24 | 221.4 | 0.073 |
| Opposite sex (OS) | −0.2489 (0.0956) | 6.78 | 283.9 | <0.01 |
| Sex * OS | 0.1993 (0.1147) | 3.02 | 401.7 | 0.083 |
| D. Follow-up, DZ twins only, n=412, R2=0.81 | ||||
|---|---|---|---|---|
| Parameter | Estimate (SE) | F | DF denominator | P |
| Intercept | 7.792 (0.1998) | |||
| Age | −0.0198 (0.0038) | 27.19 | 219.6 | <0.0001 |
| Sex | −0.1913 (0.0889) | 4.63 | 221.6 | 0.033 |
| Opposite sex (OS) | −0.1864 (0.0917) | 4.14 | 267.6 | 0.043 |
| Sex * OS | 0.2106 (0.1082) | 3.79 | 375.7 | 0.052 |
Sex was coded 0 (females) and 1 (males). Opposite sex was also coded 0 (same sex) and 1 (opposite sex). Twin identity was included as random effect in all tables, with individual identity nested in twin identity added as random effect in tables A and B.
Note the similarity of the Sex * OS estimate in all data selections. Numerator DF=1 in all cases.
Figure 1.
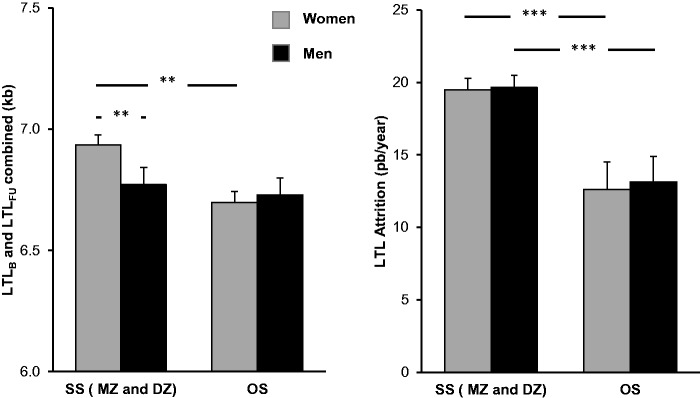
Age-adjusted leukocyte telomere length (LTL) and LTL attrition in same-sex (SS) twins (monozygotic, MZ; and dizygotic, DZ; pooled), and in opposite-sex (OS) twins. Left panel displays LTLs, bars show least square means from the model in Table 2A; right panel displays LTL attrition rate. *P < 0.05; **P < 0.01; ***P < 0.001.
Post-hoc analyses of the effect of being a co-twin of an OS twin pair showed that OS twin females had shorter LTLs than SS twin females (MZ and DZ pooled: −243 ± 82 bp, P = 0.003; DZ twins only: −228 ± 91 bp, P = 0.013; tested with a model as in Table 2, except that sex and the Sex * OS interaction were removed). However, there was no effect of OS vs SS twin status on LTL in males (P > 0.6 for either rMZ and DZ pooled, or for DZ only, tested as described above for females). In contrast, among SS twins, females had longer LTLs than males (MZ and DZ pooled: + 163 ± 63 bp, P < 0.01; DZ only: + 188 ± 90 bp, P < 0.038; tested as in Table 2C, but without the terms including OS), whereas LTLs did not differ significantly between the sexes among OS twins (males: + 32 ± 58 bp, P = 0.58).
Longitudinal analyses
One way in which twin type (SS/OS) could alter the sex association with LTL is through a sex-dependent effect on the rate of LTL attrition, i.e. if female LTL attrition were faster during the life course in OS twins than in female SS twins. We tested this possibility as follows: age at the two examinations was split into two terms [average of the two sampling point ages and deviation of each sampling point from the average for each individual (delta age)] to allow separation of between- and within-individual effects in the analyses, and variation in attrition rate was investigated by testing interactions with delta age.
Were differential LTL attrition to contribute to the dependence of the sex effect on LTL on twin type, this would be revealed by a significant three-way interaction between delta age, sex and twin type (OS/SS). However, this hypothesis was not supported (P = 0.7 for the interaction term; see ‘rejected terms’ in Table 3A). LTL attrition did depend on twin type, with LTL attrition being lower in OS twins than in SS twins, but this effect was independent of sex (indeed, the interaction between twin type and delta age was present in both sexes when tested separately, both P < 0.001), suggesting that sex association with LTL does not occur through a sex-dependent effect on the rate of LTL attrition. Repeating this analysis with DZ twins only did not change this result (Table 3B), and neither did the inclusion of BMI as an additional covariate (Table S2, available as Supplementary data at IJE online).
Table 3.
Longitudinal analysis of LTL in relation to age, sex and twin sex composition [opposite sex (OS) vs same sex (SS)]
| A. DZ and MZ twins combined, n=1535 samples, R2=0.98 | ||||
|---|---|---|---|---|
| Parameter | Estimate (SE) | F | DF denominator | P |
| Intercept | 7.900 (0.128) | 403.3 | <0.0001 | |
| Average age | −0.0221 (0.0028) | 61.400 | 403.2 | <0.0001 |
| Delta (Δ) age | −0.01955 (0.0006) | 1209.000 | 735.2 | <0.0001 |
| Sex | −0.1597 (0.0616) | 6.728 | 400.8 | 0.0098 |
| Opposite sex (OS) | −0.2286 (0.0820) | 7.764 | 490.5 | 0.0055 |
| Sex * OS | 0.1917 (0.0808) | 5.624 | 773.7 | 0.0180 |
| Δ Age * OS | 0.0067 (0.0014) | 22.260 | 734.1 | <0.0001 |
| Rejected terms | ||||
| OS * Sex * Δ Age | −0.0004 (0.00285) | 0.020 | 733.9 | 0.8880 |
| Sex * Δ Age | 0.0002 (0.00104) | 0.041 | 734.6 | 0.8400 |
| B. DZ twins only, n=845 samples, R2=0.98. | ||||
|---|---|---|---|---|
| Parameter | Estimate (SE) | F | DF denominator | P |
| Intercept | 7.84 (0.1646) | |||
| Average age | −0.0209 (0.0035) | 35.08 | 242.2 | <0.0001 |
| Delta (Δ) age | −0.0192 (0.0008) | 517.00 | 399.5 | <0.0001 |
| Sex | −0.1857 (0.0878) | 4.47 | 220.9 | 0.0356 |
| Opposite sex (OS) | −0.2173 (0.0912) | 5.67 | 281.0 | 0.0179 |
| Sex * OS | 0.2174 (0.1084) | 4.02 | 399.6 | 0.0456 |
| Δ Age * OS | 0.0063 (0.0016) | 16.61 | 399.5 | <0.0001 |
| Rejected terms | ||||
| OS * Sex * Δ Age | 0.0010 (0.0031) | 0.11 | 397.5 | 0.7400 |
| Sex * Δ Age | −0.0012 (0.0014) | 0.75 | 398.5 | 0.3800 |
Sex was coded 0 (females) and 1 (males). Opposite sex was also coded 0 (SS) and 1 (OS). Twin identity, and individual identity nested in twin identity were included as random effects. Model was obtained after backward deletion of least significant terms starting with the initial model that included the rejected terms shown in A. Numerator DF=1.
Discussion
In the present study, the female co-twins of OS twin pairs displayed LTL that was equivalent to that of their male co-twins, who did not differ in LTL from SS male twins. The underlying explanation for these findings is unclear. We cannot rule out life-course differences between OS and SS twins as a possible explanation for our findings but suggest that the observed effect is related to early-life determinants of LTL.
These determinants exert an outsize influence on LTL throughout the human life course,16,17 as evidenced by the wide variation of LTL across newborns (4–5 kb)18,19 and the strong LTL tracking in adults.16 Here, we propose that a shared intra-uterine milieu might be a potential explanation for the LTL equivalence in female and male OS co-twins. This premise is based on a body of work at the core of the twin testosterone transfer hypothesis.20 It includes studies in eutherian mammals showing that female fetuses positioned between two male fetuses are likely to express masculinization of anatomical, physiological and behavioural traits during extra-uterine life.21 Similar findings have been observed,9–11 but not always,22,23 in humans for female co-twins of OS twins. Of interest are findings in Soay sheep where the female of OS twins displayed diminished lifetime breeding success.24 A similar trend has been reported for humans where females co-twins of OS showed reduced lifetime reproductive success,9 although a large Danish twin study showed no difference.23 In line with these findings and those of the present work, a recent study suggested that women with a shorter LTL might have an earlier menopause.25 One potential explanation for these findings might be that the shared intra-uterine environment influences telomere dynamics in the female co-twins to shorten their LTL via factors originating from the male co-twins. Whether these factors include testosterone is unknown at present.
Given that embryonic and early fetal growth is marked by the most proliferative phase of the human life course, the intra-uterine milieu might have a considerable influence on LTL at subsequent ages. In addition, although the activity of telomerase, the reverse transcriptase that adds telomere repeats to the ends of chromosomes, is largely repressed in somatic tissues during extra-uterine life, the enzyme is active in these tissues during early fetal development.26,27 Accordingly, the developing fetus might be highly susceptible to factors that directly impact on telomere dynamics.
Sex hormones, i.e. estrogen and testosterone, are lipid-soluble steroids capable of crossing fetal membranes. Accordingly testosterone, produced by the male co-twin, and estrogen, generated by the female co-twin, might diffuse across fetal membranes to affect the OS co-twin. However, whereas rudimentary information is available on the role of testosterone in telomere dynamics in normal somatic cells, it is well established that estrogen stimulates telomerase and that an estrogen-response element is present in the promoter region of the catalytic subunit of the enzyme.28 The shorter LTL in female OS twins relative to that of female SS twins, together with the equivalence of LTL between male and female OS co-twins, strongly suggest that the influence on LTL is exerted by the male fetus on the female fetus and not vice versa.
We note the following limitations of the present work. First, the sample of OS twins was modest and the findings were unexpected. Thus, they require confirmation in future studies. Second, our hypothesis about the potential causes of LTL equivalence in OS twins focuses on the intra-uterine milieu. We cannot exclude the role of differences in the shared extra-uterine environment between OS and SS twins as another explanation for the equivalent LTL in OS twins, given that our subjects were adults. These might stem from different growth patterns and rates of LTL attrition between OS twins during growth and development. For instance, LTL has been shown to be inversely associated with BMI in some studies,29,30 and research in twins showed that shared environmental factors contribute to similarities in the BMI between twins.31,32 However, the effects of shared environmental factors on BMI in twins were limited to childhood and early adolescence.32 Notably, BMI in our models had no substantial effect on the findings. That said, a preferable approach to further explore our hypothesis would be to focus on LTL in newborn twins. Moreover, such a study might also examine sex hormone levels in cord blood of SS and OS twins, although their levels in cord blood may not reflect those during the embryonic and early fetal period when cellular proliferative activity is the highest. Finally, we are puzzled by and have no explanation for the lower rate of LTL attrition in OS twins (similarly low in both sexes), which was based on a follow-up period of 11.5 years. This finding suggests, however, that LTL attrition during adult life does not explain the shorter LTL in female OS twins; it reinforces the need to examine the OS twin effect not only on the LTLs of twins at birth, but also their LTL attrition prior to adulthood.
In conclusion, the equivalence of LTL in OS co-twins suggests that the female co-twins of OS twins take on the LTL characteristics of the male co-twins probably in utero. If true, this intra-uterine effect is independent of genetic factors and may explain sex differences in LTL evident at older ages. The model we propose is provisional but we hope it can serve to stimulate further research into the potential effects of the intra-uterine environment on the sex difference in LTL.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by NIH grants AG030678 and HD071180; the Danish Council for Independent Research - Medical Sciences; the INTERREG 4 A - programme Southern Denmark-Schleswig-K.E.R.N. supported by the European Regional Development Fund; and the A.P. Møller Foundation for the Advancement of Medical Science.
Conflict of interest: None declared.
Supplementary Material
References
- 1.Andrew T, Aviv A, Falchi M, et al. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Gene. 2006;78:480–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broer L, Codd V, Nyholt DR, et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet 2013;21:1163–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slagboom PE, Droog S, Boomsma DI, et al. Genetic determination of telomere size in humans: A twin study of three age groups. Am J Hum Genet 1994;55:876–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Aviv A, Susser E. Leukocyte telomere length and the father's age enigma: implications for population health and for life course. Int J Epidemiol 2013;42:457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res 2012;730:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nawrot TS, Staessen JA, Gardner JP, et al. Telomere length and possible link to X chromosome. Lancet 2004;363:507–10. [DOI] [PubMed] [Google Scholar]
- 7.Hunt SC, Chen W, Gardner JP, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell 2008;7:451–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbers CC, Garcia ME, Kimura M, et al. Comparison between Southern blots and qPCR analysis of leukocyte telomere length in the Health ABC Study. J Gerontol A Biol Sci Med Sci 2014;69:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lummaa V, Pettay JE, Russell AF. Male twins reduce fitness of female co-twins in humans. Proc Natl Acad Sci U S A 2007;104:10915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuoksimaa E, Eriksson CJ, Pulkkinen L, et al. Decreased prevalence of left-handedness among females with male co-twins: evidence suggesting prenatal testosterone transfer in humans? Psychoneuroendocrinology 2010;35:1462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro DC, Brook AH, Hughes TE, et al. Intrauterine hormone effects on tooth dimensions. J Dent Res 2013;92:425–31. [DOI] [PubMed] [Google Scholar]
- 12.Benyamin B, Sørensen TI, Schousboe K, et al. Are there common genetic and environmental factors behind the endophenotypes associated with the metabolic syndrome? Diabetologia 2007;50:1880–88. [DOI] [PubMed] [Google Scholar]
- 13.Schousboe K, Visscher PM, Henriksen JE, Hopper JL, Sorensen TI, Kyvik KO. Twin study of genetic and environmental influences on glucose tolerance and indices of insulin sensitivity and secretion. Diabetologia 2003;46:1276–83. [DOI] [PubMed] [Google Scholar]
- 14.Kimura M, Stone R, Hunt S, et al. Measurement of telomere length by the Southern blot analysis of the terminal restriction fragment lengths. Nat Protoc 2010;5:1596–607. [DOI] [PubMed] [Google Scholar]
- 15.Van de Pol M, Wright J. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim Behav 2009;77:753–58. [Google Scholar]
- 16.Benetos A, Kark JD, Susser E, et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 2013;12:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun 2013;4:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akkad A, Hastings R, Konje JC, et al. Telomere length in small-for-gestational-age babies. BJOG. 2006;113:318–23. [DOI] [PubMed] [Google Scholar]
- 19.Okuda K, Bardeguez A, Gardner JP, et al. Telomere length in the newborn. Pediatr Res 2002;52:377–81. [DOI] [PubMed] [Google Scholar]
- 20.Tapp AL, Maybery MT, Whitehouse AJ. Evaluating the twin testosterone transfer hypothesis: a review of the empirical evidence. Horm Behav 2011;60:713–22. [DOI] [PubMed] [Google Scholar]
- 21.Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci Biobehav Rev 2002;26:665–78. [DOI] [PubMed] [Google Scholar]
- 22.Gaist D, Bathum L, Skytthe A, et al. Strength and anthropometric measures in identical and fraternal twins: no evidence of masculinization of females with male co-twins. Epidemiology 2000;11:340–43. [DOI] [PubMed] [Google Scholar]
- 23.Christensen KI, Basso O, Kyvik KO, et al. Fecundability of female twins. Epidemiology 1998;9:189–92. [PubMed] [Google Scholar]
- 24.Korsten P, Clutton-Brock T, Pilkington JG, Pemberton JM, Kruuk LE. Sexual conflict in twins: male co-twins reduce fitness of female Soay sheep. Biol Lett 2009;5:663–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray KE, Schiff MA, Fitzpatrick AL, Kimura M, Aviv A, Starr JR. Leukocyte telomere length and age at menopause. Epidemiology 2014;25:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright WE, Piatyszek MA, Rainey WE, et al. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 1996;18:173–79. [DOI] [PubMed] [Google Scholar]
- 27.Ulaner GA, Giudice LC. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol Hum Reprod 1997;3:769–73. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Simpson ER, Liu JP, et al. Oestrogen, telomerase, ovarian ageing and cancer. Clin Exp Pharmacol Physiol 2010;37:78–82. [DOI] [PubMed] [Google Scholar]
- 29.Vasan RS, Demissie S, Kimura M, et al. Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: the Framingham Heart Study. Circulation 2008;117:1138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet 2005;366:662–64. [DOI] [PubMed] [Google Scholar]
- 31.Silventoinen K, Bartels M, Posthuma D, et al. Genetic regulation of growth in height and weight from 3 to 12 years of age: A longitudinal study of Dutch twin children. Twin Res Hum Genet 2007;10:354–63. [DOI] [PubMed] [Google Scholar]
- 32.Dubois L, Kyvik KO, Girard M, et al. Genetic and environmental contributions to weight, height, and BMI from birth to 19 years of age: An International study of over 12,000 twin pairs. PLoS One 2012;7:e30153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


