Abstract
Background: Animal models have suggested that undernutrition during gestation and the early postnatal period may adversely affect kidney development and compromise renal function. As a natural experiment, famines provide an opportunity to test such potential effects in humans. We assessed whether exposure to the Chinese famine of 1959–1961 during gestation and early postnatal life was associated with the levels of proteinuria among female adults three decades after exposure to the famine.
Methods: We measured famine intensity using the cohort size shrinkage index and we constructed a difference-in-difference model to compare the levels of proteinuria, measured with a dipstick test of random urine specimens, among Chinese women (n = 70 543) whose exposure status to the famine varied across birth cohorts (born before, during or after the famine) and counties of residence with different degrees of famine intensity.
Results: Famine exposure was associated with a greater risk [odds ratio (OR) = 1.54; 95% confidence interval (CI): 1.04, 2.28; P = 0.029) of having higher level of proteinuria among women born during the famine years (1959–61) compared with the unexposed post famine-born cohort (1964–65) in rural samples. No association was observed among urban samples. Results were robust to adjustment for covariates.
Conclusions: Severe undernutrition during gestation and the early postnatal period may have long-term effects on levels of proteinuria in humans, but the effect sizes may be small.
Keywords: Barker hypothesis, natural experiment, maternal undernutrition, famine, proteinuria, renal function
KEY MESSAGES.
As a natural experiment, famines provide an opportunity to test undernutrition in early life and the risk of adult chronic illness.
This study found that exposure to the Chinese famine of 1959-61 during gestation and early postnatal life was associated with higher levels of protein concentration in urine, but the effect size was small.
Severe undernutrition during critical early periods of life (pregnancy and the first 2 years) may hamper optimal development of the kidney and permanently alter renal function.
Introduction
The Barker hypothesis proposes that an adverse intrauterine environment elevates the risk for chronic disease development later in life.1 It has become increasingly evident that undernutrition during gestation and the early postnatal period may result in the abnormal development of some organs, including the kidney.2–4 Previous studies have found that maternal undernutrition was associated with a reduced number of glomeruli, which can elevate the risk for hyperfiltration injury, microalbuminuria (MA) and end-stage renal disease.5,6
Experimental data on laboratory animals suggest that prenatally undernourished rats have a higher likelihood of developing renal disease,7 and maternal protein-energy malnutrition in sheep significantly increases the risk of failure of kidney function in adult offspring.8 Studies in human subjects also suggest similar links between low birthweight, a marker of intrauterine growth retardation, and the development of renal disease or compromised kidney function.3 Low birthweight was associated with an elevated risk of microalbuminuria in Australian Aborigines,9 abnormal glomerular filtration rate in Dutch adolescents10 and end-stage renal disease in adults in the USA11and Norway.12
A recent cohort study on the Dutch famine of 1944–45 was the first to provide direct evidence suggesting that famine exposure during mid-gestation may prevent the sufficient formation of glomeruli at birth and increase the risk of microalbuminuria in adulthood, although the study failed to reach a conclusion regarding end-stage renal disease risk, due to the small sample size.5 As a natural experiment, the Chinese famine of 1959–61 presents another opportunity to evaluate the impact of severe undernutrition in utero and/or during early postnatal life on adult diseases.13,14 Unlike the Dutch famine that was imposed on a previously well-nourished population, the Chinese famine affected individuals who were already experiencing chronic undernutrition and impoverishment.14
The Chinese famine of 1959–61 was unearthed in the 1980s by scholars who inferred from census data that up to 30 million people were ‘missing’ from birth cohorts during this time.14–16 The famine was caused by massive institutional and policy changes during the Great Leap Forward campaign launched in 1958, which aimed at achieving a rapid industrialization at the expense of agriculture; weather may have aggravated the problem in some areas.14,17 These radical reforms immediately resulted in a substantial drop of grain output and caused a nationwide food shortage, and the estimated availability of food energy during the 3 following years decreased to 1500 calories daily per capita, far below average food energy requirements (∼2100 calories).15,18 Dramatic variations in famine intensity existed across regions, with rural areas experiencing a disproportionate impact of the famine and the highest severity occurring in Sichuan and Anhui provinces,14,18,19 where extreme behaviours such as eating tree bark and cannibalism were reported. Failure to respond in time to the severe food shortage contributed to the famine lasting until the end of 1961 in most regions and until as late as 1962 in some rural areas. The famine caused a substantial excess mortality of approximately 30 million deaths and a fertility reduction by 30 million lost births during this period, as seen in Figure 1.15,18,20
Figure 1.
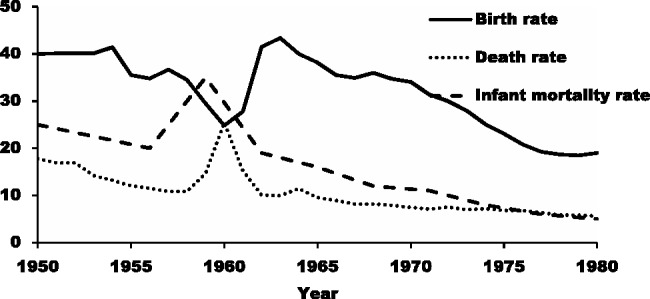
Fertility reduction and excess death rate and infant mortality (per thousand) during the Chinese Famine of 1959-61. Sources: computed from the 1982 Population Census of China and the 1988 Two-Per-Thousand National Survey of Fertility and Contraception.
In the present study, we investigated the associations between early life exposure to the Chinese famine of 1959–61 and levels of protein in urine in adulthood among a large sample of Chinese women born before, during or after the famine. We assessed the associations by birth cohorts separately for urban and rural residents.
Methods
Data, study sample and measurements
Data were derived from the China-U.S. Collaborative Project for Neural Tube Birth Defect Prevention conducted from 1993 to 1996, which examined the efficacy of periconceptual folic acid supplementation on preventing neural tube birth defects in China. The project was conducted by the U.S. Centers for Disease Control and Prevention and the Peking University Health Science Center.14,21 Chinese women living in Hebei, Zhejiang and Jiangsu provinces who were preparing for marriage registration were enrolled. Upon enrolment, a questionnaire documenting basic demographics, socio-economic status and general health conditions was completed by each participant. Anthropometric measures were obtained following standard protocols, and body weight and height were measured without shoes or heavy clothing using a calibrated scale. The body mass index (BMI) was calculated as weight (in kilograms) divided by height squared (in square metres). Hypertension was diagnosed by medical staff (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg). Urine samples were obtained by medical staff on the morning of a clinical visit after an overnight fast,21 and dipstick testing was conducted on a fresh, morning first-void urine sample, with the colour indicator turning from yellow to green progressively classifying urinary albumin concentration as negative, trace (5 to 20 mg per dl), 1+ (30 mg per dl), 2+ (100 mg per dl) or 3+ (300 mg per dl).
We restricted the study to cohorts born from 1957to 1965. The China-U.S. Collaborative Project for Neural Tube Birth Defect Prevention recruited primarily young women at their fertility peak and very few women born before 1957 were included. We divided these women into the following groups: (i) those born before the famine, in 1957–58 (the ‘pre-famine’ birth cohort) who were likely exposed to the famine during early postnatal life; (ii)those born in 1959–61 during the famine (the ‘famine’ birth cohort) who were likely exposed both in utero and during the early postnatal period; (iii) those born in 1962–63 (the ‘post-famine’ birth cohort), some of whom may have been exposed in utero in regions where the famine continued; and (iv) those born in 1964–65 who were used as the reference group. We further stratified our sample according to residency status (urban or rural)because the food supply in urban areas was guaranteed by the food rationing system, and thus urban residents were unlikely to be exposed to the famine.14,18,19
Assessing famine intensity
We quantified the regional famine intensity using the cohort size shrinkage index (CSSI).13,14 Specifically, using the 1990 Census of Chinese Population, we calculated the mean size of cohorts born during the 3 years immediately before the famine (1956–58), the 3 years immediately after the famine (1962–64), labelled Nnonfam, and the mean size of cohorts born during the famine years (1959–61), labeled Nfamine. We then generated the CSSI as the difference between Nnonfam and Nfamine, divided by Nnonfam. The validity and reliability of CSSI as a single famine intensity indicator has been tested and discussed elsewhere.13,22 Among the 35 sampled counties, the CSSI ranged from 0.24 to 0.64, with a mean of 0.42 and a standard deviation of 0.09, indicating that on average the population size in 1990 of adults who were born during the 3 years of the famine was 42% smaller compared with that of adults born during the 3 years immediately preceding or following the famine. Previous studies have suggested that the population size shrinkage among birth cohorts born during the famine years was primarily due to the fertility reduction and excess infant mortality during that time.13,18,23,24 The purpose of the CSSI was to account for the famine severity in a particular region, which likely had a direct effect on famine exposure of an individual born in that region.
Statistical analysis
We used the variation of famine exposure across cohorts and regions to construct a difference-in-difference (DID) estimator13,18 and fitted ordered logit (proportional-odds cumulative logit) models for the ordinal levels of protein concentration in urine with the DID estimator as shown below:
where Pirk refers to the level of protein concentration in urine (j = 0 refers to negative (reference level), j = 1 refers to trace level, j = 2 refers to 1 + level and j = 3 refers to 2 + and above level) for an individual born in county r and period k (k = 1 refers to birth years 1957–58, k = 2 refers to birth years 1959–61, k = 3 refers to birth years 1962–63 and the reference group refers to unexposed cohorts born in 1964–65). is the cohort fixed effect, is the region fixed effect and CSSIr is the cohort size shrinkage index in county r. A log value of CSSI was taken for a better model fit. , the coefficient of the interaction between the cohort size shrinkage index and the cohort dummy variables, captures the famine effect as a ‘treatment’ effect in a standard DID model. A detailed discussion on estimating ‘treatment’ effect using the interaction term in non-linear DID models was presented elsewhere.25,26 To estimate the average effect across 35 sampled counties, we multiplied the interaction coefficient by 0.57, the mean of the log values of CSSI across all counties. In addition, VXirk refers to a spectrum of covariates including ethnicity (han Chinese or minority), height, BMI-based weight status (<18.5 as underweight, 18.5–24.9 as normal, 25.0–29.9 as overweight and ≥30.0 as obese), hypertension (≥140/90 mmHg), education (elementary school, junior high school, or high school and above) and occupation (farmer or other), which were included in the full model to estimate the adjusted odds ratio. We also included month of birth in addition to year of birth (Cohort k) to control for birth seasonality. Standard error was adjusted for clustering by county. Cases with missing information for any of the variables included in the analysis were excluded (n = 14 002, 19.8%). Analysis suggested no significant differences in the measured characteristics between the excluded cases with missing data and those included in the sample for analyses. All analyses were conducted separately for the rural (n = 51 978) and urban (n = 4563) samples using Statistical Analysis Software (SAS Version 9.0).
Results
Characteristics of the study subjects by rural (n = 65 184)and urban residence (n = 5359) are presented in Table 1. The majority of women were born in Zhejiang Province and more than 99% of the participants were Han Chinese in both the rural and urban samples. The obesity rate was very low (<1%) among both rural and urban women. Hypertension was more prevalent among urban women (23.1%) than rural women (15.9%). Urban women tended to have a higher level of education and were less likely to be farmers than rural women. Protein in the urine was not tested for 18.3% of the rural and for 12.9% of the urban sample. Among those who had the test, 92.2% of rural women and 90.8% of urban women had no protein detected in their urine; 6.0% of rural women and 7.0% of urban women had traces of protein in their urine; and 1.8% of rural women and 2.0% of urban women had urine protein levels of 30 mg/dl or higher.
Table 1.
Characteristics of the study sample
| Characteristics | Rural sample (N = 65 184) | Urban sample (N = 5359) |
|---|---|---|
| Height (cm, mean and SD) | 158.7 (4.5) | 159.2 (4.6) |
| Province of residence (%) | ||
| Hebei | 2.3 | 3.3 |
| Zhejiang | 86.4 | 81.1 |
| Jiangsu | 11.2 | 15.5 |
| Education (%) | ||
| no schooling | 5.3 | 4.3 |
| elementary school | 36.6 | 19.5 |
| junior high school | 50.3 | 48.1 |
| high school and above | 7.5 | 27.5 |
| missing | 0.3 | 0.7 |
| Ethnicity (%) | ||
| Han | 99.3 | 99.5 |
| minority | 0.6 | 0.5 |
| missing | 0.1 | 0.0 |
| Occupation (%) | ||
| farmer | 81.5 | 50.6 |
| worker | 16.7 | 33.8 |
| other | 1.7 | 15.1 |
| missing | 0.1 | 0.5 |
| Weight status (%) | ||
| underweight | 10.7 | 14.6 |
| normal weight | 79.3 | 77.8 |
| overweight | 8.2 | 6.4 |
| obese | 0.5 | 0.4 |
| missing | 1.4 | 0.7 |
| Hypertension (%) | ||
| no | 83.3 | 76.0 |
| yes | 15.9 | 23.1 |
| missing | 0.8 | 0.9 |
| Urine protein concentration (%) | ||
| negative | 75.3 | 79.1 |
| trace (5-20 mg/dl) | 4.9 | 6.1 |
| 1 + (30 mg/dl) | 1.3 | 1.5 |
| 2 + and above(>100mg/dl) | 0.2 | 0.2 |
| missing | 18.3 | 12.9 |
Source: the China-U.S.Collaborative Project for Neural Tube Birth Defect Prevention.
The odds ratios of famine exposure and concentration of protein in the urine for the rural sample of women are presented in Table 2. Famine exposure was associated with 54% higher odds [odds ratio OR) = 1.54; 95% confidence interval (CI): 1.04, 2.28; P = 0.029] of having a higher concentration of protein in the urine for cohorts born during the famine (1959–61) compared with the unexposed birth cohort of 1964–65. Although our prior expectation was that a potential correlation between the famine exposure and the concentration of protein in urine could be partially confounded by weight or hypertension as indicated by previous literature,27,28 adjustments for these variables did not alter this association (OR = 1.53; 95% CI: 1.04, 2.26; P = 0.031). The effect size of 0.23, which equals the Ln (1.53) divided by 1.81,29 is considered small.30 We did not observe an association between the famine exposure and the concentration of protein in urine for the 1957–58 cohort who were born before the famine and exposed during the early postnatal period (OR = 1.29; 95% CI: 0.73, 2.26; P = 0.380). The 1962–63 birth cohort exhibited no statistically significant difference in the levels of proteinuria compared with the unexposed 1964–65 birth cohort (OR = 1.26; 95% CI: 0.99, 1.59; P = 0.051).
Table 2.
Famine exposure and levels of proteinuria in the rural sample (N = 51 978)
| Reference group: unexposed cohort (1964–65) | OR (95% CI) | P-value | Adjusted OR 95% CI | P-value |
|---|---|---|---|---|
| Pre-famine cohort (1957–58) | 1.29 (0.73, 2.26) | 0.380 | 1.28 (0.73, 2.25) | 0.366 |
| Famine cohort (1959–61) | 1.54a (1.04, 2.28) | 0.029 | 1.53a (1.04, 2.26) | 0.031 |
| Post-famine cohort (1962–63) | 1.26 (0.99, 1.59) | 0.051 | 1.26 (0.99, 1.59) | 0.052 |
OR, odds ratio; adjusted OR, odds ratio estimated from models controlling for height, weight status, hypertension, occupation, education, month of birth and ethnicity.
aP-values are statistically significant at the 0.05 level.
In the urban sample (Table 3), we did not find a statistically significant difference in the protein concentration in urine when the cohorts born before the famine (1957–58), during the famine (1959–61) or immediately after the famine (1962–63) were compared with the unexposed 1964–65 birth cohort.
Table 3.
Famine exposure and levels of proteinuria in the urban sample (N = 4563)
| Reference group: unexposed cohort (1964–65) | OR 95% CI | P-value | Adjusted OR 95% CI | P-value |
|---|---|---|---|---|
| Pre-famine cohort (1957–58) | 0.74 (0.20, 2.64) | 0.645 | 0.63 (0.18, 2.21) | 0.471 |
| Famine cohort (1959–61) | 0.94 (0.38, 2.34) | 0.902 | 0.90 (0.36, 2.28) | 0.824 |
| Post-famine cohort (1962–63) | 1.13 (0.64, 2.04) | 0.660 | 1.17 (0.65, 2.10) | 0.610 |
OR, odds ratio; adjusted OR, odds ratio estimated from models controlling for height, weight status, hypertension, occupation, education, month of birth and ethnicity.
A power analysis (Table 4) indicated that the sample size for rural residents ensured excellent statistical power of 100% to detect the reported associations between famine exposure and levels of proteinuria at a 95% confidence level. For the urban sample, except for the pre-famine cohorts, a two-sided test had less than 80% power to detect those reported odds ratios at 95% confidence. Therefore the null finding for the urban sample could be due to a lack of statistical power and the results regarding urban residents should be interpreted as merely suggestive.
Table 4.
| Cohort | Rural Sample |
Urban Sample |
||||
|---|---|---|---|---|---|---|
| Sample size (N = ) | Odds ratio | Power | Sample size (N = ) | Odds ratio | Power | |
| Pre-famine cohort (1957–58) | 2050 | 1.28 | 100% | 241 | 0.63 | 83% |
| Famine cohort (1959–61) | 6396 | 1.53 | 100% | 518 | 0.90 | 17% |
| Post-famine cohort (1962–63) | 24739 | 1.26 | 100% | 1594 | 1.17 | 71% |
| Unexposed cohort-reference group (1964–65) | 18793 | — | —- | 2210 | — | — |
Robustness test of the results
We excluded 18.3% of respondents in the rural sample and 12.9% in the urban sample due to missing data on levels of proteinuria, the outcome variable. To test whether these exclusions may have biased our results, we reanalysed the data using an inverse probability weighting (IPW) estimator, which is considered efficient in addressing a variety of sample selection issues.31 IPW is based on the idea that each individual has some probability (p) of being included in the sample, and that an inverse of this probability (w = 1/p) can be used to weight the sample to account for loss resulting from sample selection. Specifically, we estimated a logistic regression model, predicting the probability of being included in the analytical sample (i.e. having no missing data on the outcome variable). The explanatory variables in the predicting model included age, ethnicity, education, occupation, rural or urban residence, county of residence, height, weight and hypertension status of the women. Then we assigned each respondent a weight that was equal to the inverse of the predicted probability of being included in the analytical model. The weighted sample based on the IPW estimator yielded similar results to those reported above (Table 5), suggesting that sample attrition due to missing data on the outcome variable was unlikely to have biased our results.
Table 5.
Famine exposure and levels of proteinuria, estimated with inverse probability weighting (IPW) estimator
| Reference group: unexposed cohort (1964–65) | Rural sample |
Urban sample |
||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P-value | Adjusted OR 95% CI | P-value | |
| Pre-famine cohort (1957–58) | 1.26 (0.72, 2.18) | 0.419 | 0.63 (0.17, 2.35) | 0.494 |
| Famine cohort (1959–61) | 1.51a (1.02, 2.21) | 0.036 | 0.80 (0.28, 2.26) | 0.671 |
| Post-famine cohort (1962–63) | 1.24 (0.98, 1.56) | 0.064 | 0.99 (0.48, 2.05) | 0.985 |
OR, odds ratio; adjusted OR, odds ratio estimated from models controlling for height, weight status, hypertension, occupation, education, month of birth and ethnicity.
aP-values are statistically significant at 0.05 level
Discussion
Although increasing evidence has suggested developmental origins of many chronic diseases such as coronary heart disease and type 2 diabetes,32,33 limited evidence has been reported on undernutrition in early life due to famine exposure and the subsequent impact on renal function in adult life.5 Based on a large sample of Chinese women born before, during and after the Chinese famine of 1959–61, we found that rural women born during the famine had an elevated risk of having a higher level of proteinuria three decades after exposure to the famine; these results were independent of weight and hypertension status. Our findings were consistent with those of a previous study on the Dutch famine, which concluded that people who were exposed to famine during mid-gestation had a higher rate of microalbuminuria (defined as albumin/creatinine ratio ≥2.5 g/mmol) compared with those who were not prenatally exposed to famine (OR = 2.1; 95% CI: 1.0, 4.3), and adjustment for cardiovascular confounders including hypertension and obesity did not attenuate this association (adjusted OR = 3.2; 95% CI: 1.4, 7.7).5
These associations observed in the studies on famine cohorts suggest that nutritional deprivation in early life may damage kidney development and compromise renal function in adults, similarly to what has been observed in experiments with laboratory animals.7 In mice, the glomerular number was significantly less in protein-restricted offspring.34 In another study, pregnant rats fed a low-protein diet produced offspring with reduced glomerulogenesis and, consequently, a lower nephron number.35 Micronutrient deficiencies can also impair kidney development, which has been demonstrated in a series of studies with rats.36–39
We speculate that the elevated risk of proteinuria observed among Chinese women conceived during the famine was related to the impaired nephrogenesis that resulted from the famine exposure. In the human fetus, nephron formation starts around the 4th to 5th week of gestation and finishes around the 34th to 36th week of gestation.40 The number of nephrons increases slowly between the 10th and the 18th week, and then increases sharply from the 18th to the 32nd week.41 Once complete, nephrons and glomeruli cannot be formed later in life.41 Given that the nephrogenesis takes place before term birth, prenatal nutrient intake is likely important in determining the nephron endowment with which an individual is born and has for his/her lifetime.33,42,43 Stereological studies have suggested a direct relationship between the total glomerular number in the kidneys of adults and their birthweight, with an increase of 257 426 glomeruli associated with each 1-kg increase in birthweight.44 In turn, the glomerular filtration surface area remained low in people with less glomeruli,44 and the increased single nephron filtration rate produces an elevated transcapillary pressure that stretches and promotes scarring of enlarged glomeruli,44–47 thereby increasing the risk of glomerular hyperfiltration and hypertension in remnant nephrons.42 Subsequent effects include glomerular injury with the onset of proteinuria, systemic hypertension and glomerulosclerosis.6,42 In the long run, glomerulosclerosis would further decrease the number of nephrons, which would result in chronic kidney diseases.42
Alternative mechanisms beyond nutrition-induced effects, such as the toxic effects of food substitutes including bark and clay,48,49 may not be entirely excluded, though they are less likely to play a major role given the consistency of findings from famine studies across various contexts.48
The rural sample in our data exhibited lower levels of protein in urine compared with the urban sample despite the fact that rural residents born before or during the famine were more intensively exposed to the famine compared with their urban counterparts and the evidence that the famine exposure was associated with elevated levels of proteinuria. It is worth noting that the famine effect on proteinuria was small in terms of effect size. Other factors including hypertension, diabetes mellitus and obesity predict renal malfunction in adult Chinese populations,50,51 and the patterns of such risk factors play major roles in health disparities in chronic kidney disease across regions and populations in China.52,53 It is speculated that, although urban residents are less likely to have experienced nutritional deprivation in early life than rural residents,13 their greater risk of hypertension and diabetes and unhealthy behaviours (including sedentary lifestyle and high-fat diet) in adulthood may have led to higher levels of proteinuria being observed in the urban sample.54,55
The present study is subject to several limitations. First, we relied on a dipstick test of urine specimens, which has been widely used in outpatient settings to semi-quantitatively measure urinary protein concentrations.56 A dipstick test preferentially detects albumin, is less sensitive to globulins57 and its accuracy is lower than that of other methods including the 24-h proteinuria and the urinary protein/creatinine ratio tests used in clinical settings.56 The prevalence of proteinuria in our data is similar to the dipstick urinalysis results from International Society of Nephrology screening programmes, which suggested a prevalence of 3% proteinuria (>1+) in the Chinese population.58 The reliablity of the dipstick test was not assesed in our study, but false-negative rates of 8–18% and false-postive rates of 5% were reported in other populations in conditions where testers received minimal training in urinalysis.59
Second, we assumed that the women in our sample were living in the same county when the data were collected in the early 1990s as they did when they were born three decades earlier. This assumption is likely valid for our rural sample because the China-U.S. Collaborative Project for Neural Tube Birth Defect Prevention recruited residents with local registration (Hukou) for the study. In rural areas, women could obtain a local registration for another county through marriage but cross-county marriages were rare in these regions in the early 1990s.60,61 Urban woman may have been more likely to acquire local registration in different counties through other channels including employment by government, state-owned or collectively owned enterprises,22,62 but our analysis on the 1990 Chinese Census data suggested that a very low percentage (<4%) of women within the age range of our study subjects in the sampled provinces moved out of their county of residence during the period from 1985 to 1990.
Third, our analysis may be subject to sample selection from different sources. One is related to the fact that age and marital status affected the eligibility of participation in the US-China Collaborative Project; as a result, older women were underrepresented and unmarried women were not included. However, the bias due to this type of sample selection is expected to be modest because our difference-in-difference model substantially cancelled out differences across cohorts. In addition, compared with the post-famine birth cohorts, the famine cohorts were more likely to be born to parents who were more fertile and healthier (fertility selection); infants who were healthier or had better genetic endowment were more likely to survive the famine (survival selection). Therefore, the famine cohorts may represent a more selective and robust population compared with post-famine cohorts, which may result in an underestimation of the effects of famine exposure on adult health outcomes. Future studies comparing siblings with different exposure statuses to the famine (exposed vs unexposed) would reduce the biases due to fertility selection and survival selection and would be warranted if the data were available.
Lastly, unlike the Dutch famine that had a shorter duration (5–6 months) and clear starting- and end-points that provided evidence for a linkage between the famine exposure at different stages of gestation and health outcomes in adults, the long and imprecise duration of the Chinese famine of 1959–61 did not permit us to isolate prenatal and postnatal exposures.
Despite these limitations, the present study, as the first one to examine the effects of the Chinese famine of 1959–61 on renal function, linked exposure to the famine during gestation and early postnatal life with a higher level of proteinuria three decades later after the exposure in rural residents. Findings from our study and others suggest that undernutrition during critical early periods of life may hamper optimal development of certain organs, permanently altering their function, and may lead to elevated risks of chronic disease development.14,63–65 Such effect on a single chronic disease may be small in terms of effect size and may not be significant for individuals from a clinical point of view; however, the long-term effect of nutritional deprivation in critical early periods of life can be consequential for consideration at the population level.66 The impact of severe malnutrition in the first 1000 days of life (pregnancy and the first 2 years) on chronic illness as a whole therefore merits further investigation.
Funding
The work was funded by National Institutes of Health (5R03HD072104-02).
References
- 1.Barker D. Mothers, Babies and Health in Later Life. 2nd edn Edinburgh, UK: Churchill Livingstone, 1998. [Google Scholar]
- 2.Moritz KM, Singh RR, Probyn ME, Denton KM. Developmental programming of a reduced nephron endowment: more than just a baby's birthweight. Am J Physiol-Renal Physiol 2009;296:F1.–. [DOI] [PubMed] [Google Scholar]
- 3.Bagby SP. Developmental origins of renal disease: should nephron protection begin at birth? Clin J Am Soc Nephrol 2009;4:10.–. [DOI] [PubMed] [Google Scholar]
- 4.Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K. Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol 2005;16:2557–64. [DOI] [PubMed] [Google Scholar]
- 5.Painter RC, Roseboom TJ, Van Montfrans GA, et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J Am Soc Nephrol 2005;16:189–94. [DOI] [PubMed] [Google Scholar]
- 6.Hostetter TH, Olson J, Rennke H, Venkatachalam M, Brenner B. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. J Am Soc Nephrol 2001;12:1315–25. [DOI] [PubMed] [Google Scholar]
- 7.Magalhães JCG, Da Silveira AB, Mota DL, Paixão ADO. Renal function in juvenile rats subjected to prenatal malnutrition and chronic salt overload. Exp Physiol 2006;91:611–19. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd LJ, Foster T, Rhodes P, Rhind SM, Gardner DS. Protein-energy malnutrition during early gestation in sheep blunts fetal renal vascular and nephron development and compromises adult renal function. J Physiol 2012;590:377–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoy WE, Rees M, Kile E, Mathews JD, Wang Z. A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney Int 1999;56:1072–77. [DOI] [PubMed] [Google Scholar]
- 10.Keijzer-Veen MG, Schrevel M, Finken MJ, et al. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol 2005;16:2762–68. [DOI] [PubMed] [Google Scholar]
- 11.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birthweights contribute to the high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med 2000;160:1472. [DOI] [PubMed] [Google Scholar]
- 12.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birthweight increases risk for end-stage renal disease. J Am Soc Nephrol 2008;19:151–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Phillips MR, Zhang Y, et al. Malnutrition in early life and adult mental health: evidence from a natural experiment. Soc Sci Med 2013;97:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Li Z, Wang M, Martorell R. Early life exposure to the 1959–1961 Chinese famine has long-term health consequences. J Nutr 2010;140:1874–78. [DOI] [PubMed] [Google Scholar]
- 15.Ashton B, Hill K, Piazza A, Zeitz R. Famine in China, 1958–61. Popul Dev Rev 1984;10:613–45. [Google Scholar]
- 16.Smil V. China's great famine: 40 years later. BMJ 1999;319:1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song S, Wang W, Hu P. Famine, death, and madness: schizophrenia in early adulthood after prenatal exposure to the Chinese Great Leap Forward Famine. Soc Sci Med 2009;68:1315–21. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Zhou L-A. The long-term health and economic consequences of the 1959–1961 famine in China. J Health Econ 2007;26:659–81. [DOI] [PubMed] [Google Scholar]
- 19.Lin JY, Yang DT. Food availability, entitlements and the Chinese famine of 1959–61. Econ J 2000;110:136–58. [Google Scholar]
- 20.Peng X. Demographic consequences of the Great Leap Forward in China's provinces. Popul Dev Rev 1987;13:639–70. [Google Scholar]
- 21.Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. N Engl J Med 1999;341:1485–90. [DOI] [PubMed] [Google Scholar]
- 22.Huang C, Li Z, Venkat Narayan K, Williamson D, Martorell R. Bigger babies born to women survivors of the 1959–1961 Chinese famine: a puzzle due to survival selection? J Dev Orig Health Dis 2010;1:412–18. [DOI] [PubMed] [Google Scholar]
- 23.Song S. Does famine influence sex ratio at birth? Evidence from the 1959–1961 Great Leap Forward Famine in China. Proc Biol Sci 2012;279:2883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Y, Feng W. Famine, social disruption, and involuntary fetal loss: evidence from Chinese survey data. Demography 2005;42:301–22. [DOI] [PubMed] [Google Scholar]
- 25.Athey S, Imbens GW. Identification and inference in nonlinear difference-in-differences models. Econometrica 2006;74:431–97. [Google Scholar]
- 26.Puhani PA, Sonderhof K. The effects of a sick pay reform on absence and on health-related outcomes. J Health Econ 2010;29:285–302. [DOI] [PubMed] [Google Scholar]
- 27.Calviño J, Calvo C, Romero R, Gude F, Sánchez-Guisande D. Atherosclerosis profile and microalbuminuria in essential hypertension. Am J Kidney Dis 1999;34:996–1001. [DOI] [PubMed] [Google Scholar]
- 28.Sabharwal RK, Singh P, Arora M, Somani B, Ambade V. Incidence of microalbuminuria in hypertensive patients. Indian J Clin Biochem 2008;23:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med 2000;19:3127–31. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. A power primer. Psychol Bull 1992;112:155–59. [DOI] [PubMed] [Google Scholar]
- 31.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 2013;22:278–95. [DOI] [PubMed] [Google Scholar]
- 32.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr 2004;93:26–33. [DOI] [PubMed] [Google Scholar]
- 33.Barker D, Thornburg K, Osmond C, Kajantie E, Eriksson J. Beyond birthweight: the maternal and placental origins of chronic disease. J Dev Orig Health Dis 2010;1:360–64. [DOI] [PubMed] [Google Scholar]
- 34.Hoppe CC, Evans RG, Bertram JF, Moritz KM. Effects of dietary protein restriction on nephron number in the mouse. Am J Physiol Regul Integr Comp Physiol 2007;292:R1768–R74. [DOI] [PubMed] [Google Scholar]
- 35.Villar-Martini VC, Carvalho JJ, Neves MF, Aguila MB, Mandarim-de-Lacerda CA. Hypertension and kidney alterations in rat offspring from low protein pregnancies. J Hypertens 2009;27:S47–S51. [DOI] [PubMed] [Google Scholar]
- 36.Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat 1953;92:189–217. [DOI] [PubMed] [Google Scholar]
- 37.Koleganova N, Piecha G, Ritz E, et al. Both high and low maternal salt intake in pregnancy alter kidney development in the offspring. Am J Physiol Renal Physiol 2011;301:F344–F54. [DOI] [PubMed] [Google Scholar]
- 38.Drake KA, Sauerbry MJ, Blohowiak SE, Repyak KS, Kling PJ. Iron deficiency and renal development in the newborn rat. Pediatr Res 2009;66:619–24. [DOI] [PubMed] [Google Scholar]
- 39.Tomat AL, Inserra F, Veiras L, et al. Moderate zinc restriction during fetal and postnatal growth of rats: effects on adult arterial blood pressure and kidney. Am J Physiol Regul Integr Comp Physiol 2008;295:R543–49. [DOI] [PubMed] [Google Scholar]
- 40.Brenner B. Brenner and Rector's the Kidney. 8th edn Philadelphia, PA: Elsevier Saunders, 2008. [Google Scholar]
- 41.Gasser B, Mauss Y, Ghnassia J, et al. A quantitative study of normal nephrogenesis in the human fetus: its implication in the natural history of kidney changes due to low obstructive uropathies. Fetal Diagn Ther 1993;8:371–84. [DOI] [PubMed] [Google Scholar]
- 42.Schreuder MF. Safety in glomerular numbers. Pediatr Nephrol 2012;27:1881–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merlet-Benichou C. Influence of fetal environment on kidney development. Int J Dev Biol 1999;43:453–56. [PubMed] [Google Scholar]
- 44.Hughson M, Farris AB, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birthweight. Kidney Int 2003;63:2113–22. [DOI] [PubMed] [Google Scholar]
- 45.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 1996;49:1774–77. [DOI] [PubMed] [Google Scholar]
- 46.Cortes P, Riser B, Narins RG. Glomerular hypertension and progressive renal disease: the interplay of mesangial cell stretch, cytokine formation and extracellular matrix synthesis. Contrib Nephrol 1996;118:229–33. [DOI] [PubMed] [Google Scholar]
- 47.Adelman RD, Restaino IG, Alon US, Blowey DL. Proteinuria and focal segmental glomerulosclerosis in severely obese adolescents. J Pediatr 2001;138:481–85. [DOI] [PubMed] [Google Scholar]
- 48.Lumey L, Stein AD, Susser E. Prenatal famine and adult health. Annu Rev Public Health 2011;32:237–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu M-Q, Sun W-S, Liu B-X, et al. Prenatal malnutrition and adult schizophrenia: further evidence from the 1959–1961 Chinese famine. Schizophr Bull 2009;35:568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W, Wang H, Dong X, et al. Prevalence and risk factors associated with chronic kidney disease in an adult population from southern China. Nephrol Dial Transplant 2009;24:1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Zhang P, Wang F, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis 2008;51:373–84. [DOI] [PubMed] [Google Scholar]
- 52.Liu Q, Li Z, Wang H, et al. High prevalence and associated risk factors for impaired renal function and urinary abnormalities in a rural adult population from southern China. PLoS One 2012;7:e47100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815–22. [DOI] [PubMed] [Google Scholar]
- 54.Huang C, Yu H, Koplan JP. Can China diminish its burden of non-communicable diseases and injuries by promoting health in its policies, practices, and incentives? Lanc:783–92. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y, Huxley R, Li L, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from the China National Nutrition and Health Survey 2002. Circulation 2008;118:2679–86. [DOI] [PubMed] [Google Scholar]
- 56.Carroll MF, Temte JL. Proteinuria in adults: a diagnostic approach. Am Fam Physician 2000;62:1333–42. [PubMed] [Google Scholar]
- 57.Schrier RW, Gottschalk CW. Diseases of the Kidney. 6th edn Boston, MA: Little Brown, 1997. [Google Scholar]
- 58.Sharma SK, Zou HQ, Togtokh A, et al. Burden of CKD, proteinuria, and cardiovascular risk among Chinese, Mongolian, and Nepalese participants in the International Society of Nephrology screening programs. Am J Kidney Dis 2010;56:915–27. [DOI] [PubMed] [Google Scholar]
- 59.Halligan AW, Bell SC, Taylor DJ. Dipstick proteinuria: caveat emptor. Br J Obstet Gynaecol 1999;106:1113–15. [DOI] [PubMed] [Google Scholar]
- 60.Bossen L. Village to distant village: the opportunities and risks of long-distance marriage migration in rural China. J Contemp China 2007;16:97–116. [Google Scholar]
- 61.Fan CC, Li L. Marriage and migration in transitional China: a field study of Gaozhou, western Guangdong. Environ Plann A 2002;34:619–38. [Google Scholar]
- 62.Chan KW, Zhang L. The hukou system and rural-urban migration in China: Processes and changes. China Q 1999;160:818–55. [DOI] [PubMed] [Google Scholar]
- 63.Li QD, Li H, Li FJ, et al. Nutrition deficiency increases the risk of stomach cancer mortality. BMC Cancer 2012;12:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y, Jaddoe VW, Qi L, et al. Exposure to the Chinese famine in early life and the risk of metabolic syndrome in adulthood. Diabetes Care 2011;34:1014–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang PX, Wang JJ, Lei YX, Xiao L, Luo ZC. Impact of fetal and infant exposure to the Chinese Great Famine on the risk of hypertension in adulthood. PLoS One 2012;7:e49720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rose G. Sick individuals and sick populations. Int J Epidemiol 1985;14:32–38. [DOI] [PubMed] [Google Scholar]


