Abstract
Tissue-resident memory T (Trm) cells constitute a recently identified lymphocyte lineage that occupies tissues without recirculating. They provide a first response against infections reencountered at body surfaces, where they accelerate pathogen clearance. Because Trm cells are not present within peripheral blood, they have not yet been well characterized, but are transcriptionally, phenotypically, and functionally distinct from recirculating central and effector memory T cells. In this review, we will summarize current knowledge of Trm cell ontogeny, regulation, maintenance, and function and will highlight technical considerations for studying this population.
INTRODUCTION
The Phylogeny of Memory T Cell Subsets
Observations of phenotypic heterogeneity in the expression of homing receptors by human memory CD8+ and CD4+ T cells led to the conceptualization that memory T cells could be parsed into two subsets, which were labeled central memory (Tcm) and effector memory (Tem) cells (Sallusto et al., 1999). Importantly, this analysis was done in blood. Because Tcm and Tem cells not only expressed distinct homing receptors, but also unique effector properties, it was conceived that immunosurveillance patterns were intrinsically coupled with functional specialization. Much like naive T cells, Tcm cells patrol secondary lymphoid organs (SLOs), which include lymph nodes (LNs) and the white pulp (WP) of spleen (Figure 1) (von Andrian and Mackay, 2000; Sallusto et al., 1999). Also like naive T cells, after Ag-recognition Tcm cells undergo rapid and robust proliferation, differentiate into effector cells, and then migrate from SLOs to other tissues in search of infections to eliminate (von Andrian and Mackay, 2000). Like recently stimulated effector T cells, upon antigen recognition Tem cells remain poised for rapid execution of certain effector functions, such as cytolysis of infected host cells, rather than for proliferation. Tem cells also lack LN homing receptors (CD62L and CCR7), yet expressed distinct patterns of other homing receptors, and on that basis it was proposed that Tem cells recirculate between blood and nonlymphoid tissues (NLTs) or remain poised to mobilize to sites of inflammation (Butcher and Picker, 1996; Mackay et al., 1990). Consistent with this model, memory T cells were observed in many NLTs long after Ag clearance (Masopust et al., 2001b; Reinhardt et al., 2001). These observations provided a justification for extrapolating observations from blood Tem cells to T cells isolated from NLTs, which was convenient because blood lymphocytes are far easier to sample.
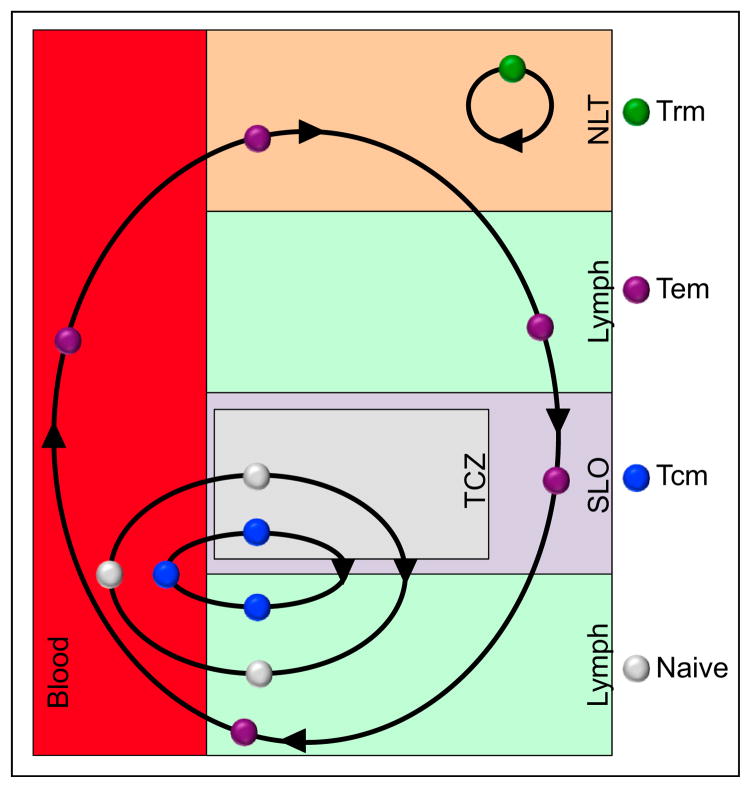
Figure 1. T Cell Migration Patterns.
T cell subsets exhibit distinct migration patterns. Like naive T cells, Tcm cells recirculate between blood, the T cell zones of secondary lymphoid organs, and lymph. Tem cells recirculate between nonlymphoid tissues, lymph, lymph nodes (where they might pass through via the sinuses, without entering the T cell zone), and blood. Trm CD8 cells do not recirculate but rather are confined to a single tissue.
However, some observations were not consistent with the model that all NLT memory cells were recirculating Tem cells. For instance, for T cells to recirculate through NLTs they must enter from the blood and exit via afferent lymphatics. Elegant work demonstrated paradoxically that CCR7 expression by T cells might be required for egress from NLT. Because the absence of CCR7 expression was a defining feature of Tem cells, it was unclear how Tem cells could recirculate between NLTs, lymph, and blood. Additionally, CD62L− cells isolated from blood and spleen did not recapitulate the panoply of phenotypes expressed by memory T cells isolated from the small intestinal mucosa, lung, and brain (Hawke et al., 1998; Hogan et al., 2001; Kim et al., 1998; Masopust et al., 2001a; van der Most et al., 2003). This prompted speculation that memory T cells permanently resided within certain NLTs rather than recirculate through blood (Masopust et al., 2001b).
These discrepancies were partly clarified upon the clear demonstration that populations of memory T cells were settled within many NLTs (Figure 1) (Gebhardt et al., 2009; Jiang et al., 2012; Masopust et al., 2010; Teijaro et al., 2011; Wakim et al., 2010). These tissue-resident memory T cells (abbreviated Trm cells to distinguish them from Tcm and Tem cells) derived from precursors that entered tissues during the effector phase of immune responses and remained positioned within this compartment. The identification of this memory T cell lineage precipitated many new questions. How are Trm cells regulated? When and how are they established? How are Trm cells maintained and for how long? How do they function and contribute to protective immunity? This review will summarize basic concepts in Trm cell biology, will draw attention to important technical considerations for their study, and will highlight remaining gaps within the field. It should be noted that the existing literature is more developed for CD8+ rather than CD4+ Trm cells, and the review will reflect this focus.
Trm Ontogeny
T cell recirculation is a dynamic and active process that is regulated during all phases of immune responses (von Andrian and Mackay, 2000; Masopust and Schenkel, 2013; Mueller et al., 2013). Naive T cells enter SLOs from blood using a combination of selectins, chemokines, and integrins, and then patrol for 12–24 hr before leaving to explore other SLOs in their continuous quest for cognate Ag (von Andrian and Mackay, 2000; von Andrian and Mempel, 2003). Exiting SLOs is an active process and depends on gradual T cell sensitization to sphingosine-1 phosphate receptor 1 (S1PR1)-dependent chemotactic gradients (Arnon et al., 2011; Lo et al., 2005; Shannon et al., 2012). In the rare event that T cells recognize cognate Ag, they transiently reduce expression of the transcription factor kruppel-like factor 2 (KLF2), a positive regulator of S1PR1 transcription (Preston et al., 2013; Skon et al., 2013; Grayson et al., 2001). S1PR1 downregulation is concomitantly associated with cell surface expression of the C-type lectin CD69 (Cyster and Schwab, 2012; Matloubian et al., 2004; Schober et al., 1999; Shiow et al., 2006; Skon et al., 2013). This prevents very recently activated T cells from leaving the SLO until they are fully primed. It should be noted that cognate antigen recognition is not the only signal that modulates CD69. For example, type I interferon (IFN), interleukin-33 (IL-33), and tumor necrosis factor-α (TNFα) and other cytokines all induce CD69 upregulation on T cells (Casey et al., 2012; Kohlmeier et al., 2010; López-Cabrera et al., 1995; Skon et al., 2013). This increases the dwell time of unactivated T cells within inflamed SLOs, putatively bettering their chance of locating cognate Ag. Thus, routine T cell egress from SLOs can be altered in the context of inflammation or antigen recognition.
When naive T cells are productively primed within SLOs, they give rise to effector cells that migrate throughout the host and leave behind a memory population that persists long after pathogen clearance (Dutton et al., 1998; Harty and Badovinac, 2008; Lefrançois, 2006; Sallusto et al., 2010; Williams and Bevan, 2007). Memory T cells can be isolated from SLO, blood-borne compartments, and NLTs (Masopust et al., 2001b; Reinhardt et al., 2001; Woodland and Kohlmeier, 2009), and at least for CD8+ T cells, this may include NLTs that were not sites of infection (Hofmann and Pircher, 2011; Kaufman et al., 2008; Liu et al., 2006; Masopust et al., 2010; 2004; Zhu et al., 2012). Memory CD8+ T cells in many NLTs lack CD62L (Casey et al., 2012), the defining phenotype of a Tem cell. However, NLT-derived memory T cells also express many markers not shared by any circulating population of memory T cells within blood (Casey et al., 2012; Gebhardt et al., 2009; Hawke et al., 1998; Hofmann and Pircher, 2011; Jiang et al., 2012; Masopust et al., 2006; Ray et al., 2004). It was difficult to reconcile this observation with the model that NLT memory T cells were recirculating between blood and tissue parenchyma.
Migration studies helped clarify this discrepancy. It was found that only effector, and not memory, CD8+ T cells were competent to enter the small intestine, brain, or salivary gland (Hickey et al., 1991; Hofmann and Pircher, 2011; Masopust et al., 2010; Wakim et al., 2010). Moreover, T cell populations within NLT grafts were not in equilibrium with host tissues when transplanted into isogenic recipients (Gebhardt et al., 2009; Masopust et al., 2010). And when the vasculature of two mice was conjoined via parabiotic surgery, T cells did not equilibrate between the skin epidermis, female reproductive tract, or lung of each mouse (Iijima and Iwasaki, 2014; Jiang et al., 2012; Schenkel et al., 2013; Teijaro et al., 2011). Taken together, these data indicate that NLTs contained populations of memory T cells that were not in circulation. Rather, they seeded NLTs during the effector phase of the response, and then became permanently established in situ (Hofmann and Pircher, 2011; Masopust et al., 2010). These cells are now referred to as Trm.
Examining the dynamics of phenotypic changes among antiviral CD8+ T cells within the small intestinal mucosa provides some insight into the ontogeny of Trm cells. After acute viral infections in mice and humans, effector T cells transiently upregulate α4β7 integrin, which facilitates migration into the small intestine (Campbell and Butcher, 2002; Liu et al., 2006; Masopust et al., 2010). After acute viral infection in mice, α4β7 is rapidly downregulated after trafficking to gut, suggesting that recently arrived effector CD8+ T cells have lost capacity for re-entry after ingress (Masopust et al., 2010). This loss of α4β7 is coupled with the in situ upregulation of αeβ7 integrin (αe is also referred to as CD103) and CD69, the maintenance of granzyme B, and the downregulation of Ly6C and CD122 expression (Masopust et al., 2006). The functional significance of CD69 and αeβ7 expression will be discussed later in the review. These data indicate that many Trm cell phenotypic signatures are acquired only after migration to destination tissues of residence.
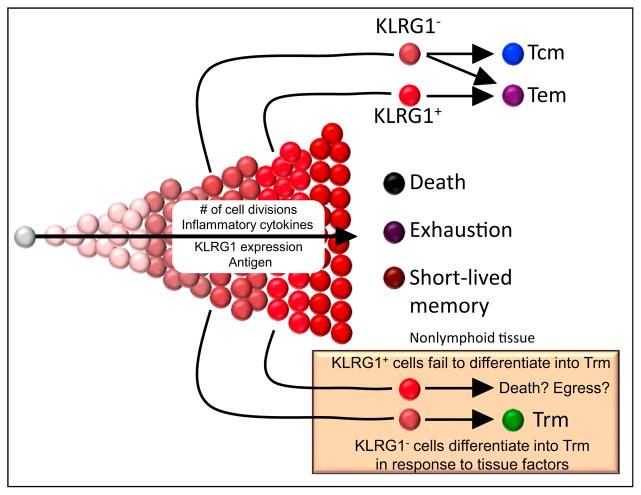
This observation supports the hypothesis that phenotype is inherently coupled with location and that NLT themselves might play an instructional role in shaping Trm cell differentiation. Indeed, when Trm cells are transferred from the intestinal mucosa to blood and then reactivated to induce proliferation and distribution throughout the host, daughter cells adopt the phenotypic signatures characteristic of their new environment (Masopust et al., 2006). In other words, effector progeny that trafficked back to the intestinal mucosa reacquired gut Trm cell-associated signatures, while daughter cells isolated from SLOs differentiated into recirculating Tcm (Masopust et al., 2006). Thus, changes in location are tied to changes in phenotype. This suggests that Trm cells, despite their effector-like phenotype, might not be terminally differentiated. Important recent observations showed that the precursors of Trm cell in the skin and small intestine are derived from less differentiated memory T cell precursors that expressed low levels of KLRG1 (Figure 2) (Mackay et al., 2013; Sheridan et al., 2014). Conversely, many recirculating memory T cells have lost the developmental capacity to acquire prototypical Trm cell-associated phenotypic signatures after restimulation and migration to the intestinal mucosa (Masopust et al., 2006). Principal component analyses revealed that Trm cells exhibited starkly different transcriptional signatures compared to naive T cells, Tcm and Tem cells, the latter being quite similar to one another (Mackay et al., 2013). While multiple Trm subsets might exist, a key finding was that Trm cells in different tissues shared many transcripts in common suggesting a core signature (Mackay et al., 2013). Many of these commonalities might be tied to the mechanisms that mediate residence.
Figure 2. Memory T Cell Differentiation.
Like Tcm cells, Trm cells derive from KLRG1− precursors in mice and are less terminally differentiated. However, Trm cell differentiation is regulated by local factors at sites of residence. KLRG1+ cells likely received more stimulation, are more terminally differentiated, and are capable of differentiating into Tem, but not Trm cells. High amounts of cell division, inflammatory cytokines, and antigen exposure ultimately result in T cell death or functional exhaustion.
Mechanisms of Trm Maintenance
In order for Trm cells to be maintained within tissues, they must adapt to local survival cues, resist shedding into the lumen at mucosal epithelial surfaces, and ignore egress signals (Masopust and Schenkel, 2013; Mueller et al., 2013). These mechanisms and their regulation represent areas of active research that have important implications for Trm cell longevity.
Epithelial cells are arranged in confluent layers that line body surfaces and cavities. The adherens protein E-cadherin tethers epithelial cells together via homophilic interactions that form tight junctions. The integrin αeβ7, one marker associated with Trm (Casey et al., 2012; Mackay et al., 2013; Wakim et al., 2010), can also bind E-cadherin (Cepek et al., 1994). Indeed, CD8+ T cells that are genetically deficient in αeβ7 are able to migrate to the small intestine epithelium, brain, and skin epidermis, but are not retained (Casey et al., 2012; Mackay et al., 2013; Schön et al., 1999; Wakim et al., 2010). One interpretation is that αeβ7 allows CD8+ T cells to physically adhere to E-cadherin expressed on epithelial cells. A non-mutually exclusive alternative hypothesis is that αeβ7 expression promotes survival of CD8+ T cells, as αeβ7+ CD8+ T cells express more of the prosurvival protein Bcl-2 early after infection in the brain and skin epidermis (Mackay et al., 2013; Wakim et al., 2010). Dependence on αeβ7 for maintenance might also be context dependent. After oral infection with Listeria monocytogenes, αeβ7 is important for the accumulation of CD8+ T cells in the small intestine epithelium but is dispensable for long-term maintenance (Sheridan et al., 2014).
Whether a CD8+ T cell requires αeβ7 for maintenance likely also depends on location and might even vary among different types of epithelium. For example, even though memory CD8+ T cells express αeβ7 in the small intestine lamina propria (LP), it is not required for T cell retention (Casey et al., 2012). Interestingly, E-cadherin is also expressed by memory CD8 T cells isolated from the salivary gland, small intestine epithelium, lung, skin, thymus, and brain and might contribute to maintenance via homophilic E-cadherin interactions between Trm cells and epithelial cells (Hofmann and Pircher, 2011; Mackay et al., 2013; Wakim et al., 2012). Other integrins that bind extracellular matrix proteins in the LP or basement membrane might also retain Trm cells in different tissues. For instance, the integrin α1β1 binds laminins and collagens and is highly expressed on CD8+ Trm in the brain, small intestine, lung, and skin (Gebhardt et al., 2009; Mackay et al., 2013; Ray et al., 2004; Wakim et al., 2012). Some evidence indicates that the majority of putative Trm cells in many organs do not even express αeβ7 (Casey et al., 2012; Schenkel et al., 2014a). To conclude, while αeβ7 expression on Ag-experienced CD8 T cells might be strongly indicative of residence, it is likely that many Trm do not depend on, nor even express, αeβ7.
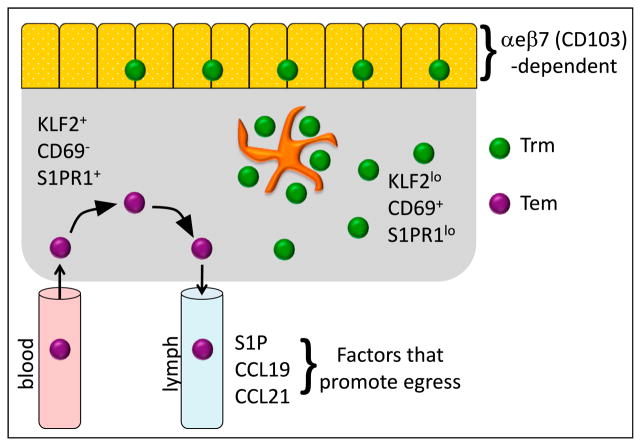
S1PR1 mediates T cell egress in lymph nodes by inducing chemotaxis to sphingosine-1-phosphate (S1P) present within efferent lymph (Cyster and Schwab, 2012). S1P is also produced by afferent lymphatic endothelial cells draining NLTs and is important for mature dendritic cells to migrate to tissue-draining lymph nodes (Czeloth et al., 2005; Lamana et al., 2011; Maeda et al., 2007; Pham et al., 2010). Thus, inhibiting S1P responsiveness might represent an important checkpoint for generating Trm (Figure 3). In support of this hypothesis, CD69 is induced on Trm after they migrate to sites of residence (Mackay et al., 2013; Masopust et al., 2006; Skon et al., 2013), and as mentioned previously, CD69 antagonizes S1PR1. Importantly, CD69 KO CD8+ T cells were not maintained in skin epidermis or lungs (Lee et al., 2011; Mackay et al., 2013). It remains to be determined whether all Trm require CD69, or whether other means of S1PR1 inhibition are sufficient for Trm maintenance. For instance, the transcription factor kruppel-like factor 2 (KLF2) is required for S1PR1 expression on T cells and once Trm precursors seed NLTs, KLF2 is downregulated (Skon et al., 2013). When S1PR1 is forcibly expressed on CD8+ T cells, Trm cells are reduced in NLTs (Skon et al., 2013). Taken together, the downregulation of KLF2 and S1PR1 and the upregulation of CD69 might both play functional roles in the development and retention of Trm cells.
Figure 3. Mechanisms of Memory T Cell Residence and Recirculation.
Trm cells express low levels of the transcription factor KLF2 and S1PR1 and high levels of the C-type lectin CD69, which collectively prevent exit from the tissue. Certain Trm populations within mucosal or epidermal epithelium require αeβ7 integrin for maintenance (αe is also referred to as CD103). Trm cells are sometimes densely clustered in leukocyte aggregates that also contain myeloid cells. In contrast, recirculating Tem cells enter tissues from blood (typically from postcapillary venules) and express high amounts of KLF2 and S1PR1, but do not express CD69, which allows them to emigrate via S1P+ draining afferent lymphatics. Presentation on lymphatic endothelium of CCR7 ligands, CCL19 and CCL21, might also promote egress of CCR7+ non-lymphoid T cells.
There might be other factors that promote residence and recirculation. Trm cells have reduced expression of another S1P receptor, S1PR5, and S1PR5 is regulated independently of KLF2 and CD69 (Mackay et al., 2013; Wakim et al., 2012). KLF2 promotes other homing markers involved in recirculation, including CCR7 expression (Carlson et al., 2006), which might promote leukocyte egress from NLT via CCL19+ and CCL21+ afferent lymphatics. Indeed, SLO T cells emigrate in a CCR7-dependent manner when transferred to footpad or lung in mice and CCR7+ T cells can be isolated from ovine afferent lymph (Bromley et al., 2005; Debes et al., 2005). Similarly, dendritic cells upregulate CCR7 after activation in tissues, which then acts in concert with S1PR1 to promote migration to downstream SLOs (Braun et al., 2011; Czeloth et al., 2005; Lamana et al., 2011; Ohl et al., 2004; Saeki et al., 1999). Thus, repression of CCR7 might also be another means of maintaining Trm cells.
Many critical questions remain. What is the longevity of Trm cells relative to recirculating T cell subsets? Unfortunately, there are few long-term studies on the durability of T cell memory in humans, even in blood (Hammarlund et al., 2003; Nanan et al., 2000; Van Epps et al., 2002), and tracking T cell memory in NLTs is less tractable. Shorter-term studies in mice and rhesus macaques revealed that tissue memory CD8+ T cells are relatively stable for 300–700 days (Jiang et al., 2012; Kaufman et al., 2008; Masopust et al., 2001b). It was not demonstrated in these studies whether recirculating memory T cells migrate to tissues to replenish Trm cell, which has been proposed for lung airways (Ely et al., 2006), or whether Trm cells were self-sustaining. However, 168 days after parabiosis surgery, there was little evidence of recirculating memory CD8+ T cell infiltration into the skin, suggesting that at least for some tissues, Trm cells might be maintained independently of recirculating memory T cells (Jiang et al., 2012). Of course, Trm cell longevity might vary considerably depending on the initial nature and site of the priming infection or vaccine, as observed for humoral immunity. Indeed, CD8+ and CD4+ T cell memory in the lung and lung airways wanes within months after influenza infection, leading to the loss of protective heterosubtypic immunity (Hogan et al., 2001; Liang et al., 1994; Wu et al., 2014).
Mechanisms of Trm Cell Differentiation
CD69 and granzyme B are signatures of recently stimulated T cells. Because these signatures are also constitutively expressed by T cells within the intestinal mucosa, it was originally proposed that mucosal T cells represented activated effectors (Montufar-Solis et al., 2007). Ag-specific studies revealed that bona fide memory T cells specific for acute infections shared these effector-like properties, raising the possibility that Ag is not required for maintenance of this effector-like phenotype (Hogan et al., 2001; Masopust et al., 2006). When naive CD8+ T cells expand homeostatically within RAG-deficient hosts, they disseminate into the intestinal mucosa in the absence of cognate antigen stimulation, the infectious milieu, and even the microbial flora, yet in each instance they still adopt their effector-like phenotypic signature (Casey et al., 2012). Homeostatically expanded CD8+ T cells in the stomach, female reproductive tract, salivary gland, pancreas, and heart also express CD69 (and other Trm cell signatures including αeβ7) (Casey et al., 2012). And when effector CD8+ T cells migrate to nonspecific inflammation in the skin or vaginal mucosa, they also upregulated αeβ7 without antigen, reinforcing the hypothesis that regulation might be independent of continual cognate Ag stimulation (Mackay et al., 2012). Taken together, it appears that localization to certain NLTs is sufficient to drive adoption of specific phenotypes, much like inductive cues that drive monocyte differentiation in the small intestine (Zigmond and Jung, 2013).
Nevertheless, these data do not preclude a role for persistent Ag in shaping Trm cell differentiation. For instance, during chronic viral infection, αeβ7 expression is reduced on virus-specific CD8+ T cells within the small intestine, which might suggest that local antigen recognition inhibits αeβ7 expression (Casey et al., 2012; Zhang and Bevan, 2013). However, the issue is far from clear, because compelling evidence demonstrates that CNS, PNS, and lung require local antigen recognition for the upregulation of αeβ7 (Lee et al., 2011; Mackay et al., 2012; Wakim et al., 2010). In one particularly elegant experiment, when antigen recognition was blocked in the lungs of influenza-infected mice using MHC-peptide specific antibodies, αeβ7 and CD69 expression was reduced (Lee et al., 2011). This loss of CD69 and αeβ7 expression was paired with a concomitant loss in antigen-specific lung CD8+ T cells. Thus, the importance of antigen in the differentiation and maintenance of Trm cells might be tissue dependent. Sites of pathogen replication might also influence Trm cell differentiation and retention. For instance, a larger number of Trm cells within the female reproductive tract are induced to express αeβ7 after local inflammation compared to systemic infection or in the presence of local inflammation (Çuburu et al., 2012; Li et al., 2008; Mackay et al., 2012; Shin and Iwasaki, 2012).
Evidence suggests that the cytokine milieu within tissues might drive Trm cell signatures, although only a limited number of signals have been defined thus far. Most definitively, transforming growth factor β (TGF-β) has been shown to induce αeβ7 expression on Trm cell precursors (Casey et al., 2012; Lee et al., 2011; Mackay et al., 2013; Sheridan et al., 2014; Zhang and Bevan, 2013). In vitro culture with TGF-β induces αeβ7 expression on primary effector CD8+ T splenocytes (but not resting memory CD8+ T splenocytes) (Casey et al., 2012). However, this αeβ7 upregulation was prevented by the addition of Ag (Casey et al., 2012). Active TGF-β is constitutively available at many epithelial surfaces, including skin epidermis and small intestine epithelium (Koyama and Podolsky, 1989; Yang et al., 1999). Moreover, TGF-βR deficiency prevents CD103 upregulation in the skin, lung, and small intestine (Casey et al., 2012; El-Asady et al., 2005; Lee et al., 2011; Mackay et al., 2013; Sheridan et al., 2014; Zhang and Bevan, 2013). Similarly, it has been demonstrated that T cells infiltrating the TGF-β rich microenvironment of tumors also express αeβ7 (Le Floc’h et al., 2007). While CD4+ T regulatory cells express CD103 in response to TGF-β, CD4+ Th1 T cells are often refractory to CD103 expression. Although it is uncertain whether this is related to a lack of TGF-βR expression, responsiveness to TGF-β is clearly dependent on T cell differentiation state (Li and Flavell, 2008). Indeed, KLRG1+ recently activated CD8 T cells, which did not give rise to CD103+ Trm, express less TGF-β receptor II (TGF-βRII) (Mackay et al., 2013; Sheridan et al., 2014). KLRG1+ effectors are also particularly vulnerable to apoptosis when treated with TGF-β in vitro (Sanjabi et al., 2009). Thus whether a T cell upregulates αeβ7 in response to TGF-β is multifactorial and intrinsically linked to differentiation state.
The cytokine mechanism(s) controlling KLF2 and the S1PR1-CD69 axis in NLTs is less well understood. CD69 expression is upregulated and KLF2 is downregulated on primary effector CD8+ T cells after short term, ex vivo culture with IL-33, TNF-α, and IFN-α and IFN-β, demonstrating that in vitro, cytokines can partially recapitulate these signatures of the Trm cell phenotype (Casey et al., 2012; Skon et al., 2013). Further, it was shown that inhibiting the PI(3)K-Akt pathway ameliorated cytokine-induced downregulation of KLF2 and S1PR1, suggesting that this signaling pathway might be important for Trm development (Skon et al., 2013). It remains possible that different cytokines might operate under different contexts for controlling KLF2 and the S1PR1-CD69 axis.
Cytokines might regulate other aspects of Trm cell differentiation and survival. The homeostatic cytokines IL-7 and IL-15 are important for maintenance of recirculating memory T cells in SLOs (Surh and Sprent, 2008). Do these cytokines also play a role in Trm cell survival? Trm cells in the skin epidermis are dependent on IL-15 (Mackay et al., 2013), whereas Trm cells positioned within SLOs persisted in the absence of IL-15 (in contrast to Tcm within SLOs) (Schenkel et al., 2014a). Thus, cytokine regulation of Trm cell persistence might be complex, varying between different tissues. Additional survival factors might also play a role in Trm cell longevity. For example, CD8+ Trm cells in the skin might require the aryl hydrocarbon receptor for long-term survival (Zaid et al., 2014). Defining the factors mediating Trm cell maintenance will help provide insight into leveraging Trm cells for clinical benefit, including vaccines. In this regard, it will be crucial to determine whether the total number of Trm cells established within NLTs is tightly regulated, as it appears to be for Tcm cells within LNs, or whether it is flexible, as shown for Tem cells (Vezys et al., 2009).
Critical Barriers to Accurately Studying Trm
Multiparameter flow cytometry allows high-throughput functional and phenotypic analyses of single cell suspensions. For this reason, studies of Trm cells often utilize populations of cells isolated from whole organs. One drawback of this approach is that anatomic and spatial information is lost, an important consideration given the potential heterogeneity of T cells within different tissue compartments within NLTs. Another drawback is that while interpretation is predicated on presumptions of near complete leukocyte extraction, isolation might be inefficient and biased toward certain organ compartments or T cell subsets. Below, we will attempt to summarize how these issues might particularly impede our understanding of Trm cell differentiation, distribution, and immunosurveillance within NLT.
Organs are complex structures composed of distinct tissues, extracellular matrices, and cell types organized for the purposes of executing unified functions. Anatomic compartments, including epithelium, lamina propria, submucosae, muscularis externae, blood and lymphatic vessels, and tertiary lymphoid organs, represent distinct environments in which memory T cells survey host cells for evidence of reinfection. Differences in cell types, cytokine milieu, adhesion molecules, and extracellular matrix proteins might all influence T cell migration and differentiation, and different compartments within organs might contain distinct memory T cell populations that become admixed when organs are dissociated for single cell analyses.
The issue of admixing compartments might be particularly confounding due to contamination from vascular compartments, and this occurs despite efforts to remove blood-borne cells by perfusion. Work from James Hogg prognosticated this important technical issue when he reported that the ratio of neutrophils to RBCs was 100-fold higher in lung capillaries than in large-bore blood vessels (Hogg et al., 1994). This was explained by the fact that neutrophils take 60–100 times longer than RBCs to transit through pulmonary capillaries. Pulmonary capillaries are narrow for a reason, and where narrowest, they are most efficient at allowing gas exchange between RBCs and the external environment. Thus even RBCs undergo deformation to navigate through this compartment. Neutrophils have a much greater cell volume than RBCs, so navigation is much slower, and perfusion is not completely effective to remove them. This issue extends to lymphocytes as well (which have a ~3-fold greater volume than RBCs; ~90 femtoliters versus ~290 femtoliters (Segel et al., 1981)). Following acute intraperitoneal LCMV challenge, up to 95% of memory CD8 T cells isolated from perfused lung were derived from vasculature (Anderson et al., 2012). Lung infections still resulted in 70% blood contamination for CD8 T cells after mTB (Anderson et al., 2014; Sakai et al., 2014) and 20% after RSV (Knudson et al., 2014). Vascular contamination by CD4+ T cells is less than CD8+ T cells in models addressed thus far (Anderson et al., 2014; Knudson et al., 2014; Teijaro et al., 2011) but is still significant. Further, it should be noted that when analyzing healthy resting lung, abundant neutrophils and naive B and T cells can be extracted; these are 100% derived from the vasculature compartment (Anderson et al., 2014). Importantly, these issues extend to other organs, particularly the liver and kidney (Anderson et al., 2014).
In animal models, this issue can be simply addressed using in vivo intravascular-staining strategies (Galkina et al., 2005; Muppidi et al., 2011; Scanlon et al., 2011), which abrogate the need to perfuse. The advantage of intravascular staining is that it refines understanding of leukocyte localization, which is essential for defining migration patterns. It also allows one to include vascular populations in analyses; these marginated pools might be simply passing through the tissue, but also might be making unique functional contributions to local immune responses as do Kupffer cells and natural killer T (NKT) cells within the liver sinusoids (Thomas et al., 2011). In fact, within lung, intravascular NKT cells precipitate allergies (Scanlon et al., 2011) and intravascular CD4 T cells produce IFN-γ in vivo in response to mTB (Sakai et al., 2014). Of course, intravascular staining won’t discriminate between leukocytes within different locations within lung tissue, including NLT parenchyma and high endothelial cell (HEV)-containing inductive sites such as inducible bronchus-associated lymphoid tissue, the latter which might contain naive lymphocytes at sites of inflammation.
Low cell yields, compared to SLOs, are a major impediment to studying NLT populations. However, these yields might underestimate the number of lymphocytes in NLTs (Ganusov and De Boer, 2007; Selby et al., 1984). Many methods exist for enzymatic and tissue dissociation but it is unclear whether any method extracts all leukocytes. Moreover, NLT populations might be particularly prone to death during lengthy or harsh processing procedures, because they do not survive well in culture (Brunner et al., 2001; Wakim et al., 2012). These issues might impact distinct leukocyte populations to varying degrees, thus distorting accurate representation of subsets. Moreover, tertiary lymphoid organs (TLOs) and aggregate structures are heterogeneously distributed throughout NLTs and might cause significant sampling bias if investigations (e.g., in humans or nonhuman primates) are limited to small biopsies.
Pairing single cell suspension analyses with imaging offers a powerful approach to overcome some of these critical barriers. The obvious advantages of imaging include the capture of spatial information, circumvention of the issue of isolation inefficiencies, and this approach is less vulnerable to subset misrepresentation. Moreover, unlabeled tissue samples can be stored for years, allowing for future staining and analyses, and there is rarely a shortage of material (i.e., mouse mucosal tissues can be cut into 100 or more thin sections). Limitations include the fact that sample processing, data acquisition, and analysis is time consuming, instrument sensitivity and antibody availability might constrain phenotypic analyses, and fewer parameters (typically 4–8) can be interrogated contemporaneously as compared to flow cytometry. Moreover, approaches to identify T cells of known specificity, such as in situ MHC tetramer staining, require significant expertise and reagents.
Lastly, Trm cells are defined by migration properties. However, stringent evaluation of in vivo recirculation involves sophisticated approaches including photoreactivation of Tg mice or T cells (Bromley et al., 2013; Ugur et al., 2014), tissue grafts (Gebhardt et al., 2009; Masopust et al., 2010), lymphatic canulation (Mackay et al., 1990), transfers of labeled T cells (Hofmann and Pircher, 2011; Masopust et al., 2004; 2010), and/or parabiotic surgery (Iijima and Iwasaki, 2014; Jiang et al., 2012; Klonowski et al., 2004; Schenkel et al., 2013). These techniques are often not pragmatic or even possible in certain animal models or humans. For this reason, truly validated markers (CD69 might not suffice) could facilitate more confident and refined analyses of Trm cells.
Trm Cell Distribution within SLO
Trm cells have been almost exclusively studied within NLTs. However, there is evidence that Trm cells might also be positioned within SLOs. In human cadavers, it was recently shown that 50%–80% of CD4+ and CD8+ T cells express CD69 in spleen and LNs (Sathaliyawala et al., 2013) and CD69+ CD8+ T cells included those with defined specificity for CMV and influenza (Turner et al., 2014). Photoconvertable fluorescence approaches have demonstrated a retained population of CD4+ T cells in Peyer’s patches and mesenteric LNs that accumulated in response to chronic antigen exposure (Ugur et al., 2014). These included, but were not limited to, T follicular helper (Tfh) cells, and expressed CD69 and low amounts of S1PR1 (Ugur et al., 2014). Indeed, Tfh might represent a major resident population of CD4+ T cells in SLOs, although they might be short-lived after Ag clearance. After murine lung infections associated with depots of residual antigen, there is evidence for retention of CD8+ T cells within draining mediastinal LNs, and CD69 and CD103 are expressed in these contexts (Takamura et al., 2010; Zammit et al., 2006). These data suggest that prolonged antigen stimulation might induce CD4+ and CD8+ Trm cells within SLOs.
There is also evidence for Trm cells in SLOs in the putative absence of persistent antigen. Parabiosis experiments demonstrated that 1%–5% of memory CD8+ T cells within spleen and LNs are Trm cells after acute LCMV infection (Schenkel et al., 2014a). These cells share many markers with Trm cells identified in the small intestinal mucosa, including expression of CD69 and low amounts of CD62L, Ly6C, the transcription factor Eomesodermin, and CD122. Unlike recirculating memory CD8+ T cells, SLO Trm cells were not dependent on IL-15 for survival.
Why would SLO contain Trm cells? CD8+ Trm cells exhibited biased distribution to splenic marginal zone and LN sinuses (Schenkel et al., 2014a), which often represent the initial sites of microbial entry to these organs (Aoshi et al., 2009; Gonzalez et al., 2010; Junt et al., 2007). T cell reactivation within these LN barrier sites precipitates rapid detection, innate immune activation, and recruitment of CXCR3+ memory T cells, all of which likely contribute to accelerating pathogen control (Kastenmüller et al., 2013; Sung et al., 2012). A network of natural killer (NK) cells, γδ T cells, and innate-like CD8+ T cells are also prepositioned near lymph node sinuses, and when activated, prevent systemic pathogen dissemination (Kastenmüller et al., 2012; Sheridan et al., 2013). Thus, occupation of SLO pathogen-entry sites might be functionally analogous to Trm cell positioning within frontline NLTs.
Trm Cell Function
In the event of reinfection, Tcm cells produce potent recall responses due to their high proliferative potential and ability to produce abundant effector progeny capable of migrating to sites of infection. However, microbes are most often encountered first at mucosal sites and other body surfaces. Thus, if Tcm cells were the only memory T cell subset, then the host would have to wait for pathogen-derived Ag to disseminate to SLOs (much like the situation for naive individuals) or inflammation induced recruitment (Nolz and Harty, 2014) before mounting an anamnestic T cell response. This fails to capitalize on the most immediate opportunity for pathogen control after initial infection, which in the case of some pathogens might be critical.
Because Trm cells occupy frontline sites of infection, they are anatomically positioned to respond most immediately. Several studies have shown that Trm cells can make critical contributions to protective immunity against local challenges (Gebhardt et al., 2009; Iijima and Iwasaki, 2014; Jiang et al., 2012; Teijaro et al., 2011). In one particularly striking example, hosts containing both Trm cells and recirculating memory T cells exhibited a 99.99% reduction in viral load 6 days after local vaccinia virus infection compared to animals containing only recirculating memory T cell (Jiang et al., 2012). In a model of influenza infection, it was shown that CD4+ Trm cell completely protected mice from lethal respiratory lung infection (Teijaro et al., 2011). Indeed, many different pathogen challenge models have shown that Trm cells mediate protection in a number of different organs (Ariotti et al., 2014; Gebhardt et al., 2009; Jiang et al., 2012; Mackay et al., 2012; Schenkel et al., 2014b; Shin and Iwasaki, 2012; Teijaro et al., 2011; Wu et al., 2014).
How are Trm cells able to effectively protect the host? Trm cells must scan host cells for evidence of reinfection and employ a variety of mechanisms to more efficiently detect pathogen. During inflammatory tissue conditions, T cell motility in the dermis increases ~5 fold and utilizes αvβ1 and β3 binding to fibronectin (Overstreet et al., 2013). Blocking integrin αv results in increased parasite burden during local Leishmania major infection, suggesting that increased T cell motility is in important for pathogen control (Overstreet et al., 2013). Chemokines also affect T cell motility. In the brains of Toxoplasma gondii-infected mice, CXCL10 expression increases the speed of local CD8 T cells (Harris et al., 2012). Moreover, T. gondii-specific T cells do not scan host brain cells via random Brownian motion, but rather move in what are known as Levy walks, greatly increasing their ability to detect pathogen (Harris et al., 2012). Additionally, CD8+ Trm cells in the epidermis are able to adopt a “dendritic-like” morphology, allowing them to scan multiple host cells at once for pathogen and thus maximizing their ability to locate infected cells (Ariotti et al., 2012; Gebhardt et al., 2011). In summary, T cells regulate their motility, directionality, and morphology to optimize pathogen detection.
Once T cells recognize infected cells, they employ a number of mechanisms to clear pathogen. Memory CD8+ T cells in certain NLT maintain constitutive granzyme B expression and like recently activated effectors, mediate ex vivo cytotoxicity (Kim et al., 1998; Masopust et al., 2006). Rapid delivery of cytotoxic molecules represents one potential mechanism to eliminate anamnestic infections. However, T cells might also clear pathogens through noncytolytic means. For example, the antiviral cytokine IFN-γ is critical for clearing L. major, HSV-2, and HBV infections (Guidotti et al., 1996; Iijima et al., 2008; Müller et al., 2012). Additionally, IFN-γ effects are not limited to infected host cells that T cells contact directly, but can also act on neighboring infected host cells (Müller et al., 2012). Other mechanisms of noncytolytic viral control exist. For instance, CD8+ T cells maintain HSV-1 viral latency by secreting lytic granules into infected neurons. Rather than killing these infected neurons and causing immunopathology, granzyme B degrades the immediate early protein ICP4, which is important for viral replication (Knickelbein et al., 2008). Thus Trm cells might employ multiple mechanisms to contribute to protective immunity.
Even though Trm cells can directly control infection, Trm cells might also activate other innate and adaptive leukocytes to protect the host. For instance, secretion of IFN-γ by Trm cells leads to endothelial upregulation of vascular cell adhesion molecule 1 (VCAM-1), an addressin that draws the recruitment of circulating memory CD8+ T cells and B cells to sites of Trm cell reactivation (Schenkel et al., 2014b). Elaboration of IFN-γ by Trm cells in the skin also causes the rapid upregulation of numerous antiviral and antibacterial genes (Ariotti et al., 2014). And Trm cell reactivation in the female reproductive tract was shown to precipitate the maturation of DCs and activation of NK cells in a cytokine-dependent manner (Schenkel et al., 2014b). Importantly, Trm cell-dependent activation of parenchymal, innate, and adaptive immunity triggered the induction of a local protective antiviral state (Ariotti et al., 2014; Schenkel et al., 2014b).
TLOs (Neyt et al., 2012) and leukocyte aggregates (TLOs have HEVs, whereas leukocyte aggregates do not) have long been noted within NLTs. Historically, they have occasionally been referred to as ectopic lymphoid structures, or abnormal sites of inflammation. Although more abundant during infection (Cromwell et al., 2011; Kobayashi et al., 2002;; Zhu et al., 2009), they are present in healthy humans and to a lesser degree in SPF mice, which have comparatively little infectious history. TLOs might comprise inductive sites for immune responses and permit naive lymphocyte trafficking. On the other hand, leukocyte aggregates contain only Ag-experienced lymphocytes. Leukocyte aggregates have been characterized in the brain, salivary gland, lung, female reproductive tract, skin, and liver under a variety of infectious or inflammatory conditions (Anderson et al., 2014; Huang et al., 2013; Iijima and Iwasaki, 2014; Natsuaki et al., 2014; Stahl et al., 2013; Wakim et al., 2010; Walton et al., 2011). There is considerable variation in the leukocyte populations found in these aggregates. For instance, aggregates generated in the vagina of HSV-2-infected mice are composed mostly of macrophages and CD4+ T cells, whereas aggregates that form in the brain after local vesicular stomatitis virus infection are composed of CD8+ T cells and CD103+ DCs (Iijima and Iwasaki, 2014; Wakim et al., 2010). Leukocyte aggregates have been proposed to be sites of in situ proliferation in a number of different models and might also control infections (Huang et al., 2013; Iijima and Iwasaki, 2014; Natsuaki et al., 2014; Stahl et al., 2013). Overall, these structures represent a unique niche for Trm cell function.
CD8+ T cells in NLTs are thus a potent occupying force that is able to efficiently scan host cells, rapidly purge infection, colocalize with other leukocytes in clusters, and alarm both hematopoietic and nonhematopoietic cells to protect the host from pathogen.
Concluding Remarks
Considerable work has begun to reveal unifying properties of Trm cells, and these are summarized in Table 1. It is becoming increasingly clear that Trm cells, which are not assayed by PBMC sampling, constitute a substantive fraction of the adaptive immune system that play a critical role in protective immunity. It is possible that Trm cells might also drive important disease processes, such as immunopathology and autoimmunity, and could also play an important role in immunosurveillance of cancer. While recent developments in understanding of Trm cells are likely to foster new translational opportunities for prophylactic or therapeutic interventions in the contexts of vaccines or established disease, critical gaps in knowledge remain.
Table 1.
Characterization of Trm Cells
| Characteristics | Exceptions, Caveats, or Anticipated Results |
|---|---|
| Phenotype: | |
| CD103+ | Trm cells in many locations are CD103− |
| CD69+ | Some Trm cells may be CD69− |
| Ly6Clo | In small intestine & SLOs, but varies by tissue |
| CD122lo | |
| CD127int | |
| CD127− | |
| CD62L− | |
| Granzyme B+ | Varies by location, maintained without Ag |
| Transcription Factors: | |
| KLF2lo | |
| Eomeslo | Varies by tissue |
| Function: | |
| Accelerate pathogen control | |
| Recruit CD8 T cells and B cells | |
| Activate innate immune system | |
| Migration Assays: | |
| Parabiosis | No T cell equilibration between parabionts |
| Transfer of effector vs. memory T cells | Only effectors migrate to sites of residence |
| Crawl out | Trm cells fail to crawl out of ex vivo cultured tissue |
| Syngeneic tissue grafts | No T cell equilibration between graft and host |
| FTY720 | Treatment does not reposition Trm cells to SLOs |
| Transfer male effector T cells to female mice | Donor cells that become Trm cells are not rejected |
| Epidermal memory T cell transfer | No emigration |
| Proliferation and Survival: | |
| Low homeostatic proliferation | Not characterized in many tissues |
| IL-15 dependence | SLOs – independent Epidermis – dependent |
| Proliferation after local reactivation | Yes, after local reactivation within dorsal root ganglia |
| Proliferation during primary effector phase | At least one CD8+ T cell division in CNS No CD4+ T cell divisions in Ag-bearing tail |
A summary of the migration assays used to define Trm, and Trm phenotypic and transcription factor signatures, proliferation potential, regulation of survival, and functions are shown.
As outlined above, a major challenge for investigations of Trm cells, particularly in humans, pertains to appropriate sampling and analytical methods of clinical specimens. The identification of stable markers and development of new protocols for imaging Ag-specific T cells within biopsies, particularly from humans of unknown haplotype, could be essential, particularly for assessing vaccine correlates of protection. There are conceptual challenges as well, as Trm cells have previously been conflated with recirculating Tem cells, although these subsets have now taken on very different meanings. To better exploit Trm cells for protective immunity via vaccination, the rules for practicably establishing this subset at the desired location will have to be better defined. In this regard, it will be critical to define the maintenance requirements and potential longevity of Trm cells within NLTs. Addressing these issues, and others, might not be straightforward, because Trm cells might be phenotypically and functionally heterogeneous, depending on their location and inducing stimulus. Although much remains to be learned, furthering investigations into Trm cell biology and function might pay dividends that can be leveraged for clinical benefit.
Acknowledgments
This work was supported by National Institutes of Health grants R01AI084913 and R01AI111671 (to D.M.)
References
- Anderson KG, Sung H, Skon CN, Lefrançois L, Deisinger A, Vezys V, Masopust D. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol. 2012;189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoshi T, Carrero JA, Konjufca V, Koide Y, Unanue ER, Miller MJ. The cellular niche of Listeria monocytogenes infection changes rapidly in the spleen. Eur J Immunol. 2009;39:417–425. doi: 10.1002/eji.200838718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Marée AFM, Zal T, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci USA. 2012;109:19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, Schumacher TN. T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- Arnon TI, Xu Y, Lo C, Pham T, An J, Coughlin S, Dorn GW, Cyster JG. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science. 2011;333:1898–1903. doi: 10.1126/science.1208248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bölter J, Münk A, Förster R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol. 2011;12:879–887. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. 2013;190:970–976. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner T, Arnold D, Wasem C, Herren S, Frutschi C. Regulation of cell death and survival in intestinal intraepithelial lymphocytes. Cell Death Differ. 2001;8:706–714. doi: 10.1038/sj.cdd.4400854. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the α E β 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Cromwell MA, Carville A, Mansfield K, Klumpp S, Westmoreland SV, Lackner AA, Johnson RP. SIV-specific CD8+ T cells are enriched in female genital mucosa of rhesus macaques and express receptors for inflammatory chemokines. Am J Reprod Immunol. 2011;65:242–247. doi: 10.1111/j.1600-0897.2010.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çuburu N, Graham BS, Buck CB, Kines RC, Pang YYS, Day PM, Lowy DR, Schiller JT. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest. 2012;122:4606–4620. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- Czeloth N, Bernhardt G, Hofmann F, Genth H, Förster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175:2960–2967. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGF-β-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganusov VV, De Boer RJ. Do most lymphocytes in humans really reside in the gut? Trends Immunol. 2007;28:514–518. doi: 10.1016/j.it.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- Gonzalez SF, Lukacs-Kornek V, Kuligowski MP, Pitcher LA, Degn SE, Kim YA, Cloninger MJ, Martinez-Pomares L, Gordon S, Turley SJ, Carroll MC. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol. 2010;11:427–434. doi: 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson JM, Murali-Krishna K, Altman JD, Ahmed R. Gene expression in antigen-specific CD8+ T cells during viral infection. J Immunol. 2001;166:795–799. doi: 10.4049/jimmunol.166.2.795. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Harris TH, Banigan EJ, Christian DA, Konradt C, Tait Wojno ED, Norose K, Wilson EH, John B, Weninger W, Luster AD, et al. Generalized Lévy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486:545–548. doi: 10.1038/nature11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Hawke S, Stevenson PG, Freeman S, Bangham CR. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med. 1998;187:1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci USA. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Coxson HO, Brumwell ML, Beyers N, Doerschuk CM, MacNee W, Wiggs BR. Erythrocyte and polymorphonuclear cell transit time and concentration in human pulmonary capillaries. J Appl Physiol. 1994;77:1795–1800. doi: 10.1152/jappl.1994.77.4.1795. [DOI] [PubMed] [Google Scholar]
- Huang LR, Wohlleber D, Reisinger F, Jenne CN, Cheng RL, Abdullah Z, Schildberg FA, Odenthal M, Dienes HP, van Rooijen N, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol. 2013;14:574–583. doi: 10.1038/ni.2573. [DOI] [PubMed] [Google Scholar]
- Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kastenmüller W, Torabi-Parizi P, Subramanian N, Lämmermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmüller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity. 2013;38:502–513. doi: 10.1016/j.immuni.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DR, Liu J, Carville A, Mansfield KG, Havenga MJE, Goudsmit J, Barouch DH. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J Immunol. 2008;181:4188–4198. doi: 10.4049/jimmunol.181.6.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Reed DS, Olson S, Schnell MJ, Rose JK, Morton PA, Lefrançois L. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc Natl Acad Sci USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrançois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CJ, Weiss KA, Hartwig SM, Varga SM. The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection. J Virol. 2014;88:9010–9016. doi: 10.1128/JVI.00329-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Darragh T, Herndier B, Anastos K, Minkoff H, Cohen M, Young M, Levine A, Grant LA, Hyun W, et al. Lymphoid follicles are generated in high-grade cervical dysplasia and have differing characteristics depending on HIV status. Am J Pathol. 2002;160:151–164. doi: 10.1016/s0002-9440(10)64359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama SY, Podolsky DK. Differential expression of transforming growth factors alpha and beta in rat intestinal epithelial cells. J Clin Invest. 1989;83:1768–1773. doi: 10.1172/JCI114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamana A, Martin P, de la Fuente H, Martinez-Muñoz L, Cruz-Adalia A, Ramirez-Huesca M, Escribano C, Gollmer K, Mellado M, Stein JV, et al. CD69 modulates sphingosine-1-phosphate-induced migration of skin dendritic cells. J Invest Dermatol. 2011;131:1503–1512. doi: 10.1038/jid.2011.54. [DOI] [PubMed] [Google Scholar]
- Le Floc’h A, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, Chouaib S, Mami-Chouaib F. α E β 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol. 2011;85:4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. TGF-β: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang M, Zhou C, Zhao X, Iijima N, Frankel FR. Novel vaccination protocol with two live mucosal vectors elicits strong cell-mediated immunity in the vagina and protects against vaginal virus challenge. J Immunol. 2008;180:2504–2513. doi: 10.4049/jimmunol.180.4.2504. [DOI] [PubMed] [Google Scholar]
- Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. 2005;201:291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Cabrera M, Muñoz E, Blázquez MV, Ursa MA, Santis AG, Sánchez-Madrid F. Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-α-responsive elements. J Biol Chem. 1995;270:21545–21551. doi: 10.1074/jbc.270.37.21545. [DOI] [PubMed] [Google Scholar]
- Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Matsuyuki H, Shimano K, Kataoka H, Sugahara K, Chiba K. Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J Immunol. 2007;178:3437–3446. doi: 10.4049/jimmunol.178.6.3437. [DOI] [PubMed] [Google Scholar]
- Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- Masopust D, Jiang J, Shen H, Lefrançois L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J Immunol. 2001a;166:2348–2356. doi: 10.4049/jimmunol.166.4.2348. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001b;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrançois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Montufar-Solis D, Garza T, Klein JR. T-cell activation in the intestinal mucosa. Immunol Rev. 2007;215:189–201. doi: 10.1111/j.1600-065X.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- Müller AJ, Filipe-Santos O, Eberl G, Aebischer T, Späth GF, Bousso P. CD4+ T cells rely on a cytokine gradient to control intracellular pathogens beyond sites of antigen presentation. Immunity. 2012;37:147–157. doi: 10.1016/j.immuni.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Muppidi JR, Arnon TI, Bronevetsky Y, Veerapen N, Tanaka M, Besra GS, Cyster JG. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J Exp Med. 2011;208:1941–1948. doi: 10.1084/jem.20111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanan R, Rauch A, Kämpgen E, Niewiesk S, Kreth HW. A novel sensitive approach for frequency analysis of measles virus-specific memory T-lymphocytes in healthy adults with a childhood history of natural measles. J Gen Virol. 2000;81:1313–1319. doi: 10.1099/0022-1317-81-5-1313. [DOI] [PubMed] [Google Scholar]
- Natsuaki Y, Egawa G, Nakamizo S, Ono S, Hanakawa S, Okada T, Kusuba N, Otsuka A, Kitoh A, Honda T, et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat Immunol. 2014;15:1064–1069. doi: 10.1038/ni.2992. [DOI] [PubMed] [Google Scholar]
- Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33:297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz JC, Harty JT. IL-15 regulates memory CD8+ T cells O-glycan synthesis and affects trafficking. 2014;124:1013–1026. doi: 10.1172/JCI72039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Förster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun YM, Lambert K, Acharya M, Billroth-Maclurg AC, Rosenberg AF, Topham DJ, et al. Inflammation-induced interstitial migration of effector CD4+ T cells is dependent on integrin αV. Nat. Immunol. 2013;14:949–958. doi: 10.1038/ni.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham THM, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston GC, Feijoo-Carnero C, Schurch N, Cowling VH, Cantrell DA. The impact of KLF2 modulation on the transcriptional program and function of CD8 T cells. PLoS ONE. 2013;8:e77537. doi: 10.1371/journal.pone.0077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, Barber DL. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol. 2014;192:2965–2969. doi: 10.4049/jimmunol.1400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJC, Bickham KL, Lerner H, Goldstein M, Sykes M, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon ST, Thomas SY, Ferreira CM, Bai L, Krausz T, Savage PB, Bendelac A. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J Exp Med. 2011;208:2113–2124. doi: 10.1084/jem.20110522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Masopust D. Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol. 2014a;192:2961–2964. doi: 10.4049/jimmunol.1400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014b;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober SL, Kuo CT, Schluns KS, Lefrançois L, Leiden JM, Jameson SC. Expression of the transcription factor lung Krüppel-like factor is regulated by cytokines and correlates with survival of memory T cells in vitro and in vivo. J Immunol. 1999;163:3662–3667. [PubMed] [Google Scholar]
- Schön MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin α E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- Segel GB, Cokelet GR, Lichtman MA. The measurement of lymphocyte volume: importance of reference particle deformability and counting solution tonicity. Blood. 1981;57:894–899. [PubMed] [Google Scholar]
- Selby WS, Janossy G, Bofill M, Jewell DP. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984;25:32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon LA, McBurney TM, Wells MA, Roth ME, Calloway PA, Bill CA, Islam S, Vines CM. CCR7/CCL19 controls expression of EDG-1 in T cells. J Biol Chem. 2012;287:11656–11664. doi: 10.1074/jbc.M111.310045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Romagnoli PA, Pham QM, Fu HH, Alonzo F, 3rd, Schubert W-D, Freitag NE, Lefrançois L. γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39:184–195. doi: 10.1016/j.immuni.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrançois L. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdicková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/ beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl FR, Heller K, Halle S, Keyser KA, Busche A, Marquardt A, Wagner K, Boelter J, Bischoff Y, Kremmer E, et al. Nodular inflammatory foci are sites of T cell priming and control of murine cytomegalovirus infection in the neonatal lung. PLoS Pathog. 2013;9:e1003828. doi: 10.1371/journal.ppat.1003828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JH, Zhang H, Moseman EA, Alvarez D, Iannacone M, Henrickson SE, de la Torre JC, Groom JR, Luster AD, von Andrian UH. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150:1249–1263. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Takamura S, Roberts AD, Jelley-Gibbs DM, Wittmer ST, Kohlmeier JE, Woodland DL. The route of priming influences the ability of respiratory virus-specific memory CD8+ T cells to be activated by residual antigen. J Exp Med. 2010;207:1153–1160. doi: 10.1084/jem.20090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, Meng F, Luster AD, Bendelac A. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur M, Schulz O, Menon MB, Krueger A, Pabst O. Resident CD4+ T cells accumulate in lymphoid organs after prolonged antigen exposure. Nat Commun. 2014;5:4821. doi: 10.1038/ncomms5821. [DOI] [PubMed] [Google Scholar]
- van der Most RG, Murali-Krishna K, Ahmed R. Prolonged presence of effector-memory CD8 T cells in the central nervous system after dengue virus encephalitis. Int Immunol. 2003;15:119–125. doi: 10.1093/intimm/dxg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps HL, Terajima M, Mustonen J, Arstila TP, Corey EA, Vaheri A, Ennis FA. Long-lived memory T lymphocyte responses after hantavirus infection. J Exp Med. 2002;196:579–588. doi: 10.1084/jem.20011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, Bevan MJ. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. 2012;189:3462–3471. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton SM, Mandaric S, Torti N, Zimmermann A, Hengel H, Oxenius A. Absence of cross-presenting cells in the salivary gland and viral immune evasion confine cytomegalovirus immune control to effector CD4 T cells. PLoS Pathog. 2011;7:e1002214. doi: 10.1371/journal.ppat.1002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2014;95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Qiu CX, Ludlow A, Ferguson MW, Brunner G. Active transforming growth factor-beta in wound repair: determination using a new assay. Am J Pathol. 1999;154:105–111. doi: 10.1016/s0002-9440(10)65256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, Manton JH, Heath WR, Mueller SN. Persistence of skin-resident memory T cells within an epidermal niche. Proc Natl Acad Sci USA. 2014;111:5307–5312. doi: 10.1073/pnas.1322292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit DJ, Turner DL, Klonowski KD, Lefrançois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, Kirk J, Eppler B, Klinman DM, Sui Y, et al. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat Med. 2012;18:1291–1296. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond E, Jung S. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol. 2013;34:162–168. doi: 10.1016/j.it.2013.02.001. [DOI] [PubMed] [Google Scholar]