Abstract
The development of B cells is dependent on the sequential DNA rearrangement of immunoglobulin loci that encode subunits of the B cell receptor. The pathway navigates a crucial checkpoint that ensures expression of a signalling-competent immunoglobulin heavy chain before commitment to rearrangement and expression of an immunoglobulin light chain. The checkpoint segregates proliferation of pre-B cells from immunoglobulin light chain recombination and their differentiation into B cells. Recent advances have revealed the molecular circuitry that controls two rival signalling systems, namely the interleukin-7 (IL-7) receptor and the pre-B cell receptor, to ensure that proliferation and immunoglobulin recombination are mutually exclusive, thereby maintaining genomic integrity during B cell development.
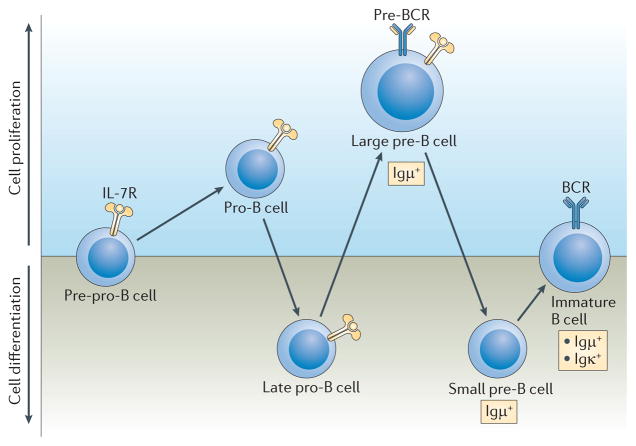
B cell lymphopoiesis generates a diverse repertoire of peripheral B cells, which can give rise to antibody-producing cells that mediate protection from pathogens but remain tolerant of self tissues1. The hallmark of B lymphopoiesis is the sequential productive DNA rearrangement of the immunoglobulin heavy chain locus (Igμ) and the immunoglobulin light chain loci (Igκ followed by Igλ), and their expression and assembly into B cell receptors (BCRs). Rearrangement of the Igμ locus involves the recombination of diversity (D) and joining (J) gene segments, and begins in pre-pro-B cells, which are not yet committed to the B cell lineage (FIG. 1). Subsequent recombination of variable (V) gene segments to rearranged (D)J regions occurs in late pro-B cells (also known as pre-BI cells). Developing B-lineage cells proliferate in response to interleukin-7 (IL-7) by interacting with bone marrow stromal cells, which are the source of this cytokine. Following an in-frame V to (D)J recombination event, the successful expression of an Igμ chain leads to its assembly with the surrogate light chain (SLC; which comprises the λ5 and VpreB proteins) and the signalling subunits Igα and Igβ to form a pre-B cell receptor (pre-BCR). The pre-BCR promotes the generation and expansion of a population of large pre-B cells (also known as pre-BII cells), which remain dependent on IL-7 signalling2,3. To initiate Igκ or Igλ gene rearrangement, these cycling pre-B cells must attenuate and/or escape the proliferative signals of the IL-7 receptor (IL-7R), which is dependent on antagonistic signalling by the pre-BCR.
Figure 1. B lymphopoiesis.
B lymphopoiesis is a highly ordered developmental process that involves sequential immunoglobulin gene recombination. Proliferation in committed B cell progenitors is dependent on the interleukin-7 receptor (IL-7R), which is first expressed in pre-pro-B cells and has a crucial role in both pro-B and large pre-B cell proliferation. Rearrangement of the Igμ locus begins with diversity (D)–joining (J) rearrangements in pre-pro-B cells that are not yet committed to the B cell lineage. Variable (V)–(D)J rearrangement occurs in the late pro-B cell pool, which contains cells that express lower levels of the IL-7R and are not proliferating. Successful in-frame rearrangements lead to expression of Igμ, which then assembles with the surrogate light chain and Igα and Igβ to form the pre-B cell receptor (pre-BCR) in large pre-B cells. Expression of the pre-BCR is associated with a proliferative burst followed by cell cycle exit and transition to the small pre-B cell stage, the latter facilitates Igκ gene recombination. Cells that undergo in-frame rearrangement of the Igκ gene, and express the Igκ protein, are selected into the immature B cell pool, where mechanisms of tolerance, such as receptor editing, purge the repertoire of self-reactive clones.
This developmental sequence enables pre-B cells to step through a crucial checkpoint that ensures expression of a signalling-competent Igμ chain before their commitment to rearrangement and expression of an immunoglobulin light chain. The checkpoint also segregates the proliferation of pre-B cells from the recombination of immunoglobulin light chain loci. Failure to do so can result in genomic instability and neoplastic transformation4.
It has long been clear that both the IL-7R and the pre-BCR are required for murine B cell lymphopoiesis2,3. However, the molecular circuits and the regulatory logic by which these two signalling systems orchestrate B cell development have remained obscure and controversial. In this Review, we describe new experimental insights that have led to the formulation of a coherent molecular framework for murine B cell development. We focus on the signalling and transcriptional regulatory networks that enable the IL-7R and pre-BCR to coordinate the pre-B cell developmental checkpoint (FIG. 2).
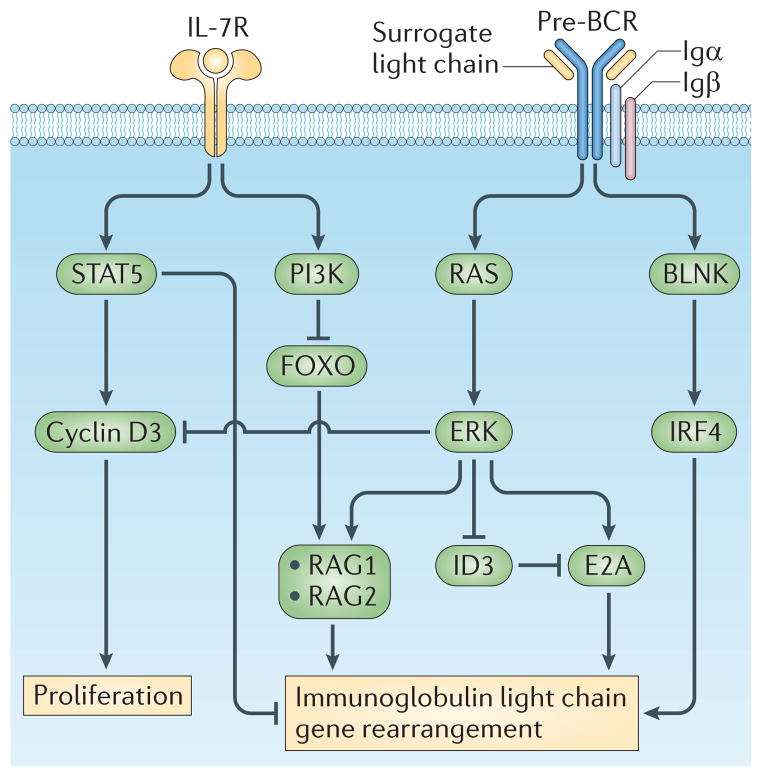
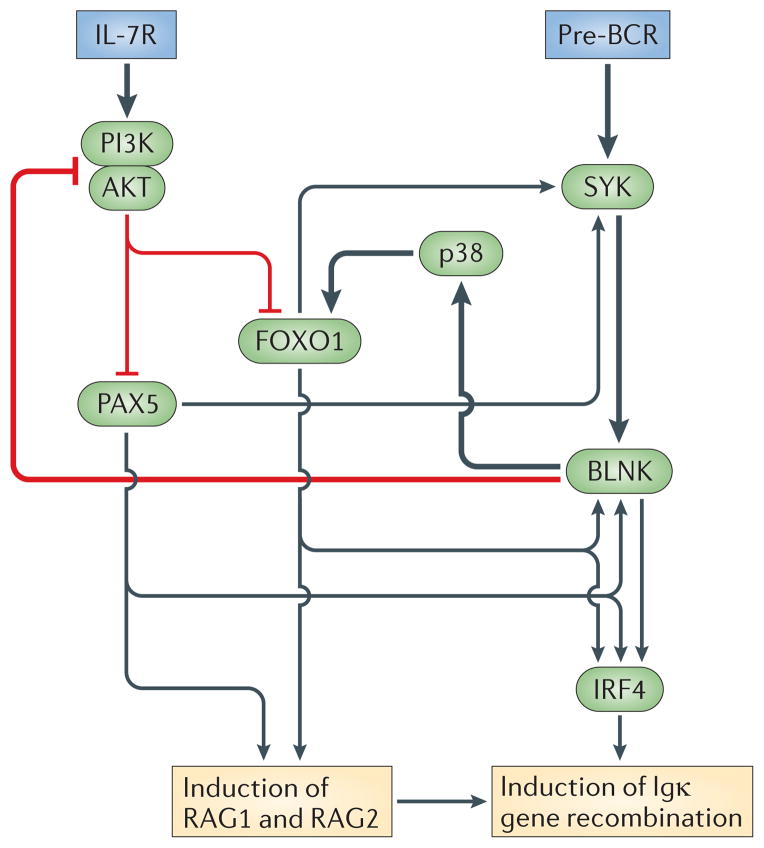
Figure 2. The IL-7R and pre-BCR coordinate proliferation with Igκ gene recombination in B lineage cells.
Downstream of each receptor, distinct signalling pathways have specific functions in proliferation and recombination. Interleukin-7 receptor (IL-7R)-mediated signal transducer and activator of transcription 5 (STAT5) activation induces transcription of cyclin D3, which promotes proliferation. In addition, STAT5 directly represses Igκ gene accessibility and recombination. The IL-7R also activates phosphoinositide 3-kinase (PI3K), which represses forkhead box protein O1 (FOXO1), an obligate inducer of recombination-activating gene 1 (RAG1) and RAG2 gene transcription. By contrast, the pre-B cell receptor (pre-BCR) is coupled to the RAS–extracellular signal-regulated kinase (ERK) signalling pathway, which represses cyclin D3 and inhibitor of DNA binding 3 (ID3) while inducing E2A. This has the effect of inhibiting proliferation and increasing levels of free nuclear E2A. E2A with interferon regulatory factor 4 (IRF4), downstream of B cell linker protein (BLNK), coordinately enhance Igκ gene accessibility. Preferential coupling of the PI3K pathway to the IL-7R, and not the pre-BCR, ensures that each receptor has opposing and antagonistic functions. The IL-7R induces proliferation and represses Igκ gene recombination while the pre-BCR represses proliferation and induces recombination.
IL-7R signalling in early B cell lymphopoiesis
The proliferation and survival of B cell progenitors is dependent on the IL-7R5, which is composed of the IL-7Rα chain (which confers specificity for IL-7) and the common-γ chain (γc). Mutation of the gene encoding IL-7Rα severely impairs B cell lymphopoiesis in the bone marrow of mice6. This defect manifests at the pre-pro-B cell stage and also in earlier intermediates, including common lymphoid progenitors (CLPs)6. Similarly, germline knockout of the gene encoding IL-7 attenuates B lymphopoiesis in the adult bone marrow, although the phenotype of IL-7Rα-deficient mice is more severe. The IL-7Rα chain can also pair with the thymic stromal lymphopoietin (TSLP) receptor chain5. Therefore, it has been postulated that TSLP might compensate for IL-7 deficiency. However, loss of TSLP does not lead to a more pronounced block of B cell lymphopoiesis in Il7−/− mice7. In keeping with these findings, mutation of ATP11c, a putative P4 ATP phospholipid flippase that is required for IL-7 responsiveness, attenuates B cell development8–10.
Many regulatory responses of pro-B cells to IL-7 signalling are conserved between mice and humans; however, whereas in mice the IL-7Rα chain is required for B cell development, in humans several patients with IL-7Rα mutations have been shown to retain peripheral B cells11,12. Therefore, B cell development in humans seems to be less dependent on IL-7R signalling (BOX 1). It remains to be determined whether another receptor system compensates for IL-7Rα loss during human B lymphopoiesis.
Box 1. IL-7 signalling and B cell development in mice versus humans.
In both humans and mice, the interleukin-7 receptor-α (IL-7Rα) chain (also known as CD127) is expressed by early B cell progenitors, and signalling via IL-7Rα activates signal transducer and activator of transcription 5 (STAT5)2,18 and drives pro-B cell proliferation, while inhibiting Igκ recombination3,4. Furthermore, attenuation of IL-7R signalling in both human and mouse pre-B cells is associated with the upregulation of forkhead box protein O1 (FOXO1) and expression of recombination-activating gene 1 (RAG1) and RAG2 (REF. 4). In mice, it has been demonstrated that B cell development requires the IL-7Rα chain. However, in humans several mutations in the IL7Ra gene have been described that are associated with normal numbers of peripheral CD19+ B cells but greatly diminished numbers of peripheral T cells and natural killer cells7,11,12,153 Many of these patients have low levels of serum immunoglobulins, which suggests that their peripheral B cells are functionally defective. These analyses suggest that, compared with mice, human B cell development is less affected by loss of IL-7R activity. It remains to be determined whether another receptor system molecularly compensates for IL-7Rα loss during human B lymphopoiesis.
Involvement of JAK3 and STAT5
The IL-7Rα and γc subunits do not have intrinsic kinase activity13. Instead, Janus kinase 3 (JAK3) is constitutively associated with γc, whereas JAK1 associates with IL-7Rα. Binding of IL-7 induces the phosphorylation of these receptor-associated kinases5,13 and of a tyrosine residue (Y449) in the IL-7Rα chain. This serves to recruit signal transducer and activator of transcription 5A (STAT5A) and STAT5B, which are the predominant STATs activated by the IL-7R, although STAT3 also contributes to IL-7R signalling14. Deletion of Stat5a and Stat5b arrests B cell development at the pre-pro-B cell stage, similarly to what is observed in Il7ra−/− mice15. Conversely, a constitutively active STAT5 can bypass many functions that are dependent on IL-7 signalling16–18.
Role of cyclin D2 and cyclin D3
Following activation by IL-7, STAT5 binds to and stimulates the transcription of Ccnd3, which encodes cyclin D3. Cyclin D3 has been shown to be required for both pro- and pre-B cell proliferation17,19. In the classical model of receptor-mediated proliferation, growth factor receptors initiate a signalling cascade that induces transit past the G1 checkpoint of the cell cycle and DNA replication20. The G1 checkpoint is regulated by the D-type cyclins, including cyclin D1, cyclin D2 and cyclin D3, which bind and activate cyclin-dependent kinase 4 (CDK4) and CDK6. Cyclin D–CDK4–CDK6 complexes initiate phosphorylation of the retinoblastoma protein (RB) family members RB, p107 and p130, resulting in their release from E2F transcription factors and the activation of cell cycle genes. The cyclin D–CDK complexes also sequester the cell cycle inhibitors cyclin-dependent kinase inhibitor 1B (CDKN1B; also known as KIP1) and CDKN1A (also known as CIP1) from the cyclin E–CDK2 and cyclin A–CDK2 complexes, respectively20. The net effect of these regulated molecular dynamics is the induction of additional cell cycle genes and transition through the G1 checkpoint21. This canonical model was initially established in biochemical studies of non-haematopoietic cell lines20. However, analysis of mice deficient in all three D-type cyclins, or in CDK4 and CDK6, showed that these regulators are only required for the proliferation of cells within the haematopoietic system and in a few additional tissues22,23.
Notably, although the genes encoding cyclin D2 and cyclin D3 are both expressed in B cell progenitors, only cyclin D3 is required for early B cell development and for the proliferation of pro-B and pre-B cells19. Intriguingly, cyclin D3 preferentially binds to CDK4 and CDK6 in pro-B cells24. This selective binding is correlated with distinct compartmentalization of cyclin D3 and cyclin D2 within pro-B cell nuclei24. These findings raise the possibility that specialized nuclear sub-compartments might facilitate the binding of cyclins to CDK4 and CDK6, thereby enabling their unique functions. We note that cyclin D3 also has a role in developing thymocytes25 and germinal centre B cells26, whereas cyclin D2 is required for mature B cell proliferation27,28. The cell cycle inhibitor KIP1 has been postulated to be an important regulator of the cell cycle3 in B lineage cells, and overexpression of KIP1 halts pre-B cell proliferation in vitro29. However, B cell lymphopoiesis in KIP1-deficient mice is essentially normal, although there is a moderate increase in the number of cycling pre-B cells30,31. Therefore, of the currently studied canonical cell cycle regulators in B cell lymphopoiesis, only cyclin D3 has been found to have an essential function.
Pro-survival role for STAT5
In addition to stimulating proliferation, STAT5 enhances the survival of B cell progenitors. Deletion of Stat5a and Stat5b using a recombination-activating gene 1 (Rag1)–Cre system (which ensures that genes are only deleted from cells expressing Rag1) showed that STAT5 is required for pro-B cell expression of the pro-survival factor myeloid cell leukaemia sequence 1 (MCL1)32. The JAK–STAT5 pathway also induces the pro-survival protein B cell lymphoma 2 (BCL-2), which additionally contributes to B cell progenitor survival16,33,34. Accordingly, constitutive expression of BCL-2 in Stat5−/− mice partially rescues pro-B cell development. Therefore, a major function of IL-7 activated STAT5 is to ensure the survival of pro-B cells by activating expression of the genes encoding MCL1 and BCL-2.
Contribution of PI3K
IL-7R signalling also activates the phosphoinositide 3-kinase (PI3K)–AKT (also known as PKB) pathway35–37. Deletion of the PI3K catalytic subunits p110α and p110δ38, or the regulatory subunit p85α39,40, impairs B cell lymphopoiesis. PI3K is required for cellular proliferation in both pre-B and mature B cells39–43. However, it does not seem to have the same role in pro-B cells. p110α and p110δ double-deficient mice show developmental arrest at the pre-B cell stage. Pro-B cells in these mice proliferate normally and IL-7-induced AKT activation is similar in the pro- and pre-B cell compartments36. Furthermore, there are no proliferative defects in pro-B cells from mice that are deficient in either p85α or the PI3K negative regulator phosphatase and tensin homologue (PTEN). Finally, pharmacological inhibition of PI3K in ex vivo cultured pro-B cells from mice deficient in the pro-apoptotic molecule BCL-2-interacting mediator of cell death (BIM; also known as BCL2L11) does not affect their proliferation24. Therefore, there is little evidence that PI3K is necessary for the proliferation of pro-B cells.
Nevertheless, the activation of PI3K–AKT signalling by the IL-7R44,45 is likely to play a part in IL-7-mediated pro-B cell survival. Activated AKT phosphorylates the forkhead box protein O (FOXO) family of transcription factors and targets them for nuclear export and degradation. FOXO factors are required for expression of BIM46,47, and so by targeting FOXO, AKT lowers BIM expression levels. AKT also directly phosphorylates and inhibits the pro-apoptotic protein BCL-2 antagonist of cell death (BAD)48. It is likely that inhibition of these pro-apoptotic targets by AKT coordinates with STAT5-induced MCL1 and BCL-2 to promote the survival of pro-B cells in response to IL-7 signalling.
Key transcription factors facilitating IL-7 signalling
A set of transcription factors, including early B cell factor 1 (EBF1), MYB and MYC-interacting zinc finger protein 1 (MIZ1; also known as ZBTB17), interplay with IL-7 signalling to promote the survival and proliferation of B cell lineage progenitors. They do so either by positively regulating the expression of Il7ra or by functioning in parallel or downstream of IL-7R signalling. EBF1 is a major transcriptional determinant that functions in B cell fate specification and commitment in concert with the transcription factors E2A and paired box 5 (PAX5), respectively49–51. The pro-B cell gene regulatory network, which involves these and other transcription factors, has been reviewed extensively and will not be covered here52,53. Conditional deletion of Ebf1 results in a marked defect in pro-B cell survival and proliferation54. This is accompanied by reduced expression of the EBF1-targeted genes E2f2, E2f8, Ccnd3 and Bcl2l1. v-ABL transformed Ebf1−/− B lineage progenitors exhibit a survival defect that is rescued by forced expression of B cell lymphoma-XL (BCL-XL; also known as BCL2L1). It should be noted that Ebf1−/− B lineage progenitors express MCL1 at normal levels, suggesting that the different BCL-2 family members are not redundant in the pro-B cell compartment. EBF1 regulates expression of FOXO1, which in turn activates Il7ra55. Intriguingly, MYB has a similarly important role to EBF1 in regulating pro-B cell survival and proliferation56,57. The conditional loss of MYB through the use of an Mb1–Cre transgene (which enables deletion of floxed Myb allele in pre-pro-B cells) results in impaired expression of Ebf1 and Il7ra. Restoring expression of EBF1 but not IL-7Rα partially rescues the block in early B cell development. MYB also functions downstream of EBF1 in rescuing the survival defect of v-ABL-transformed Ebf1−/− B cell lineage progenitors.
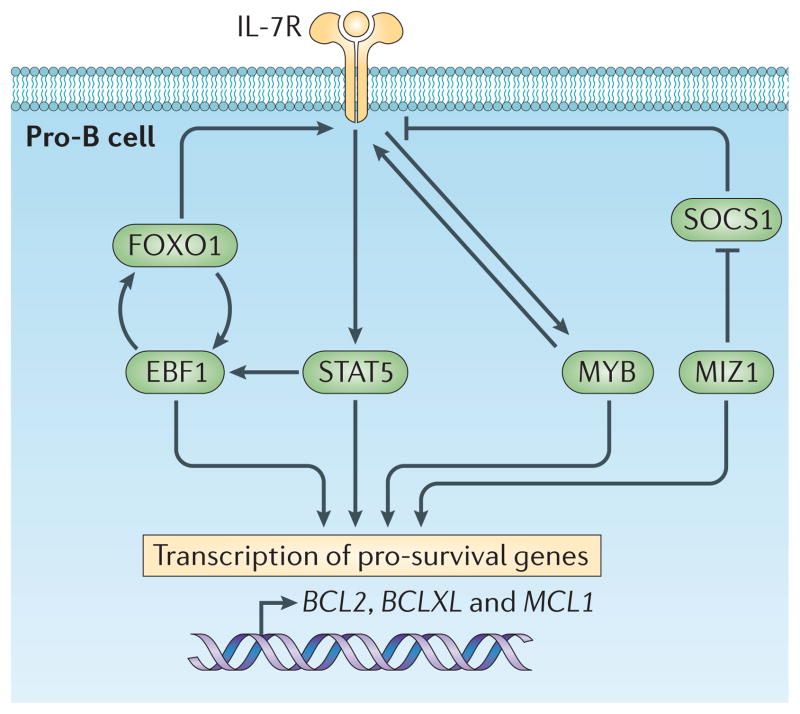
Thus, the IL-7R and the transcription factors EBF1, FOXO1 and MYB seem to represent components of a mutually reinforcing regulatory network that promotes the survival and proliferation of pro-B cells (FIG. 3). The transcription factor MIZ1 further stabilizes this network, in part, by repressing the suppressor of cytokine signalling 1 (Socs1) gene, which encodes an inhibitor of JAK signalling and STAT5 activation58.
Figure 3. Self-reinforcing network regulating pro-B cell survival.
Signal transducer and activator of transcription 5 (STAT5), which is induced by interleukin-7 receptor (IL-7R), and the transcription factors early B cell factor 1 (EBF1), forkhead box protein O1 (FOXO1) and MYB are components of a mutually reinforcing regulatory network that promotes the survival of pro-B cells. This regulatory network programmes the expression of the pro-survival genes B cell lymphoma 2 (BCL2), B cell lymphoma-XL (BCLXL) and myeloid cell leukaemia sequence 1 (MCL1), which are not mutually redundant. The transcription factor MYC-interacting zinc finger protein 1 (MIZ1) stabilizes the network by repressing the suppressor of cytokine signalling 1 (SOCS1) gene, which encodes an inhibitor of Janus kinase (JAK) signalling and STAT5 activation.
Roles for other signalling pathways
Although IL-7 signalling is the major pathway regulating pro-B cell survival and proliferation, it is likely that other signalling systems are important at this step in B cell development. There is a critical, intrinsic59 requirement for the SRC family tyrosine kinase ABL in B cell lymphopoiesis60,61. It is expressed throughout B cell development, but levels of phosphorylated ABL peak in pro-B cells62. Defective B cell development is associated with increased apoptosis and decreased proliferation in vivo62,63. However, transgenic expression of BCL-XL only partially rescues the developmental block62, indicating that ABL does more than prevent apoptosis. ABL can be activated by signalling through the antigen receptors64–66, but the high levels of phosphorylated ABL that are detected in pro-B cells suggest that other receptors can also activate ABL. It is also possible that ABL is not induced following the activation of a cell surface receptor in pro-B cells but instead in response to DNA double-strand breaks that are intermediates in immunoglobulin heavy chain gene recombination67.
Specification of pro-B cells
Given that the IL-7R is expressed in CLPs and its loss or that of IL-7 results in an early block to B cell development that is not rescued by ectopic expression of BCL-2 (REF. 68), a role for IL-7 signalling in B cell fate specification has been extensively considered52. The available data are consistent with a transient function of IL-7 signalling in CLPs that promotes the expression of EBF1 and, in turn, PAX5. In keeping with this possibility, IL-7 signalling has been shown to regulate the activity of a distal promoter in the Ebf1 gene69. Consistent with these findings, ectopic expression of EBF1 or constitutively activated STAT5 can restore B cell development in IL7−/− mice70,71.
It should be noted that conditional deletion of Stat5 using a Rag1–Cre transgene system results, as expected, in an early block to B cell development. However, unlike in IL7ra−/− or γc−/− mice, the ectopic expression of Bcl2 in mice with a Rag1–Cre-mediated deletion of Stat5 rescues the generation of pro-B cells32. Intriguingly, expression of EBF1 and PAX5 is maintained in the absence of STAT5 in these ‘rescued’ Stat5−/− pro-B cells. These two sets of experimental analyses can be reconciled with a model in which a transient pulse of IL-7 signalling in CLPs can promote B cell fate specification by inducing a key lineage determinant, namely EBF1. The expression of EBF1 could then be sustained in pro-B cells via a positive feedback loop that does not rely on continued IL-7 signalling. Such transient inductive signalling to enable cell fate specification has been widely noted in other developmental contexts, with Notch signalling being a relevant exemplar in T lineage determination72.
Rag expression and immunoglobulin heavy chain gene rearrangement
The Rag1 and Rag2 genes are directly activated by FOXO1 and FOXO3A36,55,73,74. The repression of FOXO activity and therefore Rag expression is a major, non-redundant function of the PI3K–AKT pathway. Rag expression is dysregulated in p110α and p110δ double-deficient mice38. Furthermore, attenuated IL-7R signalling leads to diminished Akt activation, upregulation of FOXO and the robust induction of the Rag genes36. The repression of Rag expression during pro-B cell proliferation in the context of IL-7R signalling (see below) is reinforced by mechanisms intrinsic to the cell cycle machinery. The cyclin A–CDK2 complex, which regulates the late G1 transition, also phosphorylates Rag2 at Thr490 (REF. 75), thereby targeting it for ubiquitylation by S phase kinase-associated protein 2 (SKP2) and subsequent proteasome-mediated degradation76,77.
The findings that IL-7 signalling via PI3K–AKT represses Rag gene expression raise a developmental conundrum. How can Igμ DNA rearrangement occur in pro-B cells that are dependent on IL-7 signalling for their survival and proliferation? Recent evidence indicates that the pro-B cell pool is heterogeneous for IL-7R surface expression; the level of IL-7R expression is positively correlated with the intracellular level of phosphorylated STAT5, but is negatively correlated with the level of Rag expression78. Such a heterogenous cellular state might be generated by a regulatory loop between IL-7R and FOXO factors. In pro-B cells that have been recently activated by IL-7 signalling, the destabilization of FOXO transcription factors, which positively regulate expression of the Il7ra gene, is likely to result in the lowering of IL-7R levels. This might result in a transient diminution of IL-7 signalling and therefore upregulation of FOXO factors and the eventual restoration of high levels of the IL-7R. Thus, pro-B cells might oscillate between IL-7R high and low states, with the low state enabling Rag expression and Igμ recombination, and subsequent transitioning into the pre-B cell pool.
In addition to inducing Rag gene expression, FOXO1 facilitates V(D)J recombination by enhancing VH gene accessibility and compaction of the Igμ locus79,80. In this regard, STAT5 that is transiently activated via IL-7 signalling in pro-B cells has been shown to be recruited to distal VH gene segments and to promote localized histone acetylation and, in turn, distal VH gene rearrangement81. Thus, the transient pulse of IL-7 signalling that has been suggested to induce B cell fate specification could also induce chromatin remodelling of distal VH gene segments via STAT5 and enable their eventual recombination in pro-B cells that express low levels of IL-7R and high levels of Rag transcripts81.
Pre-BCR signalling and development checkpoint
In-frame V(D)J gene rearrangements in pro-B cells leads to cell surface expression of a pre-BCR containing Igμ, Igα, Igβ and the SLC molecules λ5 and VpreB82–85. B cell progenitors lacking surface Igμ, Igα or Igβ undergo limited V(D)J recombination but are not selected for expansion and further development84. Assembly of the pre-BCR with the SLC, but not with Igκ, is sufficient to induce signalling in vitro86. Signalling is associated with receptor aggregation on the cell surface and is mediated by basic amino acids in the non-immunoglobulin tail of λ5 (REFS 87,88). These observations suggest that the SLC functions to induce surface aggregation and signalling by the pre-BCR in the absence of a selecting ligand. The importance of the λ5 non-immunoglobulin tail has been demonstrated in vivo89. However, it is still not clear whether it functions in vivo to self-aggregate the pre-BCR or to confer binding to one or more ligands in the bone marrow, such as heparin or galectin 1 (REFS 90–92). Galectin 1 is a particularly attractive candidate ligand because it might have a role in pre-B cell development93 and is preferentially expressed in bone marrow niches that contain pre-B cells and that are relatively devoid of IL-7 (REF. 94).
B cell development in mice lacking the SLC does occur, albeit inefficiently, and involves expression of heavy and light chains that confer autoreactivity95. This has been interpreted to indicate that the pre-BCR selects against autoreactivity and that the pre-B cell stage is a tolerance checkpoint. An alternative explanation is that, in the absence of the SLC, autoreactivity is necessary to aggregate the pre-BCR and transmit developmentally required signals96.
Clonal expansion of pre-B cells
Genetic analyses of the components of the PI3K–AKT signalling pathway discussed above do suggest a crucial need for PI3K in proliferating large pre-B cells. At this stage of B cell development, PI3K is likely to activate the cell growth and bioenergetic machinery that is necessary to support rapid cell division97,98. Downstream of PI3K, the coordinated activation of AKT by pyruvate dehydrogenase kinase 1 (PDK1) and mammalian target of rapamycin complex 2 (mTORC2)99 results in increased expression of glucose transporter 1 (GLUT1; also known as SLC2A1) and cellular uptake of glucose100, upregulation of several glycolytic enzymes101 and other changes that enhance glucose metabolism. These changes greatly augment aerobic glycolysis, which is used by most cells of the immune system for rapid cell division102. Downstream of AKT, activation of mTORC1 enhances several processes that augment protein synthesis, including cap-dependent mRNA translation initiation and ribosome synthesis. In addition, mTORC1 augments lipid biosynthesis and inhibits autophagy98,103.
The MYC proto-oncogene is likely to exert an important function in pre-B cell proliferation. Transgenic expression of MYC under the Igμ enhancer (Eμ) demonstrates that MYC can enhance protein synthesis and cell size104,105. These findings are consistent with current concepts that MYC regulates a transcriptional programme that drives biomass accumulation and enhances cell bioenergetics98,106. MYC also amplifies PI3K signalling by repressing PTEN107 and induces the expression of cyclin D3 (REF. 30). Accordingly, high expression of MYC protein defines a subset of large, rapidly dividing pre-B cells108. How is MYC expression regulated in pre-B cells? Withdrawal of IL-7 reduces MYC expression, and the overexpression of MYC blocks exit from the cell cycle in response to IL-7 withdrawal30, indicating that MYC is regulated by IL-7R signalling. However, the transient increase of MYC expression in pre-B cells is not correlated with increased phosphorylation of STAT5, a transducer of IL-7R signalling36. Although the signalling pathways leading to increased MYC expression in pre-B cells are not known, there are multiple candidates, including PI3K, extracellular signal-regulated kinase (ERK) and nuclear factor-κB105,106,109.
Transition of cycling pre-B cells to a resting state
Pre-B cell clonal expansion in vivo is limited to four to five cell divisions110. The signalling and gene regulatory mechanisms in pre-B cells that limit their clonal expansion have been intensively pursued, particularly because cell cycle arrest is coupled to the re-induction of Rag gene expression and immunoglobulin light chain gene rearrangement (see below). It has been hypothesized that the pre-BCR provides signals that not only drive the proliferation of pre-B cells but that also silence the expression of the SLC and, subsequently, the expression of pre-BCR111. In this model, subsequent cell divisions, which would dilute SLC levels, would lead to a loss of the proliferative signal and exit from the cell cycle112,113. However, experiments in which the SLC was constitutively expressed indicate that downregulation of the pre-BCR is not required for the cessation of proliferation or for the initiation of Igκ gene rearrangement114. Furthermore, loss of downstream components of the pre-BCR signalling cascade, including B cell linker protein (BLNK; also known as SLP65), Bruton tyrosine kinase (BTK) and phospholipase Cγ2 (PLCγ2), block development at the cycling pre-BCR+ pre-B cell stage115–117. These observations indicate that pre-BCR activated signalling mechanisms eventually function to limit proliferation.
Recent evidence indicates that downstream of the pre-BCR, the RAS–ERK signalling pathway functions to attenuate proliferation. It does so by inducing the developmentally restricted transcription factor Aiolos (an Ikaros family member encoded by Ikzf3)118, which represses transcription of Myc30 and Ccnd3 (REF. 17). The restricted expression of Aiolos provides an attractive molecular explanation of how, in one cell lineage and at a particular stage of development, RAS activation terminates proliferation, whereas at an earlier developmental stage, during which Aiolos is not detectably expressed119, the RAS–ERK pathway enhances proliferation109,120. Importantly, the pre-BCR can also antagonize IL-7-dependent proliferation of pre-B cells by BLNK-mediated inhibition of the PI3K–AKT pathway (see below).
As well as inducing cell cycle arrest, pre-BCR signalling is also likely to promote the survival of small resting pre-B cells that are undergoing immunoglobulin light chain rearrangement. Little is known about how the pre-BCR inhibits cell death, although the pre-TCR promotes survival by suppressing BIM and BH3-interacting domain death agonist (BID)121. It is possible that pre-BCR directed ERK activation targets BIM for degradation45,122. Additionally, DNA double-strand breaks, which arise during immunoglobulin gene recombination, lead to ataxia telangiectasia mutated (ATM)-dependent induction of the serine/threonine protein kinase PIM2, which phosphorylates BAD, targeting it for degradation34.
Following the pre-B cell proliferative burst and the loss of favourable bioenergetics, activation of AMP-activated kinase (AMPK) antagonizes mTORC1 and curtails protein synthesis. In addition to further inhibiting proliferation, this regulatory loop is likely to contribute to light chain recombination because AMPK can directly phosphorylate and activate Rag1 (REF. 123). Consistent with this model, mutation of the gene encoding the AMPK binding partner folliculin-interacting protein 1 (FNIP1) leads to excessive cell growth and a block in differentiation at the large pre-B cell stage124.
Pre-BCR signalling and Igκ recombination
Induction of Igκ gene rearrangements in resting pre-B cells
Cell cycle arrest in pre-B cells is not sufficient to induce Igκ gene recombination29. Rather, Igκ recombination seems to be directly regulated by the pre-BCR. Transgenic Igμ expression increases Igκ locus accessibility in Rag2−/− pro-B cells125,126, whereas targeted mutations of some pre-BCR signalling components can enhance proliferation of pre-B cells while impairing Igκ gene recombination116,127. Furthermore, constitutive expression of the pre-BCR results in extensive light chain recombination114.
In addition to mediating cell cycle exit, the RAS–ERK pathway is a major mediator of pre-BCR-directed Igκ gene recombination17,128,129. RAS–ERK signalling upregulates expression of the transcription factor E2A while repressing expression of its inhibitor ID3 (inhibitor of DNA binding 3)130. This results in a large increase in the level of free E2A available for DNA binding and activation of the Igκ intronic enhancer (Eκi) and the Igκ 3′ enhancer (Eκ3′)17 (FIG. 4). Gene-targeting studies have demonstrated that mutation of an enhancer box (E-box) in the Eκi, iκE2, diminishes Igκ gene rearrangement, and that mutation of iκE1 and iκE2 together affects Igκ gene rearrangement more markedly and in a similar way to the complete deletion of Eκi (REF. 131).
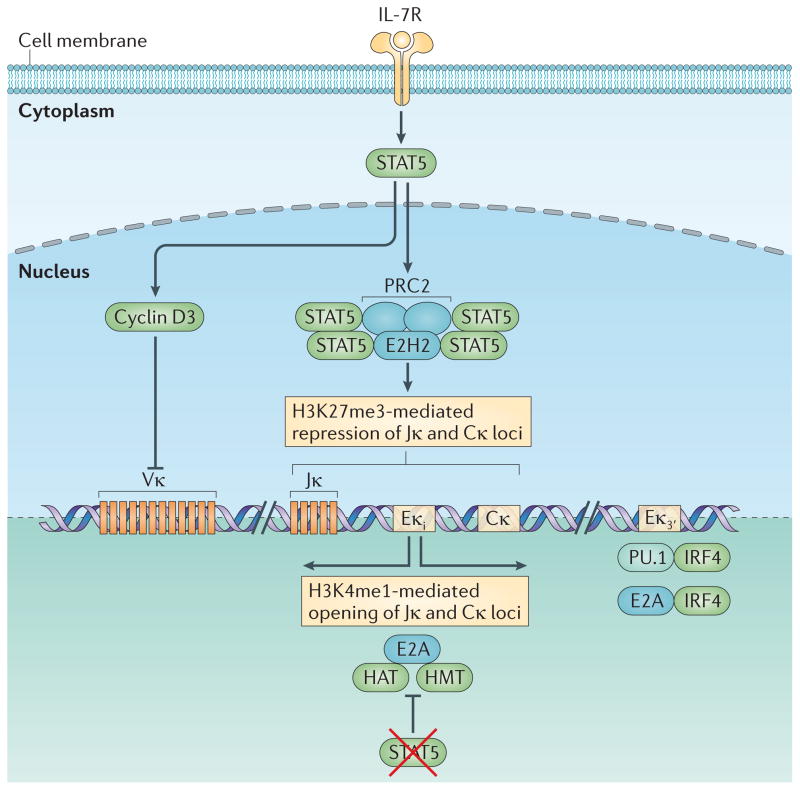
Figure 4. Regulation of Igκ locus accessibility.
Stimulation of the interleukin-7 receptor (IL-7R) induces the activation of signal transducer and activator of transcription 5 (STAT5). Within the Igκ intronic enhancer (Eκi), phosphorylated STAT5 binds as a tetramer, instead of as a dimer; this enables it to recruit Polycomb repressive complex 2 (PRC2), which contains the histone methyltransferase enhancer of zeste homologue 2 (EZH2). EZH2 marks the region, containing Jκ and Cκ, with histone H3 lysine 27 trimethylation (H3K27me3), thereby conferring local epigenetic repression. STAT5 also induces the expression of cyclin D3, which potently represses Vκ accessibility. The mechanism by which it does so is unclear and neither seems to require direct chromatin binding nor involve changes in post-translational histone modifications. Induction of Igκ requires escape from IL-7R signalling, the consequent loss of activated STAT5 and the downregulation of the STAT5 target cyclin D3. However, loss of IL-7R signalling is not sufficient for opening of the Igκ locus. Pre-B cell receptor (pre-BCR)-mediated extracellular signal-regulated kinase (ERK) activation and downstream induction of free nuclear E2A is required. With the loss of phospho-STAT5 binding, E2A binds to the Eκi, where it recruits histone acetyltransferases (HATs) and histone methyltransferases (HMTs) that provide histone marks that lead to the opening of Jκ and Cκ to recombination. E2A also binds to the Eκ3′, where it cooperates with pre-BCR-induced interferon-regulatory factor 4 (IRF4) to further enhance Igκ accessibility. IRF4 also binds in a cooperative manner with the transcription factor PU.1 to a distinct composite regulatory sequence in Eκ3′ to regulate its activation.
These and other studies132–135 implicate E2A as an important regulator of Igκ locus transcription and accessibility to recombination. When bound to the Eκi, E2A recruits the transcriptional co-activators CREB-binding protein (CBP) and p300, which modify nucleosomal histones in the flanking Cκ and Jκ regions with acetyl groups, thereby making the region accessible to the recombination machinery17,136,137. In addition to E2A, the pre-BCR induces expression of the interferon regulatory factor (IRF) family members IRF4 and IRF8, which are required in vivo for initiating Igκ gene recombination, suppressing the SLC and exiting the cell cycle138,139. The transcription factors IRF4 and IFR8 induce localized histone acetylation, Igκ transcription and recombination by binding to the Eκ3′ enhancer, thereby complementing regulation of the Eκi by E2A29. They do so by cooperative binding with the transcription factors PU.1 (also known as SPI1) or SPIB to Ets-IRF composite motifs (EICEs). IRF4 and IRF8 also similarly bind to the Eλ enhancers and are crucial regulators of Igλ transcription and recombination in pre-B cells29,138.
Importance of attenuating IL-7R signalling
Signalling through the pre-BCR, and the induction of E2A, IRF4 and IRF8, is not sufficient to induce Igκ gene recombination or to make the Igκ locus appropriately accessible to recombination. Cells must also attenuate or escape IL-7R signalling29. This is because STAT5 binds directly to the Eκi as a tetramer and recruits Polycomb repressive complex 2 (PRC2)140. Within PRC2, the methyltransferase enhancer of zeste homologue 2 (EZH2) (FIG. 4) modifies nucleosomes at the Eκi, Jκ and Cκ regions with the histone H3 lysine 27 trimethylation (H3K27me3) mark. This modification is proposed to make the regions inaccessible to both E2A binding and to access by the recombination machinery.
The elucidation of STAT5 as a repressor provides a simple bimolecular model of how E2A, downstream of the pre-BCR, and STAT5, downstream of the IL-7R, determine the activity of Eκi and accessibility of Jκ and Cκ to the recombination machinery. Furthermore, it suggests that the Eκi, which is positioned between the Jκ and Cκ regions and in close proximity to both, regulates local accessibility to the recombination machinery.
However, this model cannot be extended to Vκ gene segments because these are situated far from Eκi and are mostly devoid of either histone acetylation or H3K27me3 marks141 (M.M. and M.R.C., unpublished observations). Surprisingly, cyclin D3 is a potent repressor of Vκ transcription24. Cyclin D3 does not bind chromatin and therefore is unlikely to act as a conventional transcriptional repressor. Rather, nuclear matrix-associated cyclin D3 seems to regulate Vκ transcription. This fraction of cyclin D3 is spatially and functionally different from that associated with CDK4 and CDK6. It is not known how nuclear matrix-associated cyclin D3 regulates Vκ transcription. However, these findings raise the intriguing possibility that differential compartmentalization of cyclin D3 within the nucleus enables it to drive cell cycle progression and repress Vκ gene accessibility.
Thus, two key mediators of IL-7R-driven proliferation, namely STAT5 and cyclin D3, coordinately repress the Igκ locus and prevent recombination during pre-B cell division. Each represses a different region of the Igκ locus through apparently very different mechanisms. Furthermore, escape from both repressive mechanisms is required to open the locus to recombination24,140. Through these and other molecular mechanisms described below, cell cycle exit is tightly coupled to recombination in pre-B cells.
Light chain recombination in resting pre-B cells also requires the re-induction of the Rag genes, which, like Igκ locus accessibility, is determined by the diametrically opposed activities of the IL-7R and pre-BCR. Central to this model is the recent observation that the IL-7R is more strongly coupled to the PI3K–AKT pathway than the pre-BCR36. This conclusion is based on experiments in which pre-BCR expression in Rag2−/− pro-B cells did not enhance AKT activation36. This is in contrast to the BCR in mature B cells, which activates PI3K through CD19 and B cell adaptor for phosphoinositide 3-kinase (BCAP; also known as PIK3AP1). CD19 and BCAP are dispensable at the pro- and pre-B cell stages142. It is still possible that in large pre-B cells, when proliferation is maximal, that pre-BCR augments PI3K activation. However, presumably this would have to occur in the presence of low levels of the adaptor protein BLNK (see below)36, would not depend on CD19 or BCAP142 and would have to diminish on transition to the small pre-B cell stage. Alternatively, another unknown receptor could transiently activate PI3K in large pre-B cells.
As described above, PI3K–AKT signalling promotes nuclear export and degradation of FOXO transcription factors, thereby preventing Rag induction73,74. PI3K–AKT signalling also lowers the level of PAX5, which contributes to Rag gene activation and Igκ gene recombination36. Therefore, through STAT5-mediated Igκ repression and PI3K–AKT-mediated downregulation of FOXO and PAX5, IL-7R signalling effectively represses Rag gene induction and Igκ gene recombination in cycling pre-B cells.
Orchestrating the pre-B cell checkpoint
To avoid juxtaposing proliferation and Igκ gene recombination, a potentially unstable and dangerous developmental scenario, pre-B cells use a series of feedforward and feedback loops that seem to reinforce either IL-7R or pre-BCR directed cell fate dynamics36 (FIG. 5). In pre-B cells that have just acquired the pre-BCR as a consequence of productive immunoglobulin light chain rearrangements, both the proximal tyrosine kinase (spleen tyrosine kinase (SYK)) and the linker molecule BLNK are expressed at low levels. In this IL-7R activated celullar state, the pre-BCR cannot efficiently couple with the downstream signalling components that are needed for differentiation. On attenuation of IL-7 signalling in these cells, FOXO1 is induced and binds directly to the SYK and BLNK promoters inducing their activities. This imparts a functional SYK–BLNK module to the pre-BCR in small pre-B cells and promotes Igκ gene recombination through several mechanisms, including the induction of IRF4 and IRF8 and the activation of p38 mitogen-activated protein kinase38. p38 phosphorylates and enhances the transcriptional activity of the FOXO proteins. BLNK also feeds back and antagonizes the PI3K–AKT pathway73, thereby further upregulating FOXO and reinforcing the switch from proliferation to recombination36. This intrinsic network of counter-regulatory loops seems to be designed to reinforce the handing off of active signalling between the two receptors. This ensures that crucial cell fate decisions are dictated by a single receptor system at discrete junctures in the developmental checkpoint.
Figure 5. Regulatory network orchestrating the pre-B cell developmental checkpoint.
There are a series of feedforward and feedback regulatory loops between the interleukin-7 receptor (IL-7R) and the pre-B cell receptor (pre-BCR) that ensure dominance of one receptor at a given time. Downstream of the IL-7R, activation of phosphoinositide 3-kinase (PI3K) destabilizes both forkhead box protein O1 (FOXO1) and paired box 5 (PAX5). In addition to regulating recombination-activating gene 1 (RAG1) and RAG2 expression, both transcription factors are needed for optimal expression of spleen tyrosine kinase (SYK) and B cell linker protein (BLNK), a central signalling module of the pre-BCR. FOXO1 and PAX5 are also necessary for the induction of interferon-regulatory factor 4 (IRF4) expression through SYK–BLNK signalling. Thus, in the presence of IL-7R signalling, the pre-BCR cannot fully couple to important downstream signalling targets that are necessary for the induction of Igκ gene recombination. After a pre-B cell has attenuated or escaped IL-7R signalling thereby enabling efficient coupling of the SYK–BLNK module to the pre-BCR, regulatory loops are engaged that further repress the IL-7R and reinforce the mechanisms of recombination. The SYK–BLNK module feeds back to repress PI3K and AKT activation, and BLNK induces activation of the mitogen-activated protein kinase p38, which phosphorylates and augments FOXO1 activity. Activated FOXO1 further enhances signalling through the pre-BCR, the induction of RAG1 and RAG2 expression and commitment to Igκ gene recombination. FOXO1 also feeds back to repress IL-7Rα expression. The thick black arrows represent primary pre-BCR signalling cascades, whereas the thin black arrows denote feedforward loops. The thick red arrows represent the negative regulatory pathways through which the pre-BCR inhibits IL-7R signalling.
Contribution of distinct bone marrow niches
It is likely that the positioning of pre-B cells in relation to IL-7-expressing stromal cells within the bone marrow reinforces the switch from a fate that is directed by the IL-7R to one that is determined by the pre-BCR28,36. The bone marrow contains distinct IL-7hi and IL-7low niches143. Downstream of the pre-BCR, IRF4 induces the expression of CXC-chemokine receptor 4 (CXCR4), which confers responsiveness to CXC-chemokine ligand 12 (CXCL12)29. Notably, CXCL12-expressing stromal cells are situated away from those expressing IL-7. CXCR4 signalling, and the downstream activation of focal adhesion kinase144, might therefore facilitate the movement of pre-B cells into IL-7low niches and the attenuation of IL-7R signalling (FIG. 6).
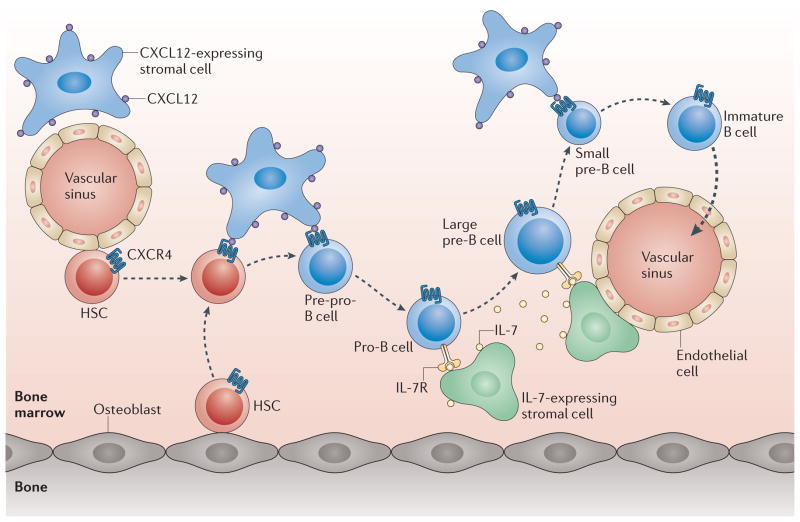
Figure 6. Movement of B cell progenitors through successive bone marrow niches.
Haematopoietic progenitor cells (HSCs) are located near to osteoblasts, endothelial cells and CXC-chemokine ligand 12 (CXCL12)-expressing stromal cells. Pre-pro-B cells with rearranged diversity (D)-joining (J) segments are proposed to reside near CXCL12-expressing cells, whereas pro-B cells are positioned beside interleukin-7 (IL-7)-expressing stromal cells. After successful variable (V)–(D)J recombination, pre-B cells express the pre-B cell receptor (pre-BCR) and proliferate in IL-7-enriched bone marrow niches (these cells are large pre-B cells). Subsequently, pre-B cells upregulate CXC-chemokine receptor 4 (CXCR4) in response to interferon-regulatory factor 4 (IRF4), which is induced by pre-BCR signalling, and migrate to CXCL12-expressing niches that are likely to be distinct from those that support HSCs. This movement might further attenuate IL-7 signalling and also ensure exit from the cell cycle, thereby enabling the small pre-B cells to efficiently induce immunoglobulin light chain recombination. Following successful immunoglobulin light chain recombination, immature B cells expressing IgM downregulate CXCR4 and exit the bone marrow.
Two signalling states for the pre-BCR
The above model defines two signalling states for the pre-BCR36 that differ in their coupling to the SYK–BLNK module. This could help to explain why pre-BCR expression is associated with an initial proliferative burst before cell cycle exit and Igκ gene recombination. However, the molecular basis of this proliferative burst remains enigmatic. It has been postulated that synergistic activation of one or more signalling pathways by the pre-BCR and IL-7R results in enhanced proliferation145. As noted above, it remains possible that in the presence of low levels of BLNK, pre-BCR signalling augments IL-7R induced PI3K activity and the proliferation of pre-B cells. Alternatively, other receptors the expression of which is induced during pre-B cell differentiation could contribute to the proliferative burst146.
Immature B cell selection and receptor editing
Successful Igκ rearrangement, and expression of an antigen-specific BCR, is necessary for selection into the immature B cell pool. In contrast to the pre-BCR, the BCR is strongly coupled to the PI3K signalling pathway, and this coupling is required for B cell selection, repression of Rag expression and allelic exclusion147. In the immature B cell compartment, cell fate decisions are determined by the antigenic specificities of the naive BCR repertoire and the need to purge it of autoreactivity. The mechanism by which this is accomplished is ingenious. Recognition of self-antigens leads to a down-modulation of BCR surface expression and to a loss of basal PI3K signalling148,149. Consequently, Rag expression is de-repressed and cells acquire some characteristics of pre-B cells. This allows cells to resume Igκ gene recombination using distal Vκ segments, followed by Igλ gene recombination. These light chain gene regions are rich in segments that encode light chain editors, which can neutralize heavy chain autoreactivity150–152. Successful editing of BCR specificity, with a loss of autoreactivity, allows surface expression and selection back into the immature B cell pool.
Differential coupling to PI3K enables the pre-BCR and BCR to direct very different cellular programmes. By substantially uncoupling from PI3K, the pre-BCR is able to initiate a signalling programme that both enhances Igκ gene accessibility and allows the induction of Rag genes. By contrast, activation of PI3K by the resting BCR on immature B cells mediates subsequent selection and the repression of further light chain recombination. Conversely, the absence of pre-BCR or BCR expression results in different cell fates that are influenced by the context in which they occur. In pro-B cells, the absence of pre-BCR expression enables IL-7-directed proliferation. By contrast, loss of BCR expression in immature B cells results in Rag expression without concurrent induction of cell cycle genes148,149. Thus, lack of pre-BCR expression results in proliferation, whereas loss of BCR expression results in immunoglobulin light chain recombination.
Concluding remarks
A clear picture is emerging of the molecular processes that drive cell fate decisions during B cell lymphopoiesis. Central to this is the assigning of specific functions and signalling programmes to the IL-7R and the pre-BCR (FIG. 1). The primary function of the IL-7R is to maintain and expand early B cell progenitor populations. It does so by activating signalling pathways that contribute crucial, non-redundant and complementary molecular functions. Activated STAT5 induces expression of anti-apoptotic factors and cyclin D3, which mediates transit through the cell cycle. Complementary signalling through the PI3K–AKT pathway represses apoptotic factors and provides the growth and energetic states that are necessary for proliferation and survival. Just as importantly, these same signalling cascades prevent Igκ gene recombination (FIG. 3) by repressing the Igκ locus and by preventing FOXO-induced Rag expression. Through these mechanisms the IL-7R potently prevents differentiation of pre-B cells.
The functions of the IL-7R are diametrically opposed by those of the pre-BCR, and these differences are reflected in the signalling pathways that the pre-BCR modulates. It activates the RAS–ERK pathway, and other signalling modules that induce the expression of Aiolos, E2A, IRF4 and IRF8. These transcription factors induce cell cycle exit and Igκ gene recombination. Furthermore, the pre-BCR antagonizes IL-7R-activated PI3K–AKT activity via the adaptor protein BLNK73. In so doing it attenuates IL-7R signalling, which is required for the induction of Rag genes and the differentiation of pre-B cells.
Because of the opposing functions of the IL-7R and the pre-BCR, and the fundamental incompatibility of DNA replication and recombination, simultaneous signalling through each receptor would risk genomic instability. However, there is a series of feedforward and feedback loops between the signalling cascades of the IL-7R and the pre-BCR (FIG. 4) that ensures the dominance of one receptor at any one time. These mechanisms reveal the regulatory logic of the pre-B cell developmental checkpoint. They begin to provide a coherent molecular framework for how cell fate decisions are directed by one receptor (the IL-7R) and then the other (the pre-BCR) and how proliferation of pre-B cells is tightly coupled to immunoglobulin light chain gene recombination and their differentiation into immature B cells.
Glossary
- Flippase
Transporter protein that flips phospholipids from the outer membrane leaflet to the cytosolic leaflet of plasma and endosomal membranes
- Recombination-activating gene 1
(RAG1). RAG1 and RAG2 encode proteins that are involved in creating the DNA double-strand breaks that are necessary for producing the rearranged gene segments that encode the complete protein chains of T cell and B cell receptors
- Autophagy
The catabolic process in which the cell degrades its own components through the lysosomal pathway
- Recombination machinery
The molecular components that mediate immunoglobulin gene recombination. They include lymphoid-specific proteins, such as the recombination-activating gene (RAG) proteins and terminal deoxynucleotidyl transferase (TdT), and non-lymphoid restricted proteins that are involved in non-homologous DNA end-joining, including the DNA-dependent protein kinase subunits Ku70 (also known as XRCC6), Ku80 (also known as XRCC5) and DNA-PKcs (DNA-dependent protein kinase catalytic subunit), as well as Artemis and DNA ligase 4
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Schlissel MS. Regulating antigen-receptor gene assembly. Nature Rev Immunol. 2003;3:890–899. doi: 10.1038/nri1225. [DOI] [PubMed] [Google Scholar]
- 2.Clark MR, Cooper AB, Wang L, Aifantis I. The pre-B cell receptor in B cell development: recent advances, persistent questions and conserved mechanisms. Curr Top Microbiol Immunol. 2005;290:87–104. doi: 10.1007/3-540-26363-2_5. [DOI] [PubMed] [Google Scholar]
- 3.Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signaling. Nature Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Reynolds TL, Shan S, Desiderio S. Coupling of V(D)J recombination to cell cycle suppresses genomic instability and lymphoid tumorigenesis. Immunity. 2011;34:163–174. doi: 10.1016/j.immuni.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corfe SA, Paige CJ. The many roles of IL-7 in B cell development; Mediator of survival, proliferation and differentiation. Semin Immunol. 2012;24:198–208. doi: 10.1016/j.smim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Peschon JJ, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. This study demonstrates the importance of IL-7 signalling in murine B lymphopoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen CT, et al. FLT3 ligand and not TSLP is the key regulator of IL-7-independent B-1 and B-2 B lymphopoiesis. Blood. 2008;112:2297–2304. doi: 10.1182/blood-2008-04-150508. [DOI] [PubMed] [Google Scholar]
- 8.Clark MR. Flippin’ lipids. Nature Immunol. 2011;12:373–375. doi: 10.1038/ni.2024. [DOI] [PubMed] [Google Scholar]
- 9.Siggs OM, et al. The P4 ATPase ATP11c is essential for B lymphopoiesis in adult bone marrow. Nature Immunol. 2011;12:434–440. doi: 10.1038/ni.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yabas M, et al. ATP11c is critical for phosphatidylserine internalization and B lymphocyte differentiation. Nature Immunol. 2011;12:441–449. doi: 10.1038/ni.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T−B+ NK+ severe combined immunodeficiency. Nature Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 12.Giliani S, et al. Interleukin-7 receptor α (IL-7Rα) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol Rev. 2005;203:110–126. doi: 10.1111/j.0105-2896.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 13.O’Shea JJ, Plenge RM. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou WC, Levy DE, Lee CK. STAT3 positively regulates an early step in B-cell development. Blood. 2006;108:3005–3011. doi: 10.1182/blood-2006-05-024430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Z, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA. 2006;103:1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goetz CA, Harmon IR, O’Neil JJ, Burchill MA, Farrar MA. STAT5 activation underlies IL7 receptor-dependent B cell development. J Immunol. 2004;172:4770–4778. doi: 10.4049/jimmunol.172.8.4770. [DOI] [PubMed] [Google Scholar]
- 17.Mandal M, et al. Ras orchestrates cell cycle exit and light chain recombination during early B cell development. Nature Immunol. 2009;10:1110–1117. doi: 10.1038/ni.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson SE, Shah N, Panoskaltsis-Mortari A, LeBien TW. Murine and human IL-7 activate STAT5 and induce proliferation of normal human pro-B cells. J Immunol. 2005;175:7325–7331. doi: 10.4049/jimmunol.175.11.7325. [DOI] [PubMed] [Google Scholar]
- 19.Cooper AB, et al. A unique function for cyclin D3 in early B cell development. Nature Immunol. 2006;7:489–497. doi: 10.1038/ni1324. [DOI] [PubMed] [Google Scholar]
- 20.Sherr CJ, Roberts J. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 21.Ciemerych MA, Sicinski P. Cell cycle in mouse development. Oncogene. 2005;24:2877–2898. doi: 10.1038/sj.onc.1208608. [DOI] [PubMed] [Google Scholar]
- 22.Kozar K, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. This study shows that the cyclin D proteins are primarily needed for haematopoiesis and are dispensable for the proliferation of most other non-haematopoietic cell lineages. [DOI] [PubMed] [Google Scholar]
- 23.Malumbres M, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Powers SE, et al. Subnuclear cyclin D3 compartments and the coordinated regulation of proliferation and immunoglobulin variable gene repression. J Exp Med. 2012;209:2199–2213. doi: 10.1084/jem.20120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sicinska E, et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 26.Peled JU, et al. Requirement for cyclin D3 in germinal center formation and function. Cell Res. 2010;20:631–646. doi: 10.1038/cr.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam EW, et al. Cyclin D3 compensates for loss of cyclin D2 in mouse B-lymphocytes activated via the antigen receptor and CD40. J Biol Chem. 2000;275:3479–3484. doi: 10.1074/jbc.275.5.3479. [DOI] [PubMed] [Google Scholar]
- 28.Solvason N, et al. Cyclin D2 is essential for BCR-mediated proliferation and CD5 B cell development. Int Immunol. 2000;12:631–638. doi: 10.1093/intimm/12.5.631. [DOI] [PubMed] [Google Scholar]
- 29.Johnson K, et al. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity. 2008;28:335–345. doi: 10.1016/j.immuni.2007.12.019. This paper elaborates a molecular framework for coupling the attenuation of IL-7 signalling with the expression of IRF4 in promoting pre-B cell differentiation. [DOI] [PubMed] [Google Scholar]
- 30.Ma S, et al. Ikaros and Aiolos inhibit pre-B cell proliferation by directly suppressing c-Myc expression. Mol Cell Biol. 2010;30:4149–4158. doi: 10.1128/MCB.00224-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakayama K, et al. Mice lacking p27kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 32.Malin S, et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nature Immunol. 2010;11:171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Q, et al. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24:6501–6513. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bednarski JJ, et al. RAG-induced DNA double-strand breaks signal through Pim2 to promote pre-B cell survival and limit proliferation. J Exp Med. 2011;209:11–17. doi: 10.1084/jem.20112078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milne CD, Paige CJ. IL-7: a key regulator of B lymphopoiesis. Semin Immunol. 2006;18:20–30. doi: 10.1016/j.smim.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Ochiai K, et al. A self-reinforcing regulatory network triggered by limiting IL-7 activates pre-BCR signaling and differentiation. Nature Immunol. 2012;13:300–307. doi: 10.1038/ni.2210. This study describes the assembly of a gene regulatory network that orchestrates the pre-B cell developmental checkpoint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corcoran AE, et al. The interleukin-7 receptor α chain transmits distinct signals for proliferation and differentiation during B lymphopoiesis. EMBO J. 1996;15:1924–1932. [PMC free article] [PubMed] [Google Scholar]
- 38.Ramadani F, et al. The PI3K isoforms p110α and p110δ are essential for pre-B cell receptor signaling and B cell development. Sci Signal. 2010;3:ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fruman DA, et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki H, et al. Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 41.Clayton E, et al. A crucial role for the p110δ subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196:753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jou ST, et al. Essential, nonredundant role of the phosphoinositide 3-kinase p110δ in signaling by the B-cell receptor complex. Mol Cell Biol. 2002;22:8580–8591. doi: 10.1128/MCB.22.24.8580-8591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 44.Huntington ND, et al. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim sustains B lymphopoiesis in the absence of IL-7. Int Immunol. 2009;21:715–725. doi: 10.1093/intimm/dxp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruse EA, et al. MEK/ERK-mediated phosphorylation of Bim is required to ensure survival of T and B lymphocytes during mitogenic stimulation. J Immunol. 2009;183:261–269. doi: 10.4049/jimmunol.0803853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 47.Essafi A, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–2329. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 48.Castellano E, Downward J. RAS interaction with PI3K: more than just another effector pathway. Genes Cancer. 2011;2:261–274. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- 50.Medina KL, et al. Assembling a gene regulatory network for specification of the B cell fate. Dev Cell. 2004;7:607–617. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Pongubala JM, et al. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nature Immunol. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]
- 52.Singh H, Medina KL, Pongubala MR. Contigent gene regulatory networks and B cell fate specification. Proc Natl Acad Sci USA. 2005;102:4949–4953. doi: 10.1073/pnas.0500480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Gyory I, et al. Transcription factor Ebf1 regulates differentiation stage-specific signaling, proliferation, and survival of B cells. Genes Dev. 2012;26:668–682. doi: 10.1101/gad.187328.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dengler HS, et al. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nature Immunol. 2008;12:1388–1398. doi: 10.1038/ni.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fahlman C, Blomhoff HK, Veiby OP, McNiece IK, Jacobsen SE. Stem cell factor and interleukin-7 synergize to enhance early myelopoiesis in vitro. Blood. 1994;84:1450–1456. [PubMed] [Google Scholar]
- 57.Fahl SP, Crittenden RB, Allman D, Bender TP. c-Myb is required for pro-B cell differentiation. J Immunol. 2009;183:5582–5592. doi: 10.4049/jimmunol.0901187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kosan C, et al. Transcription factor miz-1 is required to regulate interleukin-7 receptor signaling at early commitment stages of B cell differentiation. Immunity. 2010;33:917–928. doi: 10.1016/j.immuni.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 59.Hardin JD, et al. Bone marrow B lymphocyte development in c-abl-deficient mice. Cell Immunol. 1995;165:44–54. doi: 10.1006/cimm.1995.1185. [DOI] [PubMed] [Google Scholar]
- 60.Schwartzberg PL, et al. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell. 1991;65:1165–1175. doi: 10.1016/0092-8674(91)90012-n. [DOI] [PubMed] [Google Scholar]
- 61.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-Abl proto-oncogene. Cell. 1991;65:1153–1164. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 62.Brightbill H, Schlissel MS. The effects of c-Abl mutation on developing B cell differentiation and survival. Int Immunol. 2009;21:575–585. doi: 10.1093/intimm/dxp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lam QL, et al. Impaired V(D)J recombination and increased apoptosis among B cell precursors in the bone marrow of c-Abl-deficient mice. Int Immunol. 2007;19:267–276. doi: 10.1093/intimm/dxl143. [DOI] [PubMed] [Google Scholar]
- 64.Gu JJ, Zhang N, He YW, Koleske AJ, Pendergast AM. Defective T cell development and function in the absence of Abelson kinases. J Immunol. 2007;179:7334–7344. doi: 10.4049/jimmunol.179.11.7334. [DOI] [PubMed] [Google Scholar]
- 65.Zipfel PA, et al. The c-Abl tyrosine kinase is regulated downstream of the B cell antigen receptor and interacts with CD19. J Immunol. 2000;165:6872–6881. doi: 10.4049/jimmunol.165.12.6872. [DOI] [PubMed] [Google Scholar]
- 66.Roose JP, et al. T cell receptor-independent basal signaling via Erk and Abl kinases suppresses RAG gene expression. PLoS Biol. 2003;1:E53. doi: 10.1371/journal.pbio.0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kharbanda S, et al. Activation of the c-Abl tyrosine kinase in the stress response to DMA-damaging agents. Nature. 2002;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 68.Kondo M, Akashi K, Domen J, Sugamura K, Weissman IL. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common γ chain-deficient mice. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 69.Roessler S, et al. Distinct promoters mediate the regulation of Ebf1 gene expression by interleukin-7 and Pax5. Mol Cell Biol. 2007;27:579–594. doi: 10.1128/MCB.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dias S, Mansson R, Gurbuxani S, Sigvardsson M, Kee BL. E2A proteins promote development of lymphoid-primed multipotent progenitors. Immunity. 2005;29:217–227. doi: 10.1016/j.immuni.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nature Rev Immunol. 2008;8:9–21. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herzog S, et al. SLP-65 regulates immunoglobulin light chain gene recombination through the PI(3)K-PKB-Foxo pathway. Nature Immunol. 2008;9:623–631. doi: 10.1038/ni.1616. [DOI] [PubMed] [Google Scholar]
- 74.Amin RH, Schlissel MS. Foxo1 directly regulates the transcription of recombination-activating genes during B cell development. Nature Immunol. 2008;9:613–622. doi: 10.1038/ni.1612. References 73 and 74 demonstrate the role of PI3K and the FOXO transcription factors in regulating Rag gene expression during B lymphopoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J, Desiderio S. Cyclin A/CDK2 regulates V(D)J recombination by coordinating RAG-2 accumulation and DNA repair. Immunity. 1999;11:771–781. doi: 10.1016/s1074-7613(00)80151-x. [DOI] [PubMed] [Google Scholar]
- 76.Li Z, Dordai DI, Lee J, Desiderio S. A conserved degradation signal regulates RAG-2 accumulation during cell division and links V(D)J recombination to the cell cycle. Immunity. 1996;5:575–589. doi: 10.1016/s1074-7613(00)80272-1. [DOI] [PubMed] [Google Scholar]
- 77.Jiang H, et al. Ubiquitylation of RAG-2 by Skp2-SCF links destruction of the V(D)J recombinase to the cell cycle. Mol Cell. 2005;18:699–709. doi: 10.1016/j.molcel.2005.05.011. This study demonstrates that the mechanisms that regulate cell cycle progression also regulate the stability of Rag2. [DOI] [PubMed] [Google Scholar]
- 78.Johnson K, et al. IL-7 functionally segregates the pro-B cell stage by regulating transcription of recombination mediators across cell cycle. J Immunol. 2012;188:6084–6092. doi: 10.4049/jimmunol.1200368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reynaud D, et al. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nature Immunol. 2008;9:927–936. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alkhatib A, et al. FoxO1 induces Ikaros splicing to promote immunoglobulin gene recombination. J Exp Med. 2012;209:395–406. doi: 10.1084/jem.20110216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertolino E, et al. Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nature Immunol. 2005;6:836–843. doi: 10.1038/ni1226. [DOI] [PubMed] [Google Scholar]
- 82.Kitamura D, Roes J, Kuhn R, Rajewsky KA. B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. This paper demonstrates the importance of the pre-BCR in B lymphopoiesis. [DOI] [PubMed] [Google Scholar]
- 83.Shimizu T, Mundt C, Licence S, Melchers F, Martensson I. VpreB1/VpreB2/λ5 triple-deficient mice show impaired B cell development but functional allelic exclusion of the IgH locus. J Immunol. 2002;168:6286–6293. doi: 10.4049/jimmunol.168.12.6286. [DOI] [PubMed] [Google Scholar]
- 84.Pelanda R, Braun U, Hobeika E, Nussenzweig MC, Reth M. B cell progenitors are arrested in maturation but have intact V(D)J recombination in the absence of Ig-α and Ig-β. J Immunol. 2002;169:865–872. doi: 10.4049/jimmunol.169.2.865. [DOI] [PubMed] [Google Scholar]
- 85.Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Ig-β. Science. 1996;272:411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 86.Meixlsperger S, et al. Conventional light chains inhibit the autonomous signaling capacity of the B cell receptor. Immunity. 2007;26:323–333. doi: 10.1016/j.immuni.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 87.Ohnishi K, Melchers F. The nonimmunoglobulin portion of λ5 mediates cell-auttonomous pre-B cell receptor signaling. Nature Immunol. 2003;4:849–856. doi: 10.1038/ni959. [DOI] [PubMed] [Google Scholar]
- 88.Bankovich AJ, et al. Structural insight into pre-B cell receptor function. Science. 2007;316:291–294. doi: 10.1126/science.1139412. [DOI] [PubMed] [Google Scholar]
- 89.Vettermann C, et al. A unique role for the lambda5 non-immunoglobulin tail in early B lymphocyte development. J Immunol. 2008;181:3232–3242. doi: 10.4049/jimmunol.181.5.3232. [DOI] [PubMed] [Google Scholar]
- 90.Kohler F, et al. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity. 2008;29:912–921. doi: 10.1016/j.immuni.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 91.Bradl H, Wittmann J, Milius D, Vettermann C, Jack HM. Interaction of murine precursor B cell receptor with stroma cells is controlled by the unique tail of lambda 5 and stroma cell-associated heparan sulfate. J Immunol. 2003;171:2338–2348. doi: 10.4049/jimmunol.171.5.2338. [DOI] [PubMed] [Google Scholar]
- 92.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci USA. 2002;99:13014–13019. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Espeli M, Mancini SJC, Breton C, Poirier F, Schiff C. Impaired B-cell development at the pre-BII-cell stage in galectin-1-deficient mice due to inefficient pre-BII/stromal cell interactions. Blood. 2009;113:5878–5886. doi: 10.1182/blood-2009-01-198465. [DOI] [PubMed] [Google Scholar]
- 94.Mourcin F, et al. Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood. 2011;117:6552–6561. doi: 10.1182/blood-2010-12-323113. [DOI] [PubMed] [Google Scholar]
- 95.Keenan RA, et al. Censoring of autoreactive B cell development by the pre-B cell receptor. Science. 2008;321:696–699. doi: 10.1126/science.1157533. [DOI] [PubMed] [Google Scholar]
- 96.Eschbach C, et al. Efficient generation of B lymphocytes by recognition of self-antigens. Eur J Immunol. 2011;41:2397–2403. doi: 10.1002/eji.201041344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doughty CA, et al. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107:4458–4465. doi: 10.1182/blood-2005-12-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ward PS, Thompson CB. Signaling in control of cell growth and metabolism. Cold Spring Harb Perspect Biol. 2012;4:a006783. doi: 10.1101/cshperspect.a006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lazorchak AS, et al. Sin1-mTORC2 suppresses rag and Il7r gene expression through Akt2 in B cells. Mol Cell. 2010;39:433–443. doi: 10.1016/j.molcel.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rathmell JC, et al. Akt-directed glucose matabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gottlob K, et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Powell DJ, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iritani BM, Eisenman RN. c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc Natl Acad Sci, USA. 1999;1999:13180–13185. doi: 10.1073/pnas.96.23.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grumont RJ, Strasser A, Gerondakis S. B cell growth is controlled by phosphatidylinosotol 3-kinase-dependent induction of Rel/NF-κB regulated c-myc transcription. Mol Cell. 2002;10:1283–1294. doi: 10.1016/s1097-2765(02)00779-7. [DOI] [PubMed] [Google Scholar]
- 106.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao C, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nature Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sandoval GJ, et al. Cutting edge: cell-autonomous control of IL-7 response revealed in a novel stage of precursor B cells. J Immunol. 2013;190:2485–2489. doi: 10.4049/jimmunol.1203208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yasuda T, et al. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28:499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 110.Rolink A, Winkler T, Melchers F, Andersson J. Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. J Exp Med. 2000;191:23–31. doi: 10.1084/jem.191.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parker MJ, et al. The pre-B-cell receptor induces silencing of VpreB and λ5 transcription. EMBO J. 2005;24:3895–3905. doi: 10.1038/sj.emboj.7600850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Melchers F. The pre-B-cell receptor: selector of fitting immunoglobulin heavy chains for the B-cell repertoire. Nature Rev Immunol. 2005;5:578–584. doi: 10.1038/nri1649. [DOI] [PubMed] [Google Scholar]
- 113.Melchers F, et al. Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells. Immunol Rev. 2000;175:33–46. [PubMed] [Google Scholar]
- 114.van Loo PF, Dingjan GM, Maas A, Hendriks RW. Surrogate-light-chain silencing is not critical for the limitation of pre-B cell expansion but is for the termination of constitutive signaling. Immunity. 2007;27:1–13. doi: 10.1016/j.immuni.2007.07.018. This study demonstrates that downregulation of pre-BCR expression is not necessary for pre-B cell cycle exit or pre-B cell development. [DOI] [PubMed] [Google Scholar]
- 115.Jumaa H, et al. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity. 1999;11:547–554. doi: 10.1016/s1074-7613(00)80130-2. [DOI] [PubMed] [Google Scholar]
- 116.Xu S, Lee KG, Huo J, Kurosaki T, Lam KP. Combined deficiencies in Bruton tyrosine kinase and phospholipase Cγ2 arrest B-cell development at a pre-BCR+ stage. Blood. 2007;109:3377–3384. doi: 10.1182/blood-2006-07-036418. [DOI] [PubMed] [Google Scholar]
- 117.Bai L, et al. Phospholipase Cγ2 contributes to light-chain gene activation and receptor editing. Mol Cell Biol. 2007;27:5957–5967. doi: 10.1128/MCB.02273-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thompson EC, et al. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity. 2007;26:335–344. doi: 10.1016/j.immuni.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 119.Heng TS, Painter MW, Consortium IGP. The Immunological Genome Project: networks of gene expression in immune cells. Nature Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 120.Iritani BM, Forbush KA, Farrar MA, Perlmutter RM. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 1997;16:7019–7031. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mandal M, et al. Regulation of lymphocyte progenitor survival by the proapoptotic activities of Bim and Bid. Proc Natl Acad Sci USA. 2008;105:20840–20845. doi: 10.1073/pnas.0807557106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 123.Um JH, et al. Metabolic sensor AMPK directly phosphorylates RAG1 protein and regulates V(D)J recombination. Proc Natl Acad Sci USA. 2013;110:9873–9878. doi: 10.1073/pnas.1307928110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park H, et al. Disruption of Fnip1 reveals a metabolic checkpoint controlling B lymphocyte development. Immunity. 2012;36:1–13. doi: 10.1016/j.immuni.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stanhope-Baker P, Hudson K, Shaffer AL, Constantinescu A, Schlissel M. Cell type-specific chromatin structure determines the targeting of V(D)J recombinase activity in vitro. Cell. 1996;85:887–897. doi: 10.1016/s0092-8674(00)81272-6. [DOI] [PubMed] [Google Scholar]
- 126.Young F, et al. Influnence of immunoglobulin heavy-and light-chain expression on B-cell differentation. Genes Dev. 1994;8:1043–1057. doi: 10.1101/gad.8.9.1043. [DOI] [PubMed] [Google Scholar]
- 127.Flemming A, Brummer T, Reth M, Jumaa H. The adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nature Immunol. 2003;4:38–43. doi: 10.1038/ni862. [DOI] [PubMed] [Google Scholar]
- 128.Shaw AC, Swat W, Ferrini R, Davidson L, Alt FW. Activated Ras signals developmental progression of recombinase-activating gene (RAG)-deficient pro-B lymphocytes. J Exp Med. 1999;189:123–129. doi: 10.1084/jem.189.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shaw AC, Swat W, Davidson L, Alt FW. Induction of Ig light chain gene rearrangement in heavy chain-deficient B cells by activated Ras. Proc Natl Acad Sci USA. 1999;96:2239–2243. doi: 10.1073/pnas.96.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kee BL, Quong MW, Murre C. E2A proteins: essential regulators at multiple stages of B-cell development. Immunol Rev. 2000;175:138–149. [PubMed] [Google Scholar]
- 131.Inlay MA, Tian H, Lin T, Xu Y. Important roles for E protein binding sites within the immunoglobulin k chain intronic enhance in avtivating V-κJ-κ rearrangement. J Exp Med. 2004;200:1205–1211. doi: 10.1084/jem.20041135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ronanow WJ, et al. E2A and EBF act in synergy with the V(D)J recombinase to generate a diverse immunoglobulin repertoire in nonlymphoid cells. Mol Cell. 2000;5:215–223. doi: 10.1016/s1097-2765(00)80429-3. [DOI] [PubMed] [Google Scholar]
- 133.Lazorchak A, Jones ME, Zhuang Y. New insights into E-protein function in lymphocyte development. Trends Immunol. 2005;26:334–338. doi: 10.1016/j.it.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 134.Lazorchak AS, Schlissel MS, Zhuang Y. E2A and IRF-4/Pip promote chromatin modification and transcription of the immunoglobulin κ locus in pre-B cells. Mol Cell Biol. 2006;26:810–821. doi: 10.1128/MCB.26.3.810-821.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schlissel MS. Regulation of activation and recombination of the murine Igκ locus. Immunol Rev. 2004;200:215–223. doi: 10.1111/j.0105-2896.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 136.Beck K, Peak MM, Ota T, Nemazee D, Murre C. Distinct roles for E12 and E47 in B cell specification and the sequential rearrangement of immunoglobulin light chain loci. J Exp Med. 2009;206:2271–2284. doi: 10.1084/jem.20090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sakamoto S, et al. E2A and CBP/p300 act in synergy to promote chromatin accessibility of the immunoglobulin κ locus. J Immunol. 2012;188:5547–5560. doi: 10.4049/jimmunol.1002346. [DOI] [PubMed] [Google Scholar]
- 138.Lu R, Kay L, Lancki DW, Singh H. IRF-4,8 orchestrate the pre-B to B transition in lymphocyte development. Genes Dev. 2003;17:1703–1708. doi: 10.1101/gad.1104803. This study establishes the requirement of the transcription factors IRF4 and IRF8 at the pre-B cell stage. It describes an in vitro culture system that facilitates the molecular analysis of pre-B cell differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma S, Pathak S, Trinh L, Lu R. Interferon regulatory factors 7 and 8 induce the expression of Ikaros and Aiolos to down-regulate pre-B cell receptor and promote cell-cycle withdrawal in pre-B cell development. Blood. 2008;111:1396–1403. doi: 10.1182/blood-2007-08-110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mandal M, et al. Epigenetic repression of the Ig-κ locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nature Immunol. 2011;12:1212–1220. doi: 10.1038/ni.2136. These authors demonstrate that STAT5 can function as an important transcriptional repressor in B lymphophoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu CR, Feeney AJ. The epigenetic profile of Ig genes is dynamically regulated during B cell differentiation and is modulated by pre-B cell receptor signaling. J Immunol. 2009;182:1362–1369. doi: 10.4049/jimmunol.182.3.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- 143.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:335–344. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 144.Park SY, et al. Focal adhesion kinase regulates the localization and retention of pro-B cells in bone marrow microenvironments. J Immunol. 2013;190:1094–1102. doi: 10.4049/jimmunol.1202639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fleming HE, Paige CJ. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity. 2001;15:521–531. doi: 10.1016/s1074-7613(01)00216-3. [DOI] [PubMed] [Google Scholar]
- 146.Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Pre-B cell receptor expression is necessary for thymic stromal lymphopoietin responsiveness in the bone marrow but not in the liver environment. Proc Natl Acad Sci USA. 2004;101:11070–11075. doi: 10.1073/pnas.0402919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Llorian M, Stamataki Z, Hill S, Turner MS, Martensson IL. The PI3K p110δ is required for down-regulation of RAG expression in immature B cells. J Immunol. 2007;178:1981–1985. doi: 10.4049/jimmunol.178.4.1981. [DOI] [PubMed] [Google Scholar]
- 148.Schram BR, et al. B cell receptor basal signaling regulates antigen-induced Ig light chain rearrangements. J Immunol. 2008;180:4728–4741. doi: 10.4049/jimmunol.180.7.4728. [DOI] [PubMed] [Google Scholar]
- 149.Tze LE, et al. Basal immunoglobulin signaling actively maintains developmental stage in immature B cells. PLoS Biol. 2005;3:e82. doi: 10.1371/journal.pbio.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191:1813–1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Casellas R, et al. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 152.Casellas R, et al. Igκ allelic inclusion is a consequence of receptor editing. J Exp Med. 2007;204:153–160. doi: 10.1084/jem.20061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]