Abstract
Introduction
Male breast cancer (MBC) is a rare disease. Rates of MBC in Northern Africa vary by region. The age-standardized incidence for MBC is higher in Morocco than in Egypt, and the Egyptian rate is similar to the U.S of approximately 1/105 . This study aimed at investigating the clinical and molecular characteristics of MBC in Egypt and Morocco.
Methods
This case-case study included 211 cases from Egypt and 132 from Morocco. Tumor tissues were available for 47 Egyptian and 18 Moroccan patients. Medical record information was abstracted for patients’ demographics, medical history, and treatment. BRCA2 protein expression status was examined in Egyptian and Moroccan tumors. Androgen receptor CAG repeat length was analyzed using the tissue samples in Egyptian MBC tumors and controls. Limited amount of tissues from Morocco did not allow for the analysis of CAG repeats.
Results
Egyptian MBC patients had a significantly lower age at diagnosis (Egypt: 57.5 ± 15.1, Morocco: 63.9 ± 14.4, P = 0.0002) and a higher prevalence of liver cirrhosis (Egypt: 28.0%, Morocco: 0.8%, P =< 0.0001). MBC patients also had higher tumor grades [I (0.9%), II (81.0%), III (18.1%)] in Egypt vs. [I (10.7%), II (81.0%), III (8.3%)] in Morocco (P = 0.0017). The clinical and molecular characteristics of the groups from the 2 countries did not significantly differ. There was no significant difference with respect to BRCA2 expression amongst countries (Egypt: 28.9% non-wild type, Morocco: 27.8% non-wild type, P = 0.9297) or CAG lengths amongst BRCA2 expression types in Egyptians (Wild type: 54.6% with CAG repeat lengths of 20+, Non-wild type: 50% with CAG repeat lengths of 20+, P = 0.7947).
Conclusions
Differences in MBC between Egypt and Morocco are more likely due to differences in other risk factors such as consanguinity and use of xenoestrogenic pesticides.
Keywords: Male breast cancer, breast cancer, Egypt, Morocco, developing countries
1. Introduction
Rates of male breast cancer (MBC) are low in the U.S., representing approximately 1% of all breast cancers [27]. Although men present with breast disease at a later age than women, MBC tends to be more advanced, possibly leading to the observed worse survival outcomes [21]. In 2010, the American Cancer Society estimated that 1,970 men will be diagnosed with MBC, and 390 men will die [27]. Rates of MBC in the U.S. have also increased by approximately 26% in the last 26 years [21], with current age-adjusted incidence rates at 1.08/100,000 [27]. There is a broad variability globally in the rates of and possibly risk factors for MBC. In Africa the proportion of male breast cancer relative to all breast cancer is estimated to be 1.42% in Egypt [19], 2.3% in Rabat, Morocco [45], 2.9% in Tanzania [8], and 8.9% in South Western Nigeria [35]. This comparison of the MBC patients in Egypt and Morocco seeks to explain this variability.
Similar to the U.S., rates of MBC in Egypt are also low. Data from the Middle East Cancer Consortium show an age-standardized incidence rate of MBC to be 0.8/100,000 in both the U.S. and Egypt [19]. More recently, the Gharbiah Population-based Cancer Registry reported the age-standardized incidence rate of MBC to be 0.7/100,000, which represented about 1.1% of all breast cancers [26]. In previous decades, the proportion of MBC to all breast cancers in Egypt were reported to be up to 12-times that of the United States [15]. While this shift in MBC proportion may be attributed in part to hospital-based data of earlier studies and the more reliable population-based data of later studies in Egypt [15,42], others point to the recent significant decline in the Schistosoma parasitic infection and its associated liver cirrhosis [1,3,13,23,33,39]. Liver disease is believed to be a risk factor for MBC because of the associated high circulating level of estrogen, which stimulates breast tissue growth [44].
In Morocco, the age-standardized incidence of MBC is 1.0/100,000 [45]; about 42% higher than rates in Egypt [26]. Further, the proportion of male breast cancer relative to all breast cancer is estimated to be 2.3% in Rabat, Morocco, [45] compared to 1.1% of all breast cancers in Egypt [26]. This discrepancy in rates between Egypt and Morocco may be explained by differences in environmental exposures and/or genetic risk factors related to MBC.
The molecular etiology of MBC is not well defined. A variety of studies offer conflicting results in part due to the rarity of the disease [5] and lack of national population-based registries [14,28]. Certain genetic factors that have been hypothesized to contribute to risk for MBC include Klinefelter syndrome, mutations in the BRCA2 tumor suppressor gene, and increased CAG repeats in the polymorphic androgen receptor (AR). Men with BRCA2 mutations have an 8.4% risk of MBC up to the age of 80 years [16]. Hormonal factors are especially important in breast cancer development. While estrogens promote breast tissue growth, androgens restrain tissue growth in the breast, thereby exerting antimitogenic effects [18]. The transactivational power of the AR is inversely related its length, with longer receptors being worse at restraining tissue growth [9,18,32]. Within the AR, the highly polymorphic region of glutamine repeats encoded by a series of CAG trinucleotide repeats is a candidate as a risk modulator. CAG repeat lengths are known to vary according to ethnicity [7,15], and any individual’s CAG repeat length can vary from 6–39 glutamines, resulting in differing androgen receptor activity [7]. A longer CAG repeat length would then result in a longer and weaker AR, which may be a predisposition for breast cancer. Our recent study on Egyptian MBC showed no significant difference in the CAG repeat length between MBC patients and healthy men [20]. This study aimed to describe and compare the clinical and selected molecular features, including BRCA2 expression, of MBC patients in Egypt and Morocco from 1999-2006 to explain the different incident rates in the two countries.
2. Materials and methods
2.1. Study population
The collaborating institutions were the National Cancer Institute in Cairo (NCI-Cairo) and the Tanta Cancer Center (TCC) in the Nile delta region of Egypt as well as the National Institute of Oncology in Rabat and the Ibn Rochd University Hospital in Casablanca, Morocco (Fig. 1). NCI-Cairo is the largest cancer center in Egypt with referrals from all regions of the country [17]. TCC is located in Gharbiah Province in the center of the Nile delta region and is the home to the Gharbiah Population-Based Cancer Registry, the only population-based cancer registry in Egypt, which is funded by the National Cancer Institute of the U.S. [19]. Data were abstracted from medical records of 211 Egyptian and 132 Moroccan patients with corresponding formalin-fixed paraffin-embedded tumor tissue for 47 Egyptian and 18 Moroccan patients. Patients were included in the study if they were 18 or older and were diagnosed with breast cancer between the years of 1999–2008 in Egypt and 2002–2008 in Morocco. Medical records of all patients diagnosed at the collaborating institutions for the above-listed time periods were retrieved and abstracted. In the 16 years from 1970–1985, NCI-Cairo diagnosed 228 cases of MBC, or 14.25 a year [40]. In the 5 years from 1985– 1989, NCI-Cairo diagnosed 65 cases of MBC, or 13 per year [34]. The 140 cases in this study collected over the 10-year period from NCI-Cairo diagnosed, then, represent all diagnosed cases. In the 3 years from 2000– 2002, TCC diagnosed 21 MBC cases, or 7 cases per year [26]. The 70 cases in this study collected over the 10-year period TCC also represent all diagnosed cases. In Morocco, we included 85 cases from the National Institute of Oncology in Rabat diagnosed over the 7 years preceding our study and 47 cases from Ibn Rochd Hospital in Casablanca over the same 7 years preceding our study. These cases from Rabat and Casablanca represented all MBC patients seen at the two institutions during the 7-year period. Tumor tissue paraffin blocks were retrieved for all cases that underwent mastectomy and were available only for patients diagnosed in the 3–4 years preceding 2006 due to institutional limitations on paraffin block storage periods in Egypt and Morocco.
Fig. 1.
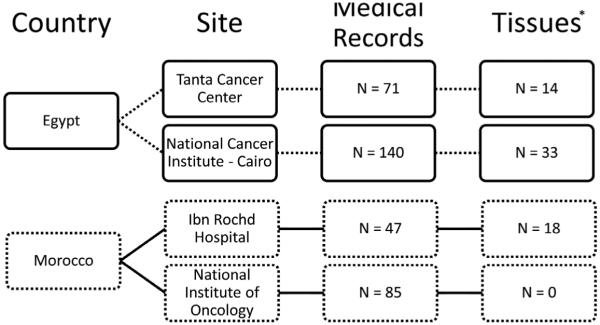
Flow chart depicting the breakdown of study participants. *Formalin-fixed paraffin-embedded tissue samples were available 3–4 years preceding 2006 while medical records were available from 1999 in Egypt and 2002 in Morocco.
This case-case study included MBC cases from Egypt compared to MBC cases from Morocco. Information was abstracted from medical records about clinical features of tumors, histopathological characteristics, method of treatment, patient family and medical histories, and demographics. Slides from tumor tissues were prepared and used for staining for ER and PR status, BRCA2 protein expression, and androgen receptor CAG repeat length determination according to the following laboratory methods:
2.1.1. Androgen receptor CAG repeat length
On the molecular level, the glutamines that appear in the polyglutamine tract of the androgen receptor are encoded by a series of CAG repeats in the DNA of the AR gene. Each sequence of CAG base pairs in this region of the AR gene will result in one glutamine amino acid in the androgen receptor. The CAG repeat region is located in the first exon of the AR gene on the X chromosome [4]. PCR amplification of isolated patient DNA followed by fragment length analysis according to the methods described by Gilbert et al., 2011, reveal the number of CAG repeats in this region. A case-control study comparing the CAG repeat lengths in the 44 Egyptian MBC tumor and 43 non-MBC controls found no significant difference in amount of repeats (MBC cases: 19.5 ± 2.8 repeats, Controls: 19.3 ± 4.2 repeats, P = 0.843), but did note a higher proportion of short repeat lengths among controls, possibly suggesting a protective effect of short CAG repeat lengths [20]. A similar case-control study of the androgen receptor CAG repeat length was not done with the Moroccan samples due to the limited amount of tissues available.
2.1.2. BRCA2 protein expression
Tissue sections of the tumor blocks were analyzed for truncations in the BRCA2 protein through N-terminal and C-terminal antibody staining. Detecting BRCA2 expression in this manner has been shown to distinguish between cancers with and without the wild type BRCA2 protein. Although this method does not identify the specific mutation, wild type BRCA2 protein is suggestive of a normal BRCA2 gene expression. Non-wild type BRCA2 protein is suggestive of a BRCA2 gene with a mutation somewhere that at the very least results in protein under-expression [46]. When stained with either antibody, truncation is indicated through the visual presence of the beginning of the protein but not the end portion. The formalin-fixed paraffin-embedded sections were mounted on aminopropyltriethosylane-coated glass slides and subjected to a standard wash and incubation protocol. Slides were de-paraffinized and re-hydrated, immersed in 3% H2O2 for 20 min, followed by pre-incubated with normal goat serum diluted 1:10 in PBS with 5% BSA for 15 min. Slides were incubated with the C-terminal BRCA2 antibody (EMD Sciences, Cat#CA1033, Lot#D00076432) at a dilution of 1:30. Another set of slides was also incubated with the N-terminal antibody (R&D Systems, Cat#MAB2476, Lot#UFN01) at a concentration of 25 μg/mL. After staining, the slides were incubated for 30 min with biotinylated goat anti-rabbit immunoglobulin in a dilution of 1:100 followed by incubation for 30 min with peroxidase-conjugated avidin-biotin complex in a dilution of 1:50. The slides were then washed in an acetate buffer and placed in 10% AEC. Counter-staining with Mayer’s hematoxylin allowed for visual determination of truncation. Staining patterns were exclusively nuclear, perinuclear, and intranuclear hernia staining.
2.1.3. ER and PR status determination
Paraffin-embedded samples were cut to 4 μ thick and placed on positively charged slides. Slides were immunostained using the Dako Cytomation En Vision System (Dako, Carpinteria, CA). After deparaffinization, sections were rehydrated and endogenous peroxidase was blocked with 1% H2O2 in methanol. After pressure induced epitope retrieval (Biocare Medical Decloaking Chamber, Concord CA; citrate buffer, pH 6.0), sections were incubated with anti-ER antibody (clone 1D5, dilution 1:100; Dako) or anti-PR antibody (clone 636, dilution 1:200; Dako) at room temperature. The reaction was visualized using the EnVision kit and 3, 3-diaminobenzidine as chromogen, followed by light counterstaining with haematoxylin. Positive and negative controls were used in each staining run. Negative controls consisted of eliminating primary antibody and positive controls were known ER/PR positive human tumors. In a blinded fashion, the samples were read and scored by light microscopy. Each slide was scored visually and independently by two pathologists blinded to all clinicopathologic data.
2.2. Statistical analysis
New variables were created to summarize complex data such as method of treatment. Missing data were not included in the final analysis. The number of patients who were included relative to the total number is indicated in the tables for the corresponding analyses. The student’s t-test was used to determine the significance of differences in mean values. A χ2-test for independence was used to determine the significance of differences in frequency distributions. The cut-off value for statistical significance was P value = 0.05. SAS version 9.2 (SAS Institute, Cary, NC) was used.
3. Results
Table 1 shows the comparison of age and pathological diagnosis of MBC cases between Egyptian and Moroccan patients. There was a significant difference with respect to mean age between the two countries (Egypt: 57.5 ± 15.1, Morocco: 63.9 ± 14.4, p = 0.0002). There was no significant difference with respect to the pathologic diagnosis between Egypt and Morocco, with a majority in both countries having invasive ductal carcinoma (Egypt: 87.1%, Morocco: 93.5%, p = 0.0859).
Table 1.
Age and pathologic information for Egyptian and Moroccan MBC patients
| Characteristic(N=Egypt,N=Morocco) | Egypt (N=211) | Morocco (N=132) | p-value |
|---|---|---|---|
| Age (mean ± SD, Years) (N=186,N=128) | 57.5 (± 15.1) | 63.9 (± 14.4) | 0.0002a |
| Pathological type (N=170,N=108) | |||
| Invasive ductal carcinoma | 148 (87.1%) | 101 (93.5%) | 0.0859b |
| Invasive lobular carcinoma | 5 (2.9%) | 0 (0%) | 0.0721b |
| Papillary/Apocrine carcinoma/Other | 17 (10.0%) | 7 (6.5%) | 0.3086b |
t-test.
χ2-test.
Table 2 shows the comparison of patient characteristics including symptom and treatment methods among MBC patients from TCC and Morocco. The data for MBC patients treated at NCI-Cairo were unavailable and not included in this analysis. There was no significant difference between the two countries in the proportion of those living in an urban area (TCC: 33.9%, Morocco: 32.1%, P = 0.8231) or who had a family history of breast cancer (TCC: 2.0%, Morocco: 3.9%, P = 0.5215). However, there was a significant difference with respect to the proportion of MBC patients who have had liver cirrhosis (TCC: 28.0%, Morocco: 0.8%, P =< 0.0001). There was no significant difference between the two countries in the proportions the most common symptoms as diagnoses (palpable mass, nipple retraction, redness or darkness of skin). With regard to the comparison of the treatment characteristics, Egyptian and Moroccan MBC patients were treated similarly. There was no significant difference between the proportion of patients receiving chemotherapy and/or radiation (TCC: 9.4%, Morocco: 20.4%, P = 0.0833). There was also no significant difference between the proportion of patients receiving surgery and/or hormone therapy concurrent with chemotherapy and/or radiation (TCC: 81.1%, Morocco: 73.5%, P = 0.2916).
Table 2.
Comparison between Moroccans and Egyptians from TCC with regards to demographic, diagnosis, and treatment variables
| Characteristic(N=Egypt,N=Morocco) | Tanta, Egypt (N=71) | Morocco (N=132) | p-valuea |
|---|---|---|---|
| Lives in an urban area (N=62,N=81) | 21 (33.9%) | 26 (32.1%) | 0.8231 |
| Liver Cirrhosis (N=50,N=127) | 14 (28.0%) | 1 (0.8%) | < 0.0001 |
| Family History of Breast Cancer (N=50,N=127) | 1 (2.0%) | 5 (3.9%) | 0.5215 |
| Symptom at Diagnosis | |||
| Palpable mass (N=50,N=125) | 22 (44.0%) | 53 (42.4%) | 0.8468 |
| Nipple retraction(N=50,N=124) | 8 (16.0%) | 10 (8.1%) | 0.1198 |
| Redness or darkness(N=50,N=124) | 0 (0%) | 6 (4.8%) | 0.1134 |
| Treatment Characteristicb (N=53,N=98) | |||
| Chemotherapy and/or radiotherapy | 5 (9.4%) | 20 (20.4%) | 0.0833 |
| Surgery and/or hormone therapy and/or | 43 (81.1%) | 72 (73.5%) | 0.2916 |
| Chemotherapy/radiotherapy |
χ2-test.
Treatment characteristic not available for 5 patients (9.4%) in Tanta, Egypt, and 6 patients (6.1%) in Morocco.
Table 3 shows the comparison of histopathologic data between Egyptian and Moroccan MBC patients. There was no significant difference in the proportions of those who had normal BRCA2 protein expression (Egypt: 28.9%, Morocco: 27.8%, P = 0.9297), lymph node involvement (Egypt: 37.7%, Morocco: 38.2%, P = 0.9349), laterality (P = 0.0967), ER status (P = 0.5264), PR status (P = 0.2461), or tumor size (Egypt: 3.8 cm, Morocco: 3.6 cm for the largest diameter, P = 0.6845). There was, however, a significant difference between Egyptian and Moroccan MBC cases with respect to the tumor grade (P = 0.0017). The mean length of the Egyptian MBC patients’ AR CAG repeat polymorphism is 19.45 ± 2.79.
Table 3.
Comparison between Egyptian and Moroccan MBC patients with regards to BRCA2 expression and histopathological characteristics
| Characteristic(N=Egypt,N=Morocco) | Egypt | Morocco | p-value |
|---|---|---|---|
| BRCA2 Status(N=45,N=18) | |||
| Wild-type | 32 (71.1%) | 13 (72.2%) | 0.9297a |
| Non-wild-type | 13 (28.9%) | 5 (27.8%) | |
| Lymph Node Involvement (N=122,N=123) | |||
| Negative | 76 (62.3%) | 76 (61.8%) | 0.9349a |
| Positive | 46 (37.7%) | 47 (38.2%) | |
| Laterality (N=152,N=127) | |||
| Right breast | 66 (43.4%) | 64 (50.4%) | 0.0967a |
| Left breast | 83 (54.6%) | 56 (44.1%) | |
| Both breasts | 3 (2.0%) | 7 (5.5%) | |
| Tumor Grade (N=116,N=84) | |||
| I | 1 (0.9%) | 9 (10.7%) | 0.0017a |
| II | 94 (81.0%) | 68 (81.0%) | |
| III | 21 (18.1%) | 7 (8.3%) | |
| ER Status (N=69,N=55) | |||
| Negative | 13 (18.8%) | 8 (14.5%) | 0.5264a |
| Positive | 56 (81.2%) | 47 (85.5%) | |
| PR Status (N=68,N=53) | |||
| Negative | 19 (27.9%) | 10 (18.9%) | 0.2461a |
| Positive | 49 (72.1%) | 43 (81.1%) | |
| Tumor Size (cm) (N=61,N=61) | 3.8 | 3.6 | 0.6845b |
| Mean AR CAG repeat Length (N=44,N=0) | 19.45 | – | – |
χ2-test.
t-test.
Table 4 shows the comparison of histopathologic data between MBC cases with abnormal BRCA2 protein expression (non-wild type BRCA2) and those with normal BRCA2 protein expression (wild-type BRCA2) without regard to country. Statistics were calculated with regard to country as well (data not shown), but found to have no difference when compared to statistics for both countries (shown in Table 4). There was no significant difference between those with and without abnormal BRCA2 protein expression in the proportions of age divided at the median (P = 0.1462), tumor grade (P = 0.2072), laterality (P = 0.4844), lymph node involvement (P = 0.6771), ER status (P = 0.2593), PR status (P = 0.2393), tumor size divided at the median (P = 0.7450), or AR CAG repeat length dived at the median (P = 0.7947).
Table 4.
Comparison of patient and tumor characteristics by BRCA2 expression
| Characteristic | Number of patients |
Number with non-wild type BRCA2 (protein under- expression) |
Number with wild type BRCA2 (normal protein expression) |
p-value |
|---|---|---|---|---|
| Age at Diagnosis | ||||
| < 61 years | 22 | 4 (28.6%) | 18 (51.4%) | 0.1462a |
| ≥ 61 years | 27 | 10 (71.4%) | 17 (48.6%) | |
| Mean (years) | 60.0 | 62.6 (N = 14) | 58.9 (N = 35) | 0.4502b |
| Median (years) | 62 | 63.5 | 59 | |
| Tumor Grade | ||||
| I | 2 | 0 (0%) | 2 (5.9%) | 0.2072a |
| II | 37 | 11 (100%) | 26 (76.5%) | |
| III | 6 | 0 (0%) | 6 (17.7%) | |
| Laterality | ||||
| Right breast | 24 | 7 (63.6%) | 17 (51.5%) | 0.4844a |
| Left breast | 20 | 4 (36.4%) | 16 (48.5%) | |
| Both breasts | 0 | 0 (0%) | 0 (0%) | |
| Lymph Node Status | ||||
| Negative | 24 | 6 (46.1%) | 18 (52.9%) | 0.6771a |
| Positive | 23 | 7 (53.9%) | 16 (47.1%) | |
| ER Status | ||||
| Negative | 3 | 0 (0%) | 3 (21.4%) | 0.2593a |
| Positive | 16 | 5 (100%) | 11 (78.6%) | |
| PR Status | ||||
| Negative | 3 | 0 (0%) | 3 (23.1%) | 0.2393a |
| Positive | 15 | 15 | 10 (76.9%) | |
| Tumor Size (cm) | ||||
| < 3 cm | 12 | 4 (44.4%) | 8 (38.1%) | 0.7450a |
| ≥ 3 cm | 18 | 5 (55.6%) | 13 (61.9%) | |
| Androgen receptor CAG repeat Length | ||||
| < 20 | 21 | 5 (45.5%) | 16 (50%) | 0.7947a |
| ≥ 20 | 22 | 6 (54.6%) | 16 (50%) |
χ2-test.
t-test.
4. Discussion
This is the first and largest study on MBC in North Africa. The study revealed the following observations: First, MBC appears to affect significantly younger Egyptian than Moroccan males and MBC tumors have higher grades compared to tumors of Moroccan patients. Second, Egyptian MBC patients have significantly higher rates of liver cirrhosis than Moroccan MBC patients. Third, Egyptian and Moroccan MBC patients received similar treatments. Fourth, there was no observed difference in the proportion of tumors with abnormal BRCA2 protein expression in Egypt compared to Morocco. Fifth, in comparing MBC patients with and without abnormal BRCA2 protein expression, there was no significant difference with respect to their histopathologic characteristics or androgen receptor CAG repeat length.
This study is, for the most part, consistent with other studies describing MBC in Morocco and Egypt. One study looking at 12 Moroccan MBC patients at the Ibn Rochd University Hospital from 1988–1999 found the mean age of diagnosis to be 60 [24]. They also note axillary lymph node involvement in 8 of the 12 patients (67%) and surgery as the usual treatment [24]. While the current study found lymph node involvement in only 47 of 123 Moroccan MBC patients (38%), the mean age at diagnosis of 63.9 ± 14.4 years in our sample fits with the previous finding. Another study looking at 71 MBC patients treated at the National Institute of Oncology in Rabat, Morocco, from 1985–1998 similarly found a mean age at diagnosis of 60 years but saw lymph node involvement in 65% of cases [14]. However, similar to the current study, liver cirrhosis was seen in only 1 of the 71 Moroccan MBC patients (1.4%), grade II tumors represented 82% of their cases, and of 5 MBC cases examined for hormone receptor status, only 1 (20%) had negative receptors [14]. The younger age of diagnosis for patients in Egypt compared to Moroccans seen in our study (6.4 years difference in average age at diagnosis) is not seen as a result of differing life expectancies. This is considering the World Health Organization reports the 2009 average life expectancy for Egyptian males to be 69 years and Moroccan males at 71 years [49]. MBC has not been as well-studied in Egypt. In comparing Egyptian and Moroccan MBC tumors, their histopathologic characteristics are quite similar. There were approximately the same proportion of tumors with abnormal BRCA2 expression, lymph node involvement, a positive ER, and a positive PR. However, it is interesting to note that the grade of the Egyptian MBC tumors at diagnosis was higher than that of the Moroccans.
Male breast cancer has a large degree of genetic and hormonal risk factors. Studies utilizing SEER data [38], from Sweden [25], and a recent treatment review out of Spain [22] have shown that 10–20% of all MBC are familial. Klinefelter’s syndrome (47XXY) has been associated with MBC with a relative risk 20– 50 times higher than normal 46XY males [5,6,22]. This is probably due to altered endogenous hormones because of the extra X chromosome [6]. Other suspected risk factors for MBC include gynecomastia, diabetes, obesity, and orchitis/epididymitis [5,22,37]. A recent large epidemiologic investigation looked at 642 cases of MBC in men in the U.S. Veterans Affairs inpatient hospitalization database [5]. The study confirmed the usual MBC risk factors such as diabetes, obesity, orchitis/epididymitis, Klinefelter’s syndrome, and gynecomastia but did not show an association with thyroid diseases, smoking-related conditions, alcohol use, liver cirrhosis, prostate disease, or broken bones, which are hypothesized to have an association with MBC [5]. Our study found that 14 of 71 (28%) of MBC patients in our population had liver cirrhosis. This rate is significantly higher than in MBC patients from Morocco. However, the rate of liver cirrhosis in the general population of in Egypt is high, estimated to be 5% in those over 30 years of age, due to high prevalence of hepatitis C infections [1,13,31,43,47]. Rates of hepatitis C in Egypt range between 6% and 40% [31]. Because of the high rate of cirrhosis in the general population in Egypt, MBC patients may have susceptibility or other environmental and genetic risk factors that increase their risk for MBC. However, cirrhosis may not increase the risk for MBC in this population as no significant increase in MBC has been observed in recent years in Egypt parallel to the significant increase in cirrhosis in the general population in Egypt [1,13,26,31]. There was also no significant difference in treatment characteristics between Egyptian and Moroccan MBC patients. MBC is only diagnosed after histopathologic treatment in both Egypt and Morocco and type of treatment is based on hormonal status of tumors and stage of diagnosis [36].
The present study also did not find a difference in BRCA2 protein expression between Egyptian and Moroccan MBC patients. This is consistent with other studies. For example, Haraldsson et al. analyzed 34 Swedish MBC patients with and without BRCA2 mutations found no significant difference with respect to clinical stage, ER status, or PR status, but did find a younger age of onset for those with BRCA2 mutations [25]. Seven of the 34 (21%) MBC patients had BRCA2 mutations [25], which is similar to the proportion reported in our study. Kwiatkowska et al. similarly did not observe a difference in clinicopathological features between BRCA2 mutation carriers and non-carriers [29]. A more recent study of BRCA2 mutations and AR expression in MBC examined 43 MBC patients, 12 with BRCA2 mutations (27.9%), which is also approximately the same proportion of those with abnormal BRCA2 expression in our study [30]. Kwiatkowska et al. reported that BRCA2 mutations and AR expression in tumor tissues to be independent risk factors for MBC, and likewise did not find a significant difference between MBC patients with and without BRCA2 mutations with respect to histopathologic characteristics [30]. However, they also found a significantly earlier age of diagnosis for BRCA2-related MBC compared to non-BRCA2-related cancer [30]. A younger age of diagnosis for MBC patients with abnormal BRCA2 protein expression has not been corroborated by the current study most likely due to the limited sample size.
The distribution of androgen receptor CAG repeat lengths between those with wild-type BRCA2 (normal protein expression) and those with non-wild-type BRCA2 (under-expression) is also noteworthy. It has been shown that wild-type BRCA2 can enhance AR activity as part of its tumor-suppression functions [41]. Therefore, looking at CAG repeat length distributions, which modify AR activity [7], in those with and without normal BRCA2 protein expression, may clarify the relationship between the AR CAG repeat region, BRCA2, and MBC. Shorter CAG repeat lengths of the AR gene were found among MBC patients with abnormal BRCA2 expression compared to patients with normal BRCA2 expression [25]. No such difference between patients with and without normal BRCA2 protein expression was found in our study among the Egyptian patients. CAG repeat length data on the Moroccan patients were not available because of the limited amount of tissues available for this study. However, in previous studies, it has been seen that short CAG repeat lengths are more prevalent among non-MBC controls compared to MBC patients [20,48]. It should also be noted that our study’s finding of CAG repeat lengths of Egyptian MBC patients (19.5 ± 2.8) and controls (19.3 ± 4.2) are in agreement with a previous study that found an average CAG repeat length of 18.18 ± 3.63 for healthy Egyptian men [2].
This study had the following strengths: First, the large sample size and representativeness of the sampling provides more generalizability to MBC in these Egypt and Morocco. Second, the comparison of MBC between two countries of varying incidence rates and risk factors allowed for a comparison to the specific risk factors involved. Third, the inclusion genetic factors like those relating to BRCA2 or AR, enhanced the analysis. Limitations included the availability of tissue limited to the 3–4 period before 2006, only for those patients who underwent surgical treatment, the limited information available in the medical records and the lack of androgen receptor CAG repeat length information for Moroccan MBC patients. Further, even though this study is large compared to the current literature of MBC, it lacked the power to assemble a multivariate model for the disease.
The high degree of liver cirrhosis in Egyptian patients, and the non-significant difference in the clinical, histopathological, or genetic risk factors between Egypt and Morocco suggest that MBC is not two distinct diseases in these populations. Future studies into the etiology of MBC should include more patient tissue samples and investigate more environmental exposures, such as consanguinity and environmental sources of hormonal exposures to better compare the disease between populations.
Acknowledgments
Samuel F. Gilbert, Meaghen Quinlan-Davidson, and Ashley Strahley were supported by the Cancer Epidemiology Education in Special Populations Program of the University of Michigan, an R25 Cancer Education Grant from the National Cancer Institute (R25 CA112383).
References
- [1].Anwar WA, Khaled HM, Amry HA, El-Nezami H, Loffredo CA. Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: Possibilities for prevention. Mutat Res. 2008;659(1–2):176–184. doi: 10.1016/j.mrrev.2008.01.005. [DOI] [PubMed] [Google Scholar]
- [2].Badran WA, Fahmy I, Abdel-Megid WM, Elder K, Mansour R, Kent-First M. Length of androgen receptor-CAG repeats in fertile and infertile Egyptian men. J Androl. 2009;30(4):416–425. doi: 10.2164/jandrol.108.005843. [DOI] [PubMed] [Google Scholar]
- [3].Barakat R, Farghaly A, El Masry AG, El-Sayed MK, Hussein MH. The epidemiology of schistosomiasis in Egypt: Patterns of Schistosoma mansoni infection and morbidity in Kafer el-Sheikh. Am J Trop Med Hyg. 2000;62(2 Suppl):21–27. doi: 10.4269/ajtmh.2000.62.21. [DOI] [PubMed] [Google Scholar]
- [4].Blanco S, Suarez A, Gandia-Pla S, Gomez-Llorente C, Antunez A, Gomez-Capilla JA, Farez-Vidal ME. Use of capillary electrophoresis for accurate determination of CAG repeats causing Huntington disease. An oligonucleotide design avoiding shadow bands. Scand J Clin Lab Invest. 2008;9:1–8. doi: 10.1080/00365510801915171. [DOI] [PubMed] [Google Scholar]
- [5].Brinton LA, Carreon JD, Gierach GL, McGlynn KA, Gridley G. Etiologic factors for male breast cancer in the U.S. veterans affairs medical care system database. Breast Cancer Res Treat. 2010;119(1):185–192. doi: 10.1007/s10549-009-0379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brinton LA. Breast cancer risk among patients with Klinefelter syndrome. Acta Paediatr. 2011;100(6):814–818. doi: 10.1111/j.1651-2227.2010.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buchanan G, Yang M, Cheong A, Harris JM, Irvine RA, Lambert PF, Moore NL, Raynor M, Neufing PJ, Coetzee GA, Tilley WD. Structural and functional consequences of glutamine tract variation in the androgen receptor. Hum Mol Genet. 2004;13:1677–1692. doi: 10.1093/hmg/ddh181. [DOI] [PubMed] [Google Scholar]
- [8].Burson AM, Soliman AS, Ngoma TA, Mwaiselage J, Ogweyo P, Eissa MS, Dey S, Merajver SD. Clinical and epidemiologic profile of breast cancer in Tanzania. Breast Dis. 2010;31(1):33–41. doi: 10.3233/BD-2009-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22(15):3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Darwish MA, Faris R, Darwish N, Shouman A, Gadallah M, El-Sharkawy MS, Edelman R, Grumbach K, Rao MR, Clemens JD. Hepatitis c and cirrhotic liver disease in the Nile delta of Egypt: a community-based study. Am J Trop Med Hyg. 2001;64(3-4):147–153. doi: 10.4269/ajtmh.2001.64.147. [DOI] [PubMed] [Google Scholar]
- [11].Dey S, Zhang Z, Hablas A, Seifeldein IA, Ramadan M, El-Hamzawy H, Soliman AS. Geographic patterns of cancer in the population-based registry of Egypt: Possible links to environmental exposures. Cancer Epidemiol. 2011;35(3):254–264. doi: 10.1016/j.canep.2010.09.010. [DOI] [PubMed] [Google Scholar]
- [12].El-Gazayerli MM, Abdel-Aziz AS. On bilharziasis and male breast cancer in Egypt: A preliminary report and review of the literature. Br J Cancer. 1963;17:566–571. doi: 10.1038/bjc.1963.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].El-Khoby T, Galal N, Fenwick A, Barakat R, El-Hawey A, Nooman Z, Habib M, Abdel-Wahab F, Gabr NS, Hammam HM, Hussein MH, Mikhail NN, Cline BL, Strickland GT. The epidemiology of schistosomiasis in Egypt: Summary findings in nine governorates. Am J Trop Med Hyg. 2000;62(2 Suppl):88–99. doi: 10.4269/ajtmh.2000.62.88. [DOI] [PubMed] [Google Scholar]
- [14].El Omari-Alaoui H, Lahdiri I, Nejjar I, Hadadi K, Ahyoud F, Hachi H, Alhilal M, Errihani H, Benjaafar N, Souadka A, El Gueddari BK. Male breast cancer. A report of 71 cases. Cancer Radiother. 2002;6(6):349–351. doi: 10.1016/s1278-3218(02)00250-0. [DOI] [PubMed] [Google Scholar]
- [15].Esteban E, Rodon N, Via M, Gonzalez-Perez E, Santamaria J, Dugoujon JM, Chennawi FE, Melhaoui M, Cherkaoui M, Vona G, Harich N, Moral P. Androgen receptor CAG and GGC polymorphisms in Mediterraneans: Repeat dynamics and population relationships. J Hum Genet. 2006;51(2):129–136. doi: 10.1007/s10038-005-0336-7. [DOI] [PubMed] [Google Scholar]
- [16].Evans DG, Susnerwala I, Dawson J, Woodward E, Maher ER, Lalloo F. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47(10):710–711. doi: 10.1136/jmg.2009.075176. [DOI] [PubMed] [Google Scholar]
- [17].Felix AS, Soliman AS, Khaled H, Zaghloul MS, Banerjee M, El-Baradie M, El-Kalawy M, Abd-Elsayed AA, Ismail K, Hablas A, Seifeldin IA, Ramadan M, Wilson ML. The changing patterns of bladder cancer in Egypt over the past 26 years. Cancer Causes Control. 2008;19(4):421–429. doi: 10.1007/s10552-007-9104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ferro P, Catalano MG, Dell’Eva R, Fortunati N, Pfeffer U. The androgen receptor CAG repeat: A modifier of carcinogenesis? Mol Cell Endocrinol. 2002;193(1-2):109–120. doi: 10.1016/s0303-7207(02)00104-1. [DOI] [PubMed] [Google Scholar]
- [19].Freedman L, Edwards BK, Ries LAG, Al-Kayed S, Barchana M, Ibrahim AS, Komodiki C, Young JL. Overview and summary data. In: Freedman LS, Edwards BK, Ries LAG, Young JL, editors. Cancer incidence in four member countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) compared with US SEER. NIH; Maryland: 2006. pp. 1–27. [Google Scholar]
- [20].Gilbert SF, Soliman AS, Iniesta MD, Eissa M, Hablas A, Seifeldin IA, Strahley A, Banerjee M, Merajver SD. Androgen receptor polyglutamine tract length in Egyptian male breast cancer patients. Breast Cancer Res Treat. 2011;129(2):575–581. doi: 10.1007/s10549-011-1510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: A population-based study. Cancer. 2004;101(1):51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- [22].Gomez-Raposo C, Zambrana Tevar F, Sereno Moyano M, Lopez Gomez M, Casado E. Male breast cancer. Cancer Treat Rev. 2010;36(6):451–457. doi: 10.1016/j.ctrv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [23].Gouda I, Mokhtar N, Bilal D, El-Bolkainy T, El-Bolkainy NM. Bilharziasis and bladder cancer: A time trend analysis of 9843 patients. J Egypt Natl Canc Inst. 2007;19(2):158–162. [PubMed] [Google Scholar]
- [24].Hali F, Chiheb S, El Ouazzani T, Lakhdar H. Male breast cancer in Morocco. Ann Dermatol Venereol. 2002;129(5):699–702. Pt 1. [PubMed] [Google Scholar]
- [25].Haraldsson K, Loman N, Zhang QX, Johannsson O, Olsson H, Borg A. BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res. 1998;58(7):1367–1371. [PubMed] [Google Scholar]
- [26].Ibrahim AS. Cancer in the Nile Delta Region: a report from the Gharbiah Population-Based Cancer Registry, 2000–2002. Tanta, Egypt: 2007. [Google Scholar]
- [27].Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- [28].Khatib O, Aljurf M. Cancer prevention and control in the eastern Mediterranean region: The need for a public health approach. Hematol Oncol Stem Cell Ther. 2008;1(1):44–52. doi: 10.1016/s1658-3876(08)50060-4. [DOI] [PubMed] [Google Scholar]
- [29].Kwiatkowska E, Teresiak M, Lamperska KM, Karczewska A, Breborowicz D, Stawicka M, Godlewski D, Krzyzosiak WJ, Mackiewicz A. BRCA2 germline mutations in male breast cancer patients in the Polish population. Hum Mutat. 2001;17(1):73. doi: 10.1002/1098-1004(2001)17:1<73::AID-HUMU12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- [30].Kwiatkowska E, Teresiak M, Filas V, Karczewska A, Breborowicz D, Mackiewucz A. BRCA2 Mutations and Androgen Receptor Expression as Independent Predictors of Outcome of Male Breast Cancer Patients. Clin Cancer Res. 2003;9(12):4452–4459. [PubMed] [Google Scholar]
- [31].Lehman EM, Wilson ML. Epidemiology of hepatitis viruses among hepatocellular carcinoma cases and healthy people in Egypt: a systematic review and meta-analysis. Int J Cancer. 2009;124(3):690–697. doi: 10.1002/ijc.23937. [DOI] [PubMed] [Google Scholar]
- [32].Lindstrom S, Ma J, Altshuler D, Giovannucci E, Riboli E, Albanes D, Allen NE, Berndt SI, Boeing H, Buenode-Mesquita HB, Chanock SJ, Dunning AM, Feigelson HS, Gaziano JM, Haiman CA, Hayes RB, Henderson BE, Hunter DJ, Kaaks R, Kolonel LN, Le Marchand L, Martinez C, Overvad K, Siddiq A, Stampfer M, Stattin P, Stram DO, Thun MJ, Trichopoulos D, Tumino R, Virtamo J, Weinstein SJ, Yeager M, Kraft P, Freedman ML. A large study of androgen receptor germline variants and their relation to sex hormone levels and prostate cancer risk. Results from the national cancer institute breast and prostate cancer cohort consortium. J Clin Endocrinol Metab. 2010;95(9):E121–E127. doi: 10.1210/jc.2009-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Michelson MK, Azziz FA, Gamil FM, Wahid AA, Richards FO, Juranek DD, Habib MA, Spencer HC. Recent trends in the prevalence and distribution of schistosomiasis in the Nile delta region. Am J Trop Med Hyg. 1993;49(1):76–87. doi: 10.4269/ajtmh.1993.49.76. [DOI] [PubMed] [Google Scholar]
- [34].Mokhtar M. Cancer Incidence from the National Cancer Institute – Cairo, Department of Pathology, 1985–1989. University of Cairo; 1991. [Google Scholar]
- [35].Oguntola AS, Aderonmu AO, Adeoti ML, Olatoke SA, Akanbi O, Agodirin SO. Male breast cancer in LAUTECH teaching hospital Osogbo, South Western Nigeria. Niger Postgrad Med J. 2009;16(2):166–170. [PubMed] [Google Scholar]
- [36].Omar S. Breast Cancer, National Cancer Institute – Cairo. University of Cairo; 2000. [Google Scholar]
- [37].Onami S, Ozaki M, Mortimer JE, Pal SK. Male breast cancer: An update in diagnosis, treatment and molecular profiling. Maturitas. 2010;65(4):308–314. doi: 10.1016/j.maturitas.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rosenblatt KA, Thomas DB, McTiernan A, Austin MA, Stalsberg H, Stemhagen A, Thompson WD, Curnen MG, Satariano W, Austin DF. Breast cancer in men: Aspects of familial aggregation. J Natl Cancer Inst. 1991;83(12):849–854. doi: 10.1093/jnci/83.12.849. [DOI] [PubMed] [Google Scholar]
- [39].Sherif M, Ibrahim AS, El-Aaser AA. Prostatic carcinoma in Egypt: Epidemiology and etiology. Scand J Urol Nephrol Suppl. 1980;55:25–26. [PubMed] [Google Scholar]
- [40].Sherif O, Ibrahim AS. Cancer Incidence from the National Cancer Institute – Cairo, 1970–1985. University of Cairo; 1987. [Google Scholar]
- [41].Shin S, Verma IM. BRCA2 cooperates with histone acetyltransferases in androgen receptor-mediated transcription. Proc Natl Acad Sci USA. 2003;100(12):7201–7206. doi: 10.1073/pnas.1132020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Soliman AS, Bondy ML, Raouf AA, Makram MA, Johnston DA, Levin B. Cancer mortality in Menofeia, Egypt: Comparison with US mortality rates. Cancer Causes Control. 1999;10(5):349–354. doi: 10.1023/a:1008968701313. [DOI] [PubMed] [Google Scholar]
- [43].Soliman AS, Hung CW, Tsodikov A, Seifeldin IA, Ramadan M, Al-Gamal D, Schiefelbein EL, Thummalapally P, Dey S, Ismail K. Epidemiologic risk factors of hepatocellular carcinoma in a rural region of Egypt. Heptaol Int. 2010;4(4):681–690. doi: 10.1007/s12072-010-9187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, Trichopoulos D, Vilstrup H, Olsen J. Risk of breast cancer in men with liver cirrhosis. Am J Gastroenterol. 1998;93(2):231–233. doi: 10.1111/j.1572-0241.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- [45].Tazi MA. Incidence Des Cancer a Rabat, 2005. Rabat, Morocco: 2009. Cancer du Sein; pp. 45–47. [Google Scholar]
- [46].Watson P, Lieberman R, Snyder C, Clark VJ, Lynch HT, Holt JT. Detecting BRCA2 protein truncation in tissue biopsies to identify breast cancers that arise in BRCA2 gene mutation carriers. J Clin Oncol. 2009;27(24):3894–3900. doi: 10.1200/JCO.2008.20.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Williams R. Global challenges in liver disease. Hepatology. 2006;44(3):521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- [48].Young IE, Kurian KM, Mackenzie MA, Kunkler IH, Cohen BB, Hooper ML, Wyllie AH, Steel CM. The CAG repeat within the androgen receptor gene in male breast cancer patients. J Med Genet. 2000;37(2):139–140. doi: 10.1136/jmg.37.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Global Health Observatory. 2011 in: http://www.who.int/countries/en/


