Abstract
In this descriptive analysis, data in the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database pertaining to patients who underwent reoperative cardiac surgery were analyzed. Practice patterns and outcomes are described. Reoperative cardiac surgery for congenital heart disease is common, with one third of index operations in the database occurring subsequent to prior cardiothoracic operation(s) performed on cardiopulmonary bypass. This analysis suggests that a history of previous cardiac surgery does not independently confer a significant incremental risk of operative mortality, but that patients with greater number of previous operations appear to be at higher risk.
Background
Reoperations on patients with congenital heart disease are sometimes anticipated as part of a planned staged approach to some common types of complex congenital heart disease. In other instances, they address residual or recurrent hemodynamic burdens following reparative operations. And in some instances, they are mandated because of the limited durability and lack of growth potential of materials or devices implanted as part of a strategy of repair.1 In the present analysis, the Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD) was queried with the goal of obtaining multi-institutional descriptive data about practice patterns and outcomes associated with reoperative cardiac surgery.
The STS-CHSD is the largest database of pediatric and congenital cardiac operations in the world and, as of June 30, 2013, includes data from 120 congenital heart surgery hospitals in North America, 117 in the United States and three in Canada.2 The Report of the 2010 STS Congenital Heart Surgery Practice and Manpower Survey, undertaken by the STS Workforce on Congenital Heart Surgery, documented that 125 hospitals in the United States and eight hospitals in Canada perform pediatric and congenital heart surgery.3 Thus, the STS-CHSD contains data from 117 of the 125 hospitals (93.6% penetrance by hospital) in the United States and three of the eight centers in Canada.2
The purpose of this analysis was to query the STS-CHSD with the goal of obtaining multi-institutional descriptive data about practice patterns and outcomes associated with reoperative cardiac surgery in patients with pediatric and congenital cardiac disease.
Methods and Methods
Study Population
The STS-CHSD was queried for all index cardiac operations in the 5-year analytic window of January 1, 2007 to December 31, 2011, inclusive. The index operation of a hospitalization is defined as the first cardiac operation of that admission (that is, the first operation of that admission with operation type “CPB” or “No CPB Cardiovascular” [CPB = cardiopulmonary bypass]). Operations were excluded if any of the following criteria were met:
a Society of Thoracic Surgeons - European Association for Cardio-Thoracic Surgery Congenital Heart Surgery Mortality Category (STAT Mortality Category)4,5 is not available;
procedure is missing;
mortality status at hospital discharge is missing;
the number of prior CPB cardiothoracic operations is missing; or
the patient is ≤ 2,500g and undergoing ligation of a patent ductus arteriosus as their primary procedure.
Analytic Methods
As a surrogate for reoperation, operations in the STS-CHSD were stratified by the variable “Number of prior CPB cardio-thoracic operations.” This analysis is based on 92,603 index cardiac operations performed in the 5-year analytic window of 2007–2011, inclusive, and includes 61,930 index cardiac operations with zero prior CPB cardiothoracic operations and 30,673 index cardiac operations with one or more prior CPB cardiothoracic operations.
Because of the descriptive nature of this analysis, statistical comparisons are not made between subgroups. Mortality in this manuscript indicates mortality prior to discharge from the hospital and is reported in absolute numbers and as a percentage. Postoperative length of stay is reported as mean, median, and interquartile range.
Institutional Review Board Approval
This study was approved by the Duke University Health System Institutional Review Board. Because the data used in analysis represent a limited data set (no direct patient identifiers) that was originally collected for non-research purposes, and the investigators do not know the identity of individual patients, the analysis of these data was declared by the Duke University Health System Institutional Review Board to be research not involving human subjects.6
Results
In the 5-year analytic window of 2007–2011, of 92,603 index cardiac operations in the STS-CHSD, 67% (61,930) are with zero prior CPB cardiothoracic operations and 33% (30,673) are with one or more prior CPB cardio-thoracic operations.
Table 1 documents the number of patients who had 0, 1, 2, 3, 4, 5, or 6 or more prior CPB cardiothoracic operations and their discharge mortality.
Table 1.
Number of patients who had 0, 1, 2, 3, 4, 5, or 6 or more prior CPB cardiothoracic operations and their discharge mortality
| N | Discharge Mortality | Discharge Mortality (%) | |
|---|---|---|---|
| All index cardiac operations | 92,603 | 3,175 | 3.43 |
| Number of prior CPB cardiothoracic operations | |||
| 0 | 61,930 | 2,463 | 3.98 |
| 1 | 14,620 | 348 | 2.38 |
| 2 | 9,031 | 151 | 1.67 |
| 3 | 4,070 | 98 | 2.41 |
| 4 | 1,571 | 52 | 3.31 |
| 5 | 710 | 29 | 4.08 |
| 6 or more | 671 | 34 | 5.07 |
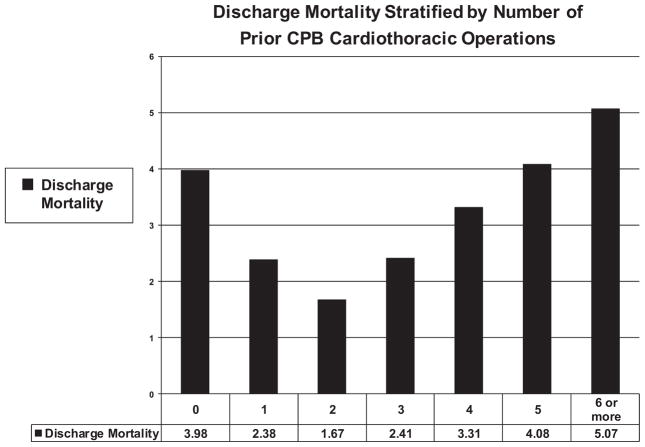
Figure 1 documents discharge mortality stratified by number of prior CPB cardiothoracic operations. Discharge mortality in patients with 0, 1, 2, 3, 4, 5, and 6 or more prior cardiopulmonary bypass operations was 3.98%, 2.38%, 1.67%, 2.41%, 3.31%, 4.08%, and 5.07%, respectively.
Figure 1.
Discharge mortality stratified by number of prior CPB cardiothoracic operations. Discharge mortality in patients with 0, 1, 2, 3, 4, 5, and 6 or more prior cardiopulmonary bypass operations was 3.98%, 2.38%, 1.67%, 2.41%, 3.31%, 4.08%, and 5.07%, respectively.
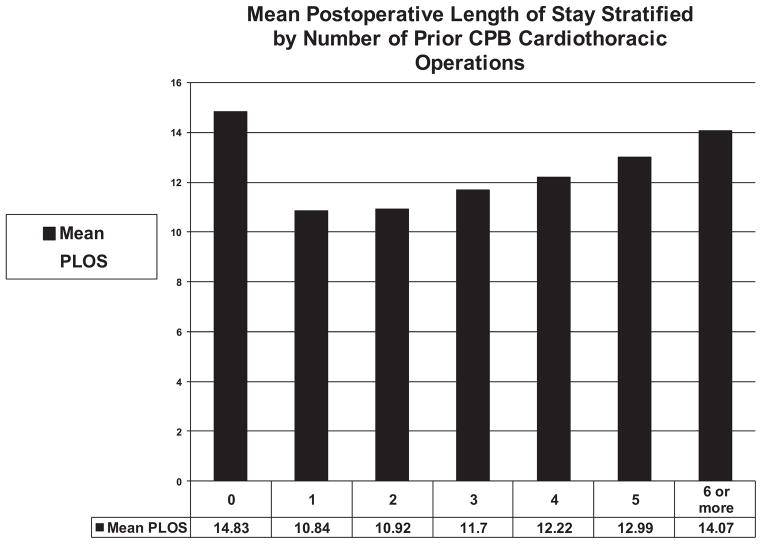
Figure 2 documents mean postoperative length of stay stratified by number of prior CPB cardiothoracic operations.
Figure 2.
Mean postoperative length of stay stratified by number of prior CPB cardiothoracic operations.
Table 2 documents the 10 most common primary procedures where the number of prior CPB cardiothoracic operations is ≥ 1, for all patients, and then stratified by the following four age groupings: neonates (0–30 days), infants (31 days–1 year), children (>1 year – < 18 years), and adults (≥ 18 years). Readmission for reoperative cardiac surgery is rare in neonates. In infants, the most common reoperation is superior cavopulmonary anastomosis(es). In children, the most common reoperation is the Fontan operation, and in adults, the most common reoperation is pulmonary valve replacement.
Table 2.
Ten Most Common Primary Procedures of Index Operations with One or More Previous Sternotomy
| Procedure | No. Eligible | No. with Mortality Status at Discharge = Dead | Discharge Mortality(%) | Postop LOS (days)
|
|||
|---|---|---|---|---|---|---|---|
| Median | 25th | 75th | Mean | ||||
| All Patients | |||||||
| BDCPA (bidirectional Glenn) | 2480 | 33 | 1.33 | 6 | 4 | 9 | 10.74 |
| PVR | 1970 | 8 | 0.41 | 4 | 3 | 5 | 5.24 |
| Fontan, TCPC, External conduit, Fenestrated | 1870 | 24 | 1.82 | 9 | 7 | 15 | 14.23 |
| Conduit reoperation | 1732 | 14 | 0.81 | 5 | 4 | 7 | 7.26 |
| Pacemaker procedure | 1642 | 5 | 0.30 | 1 | 0 | 2 | 2.77 |
| Fontan, TCPC, External conduit, Nonfenestrated | 1403 | 22 | 1.57 | 9 | 7 | 14 | 13.29 |
| Pacemaker implantation, Permanent | 927 | 13 | 1.40 | 3 | 1 | 4 | 4.73 |
| Fontan, TCPC, Lateral tunnel, Fenestrated | 895 | 6 | 0.67 | 9 | 7 | 13 | 12.38 |
| Superior Cavopulmonary anastomosis(es) + PA reconstruction | 856 | 20 | 2.34 | 7 | 5 | 13 | 14.46 |
| Valvuloplasty, Mitral | 826 | 16 | 1.94 | 5 | 4 | 9 | 11.18 |
| Neonates (0 to 30 days) | |||||||
| ASO | 48 | 1 | 2.08 | 12 | 8 | 21 | 21.5 |
| MBTS | 38 | 5 | 13.16 | 15 | 8 | 30 | 22.7 |
| Norwood procedure | 37 | 8 | 21.62 | 35 | 15 | 58 | 43.7 |
| TAPVC repair | 18 | 10 | 55.56 | 28.5 | 12 | 45 | 35.1 |
| ASO and VSD repair | 18 | 0 | 0.00 | 12 | 9 | 27 | 24 |
| Aortic arch repair | 11 | 2 | 18.18 | 20 | 14 | 63 | 54.9 |
| PAB | 11 | 4 | 36.36 | 29 | 14 | 51 | 36.3 |
| Coarctation repair, end-to-end, Extended | 8 | 0 | 0.00 | 26 | 7 | 50.5 | 32.4 |
| PDA closure, Surgical | 8 | 2 | 25.00 | 12.5 | 10 | 36.5 | 26 |
| Coarctation repair, end-to-end | 7 | 1 | 14.29 | 18 | 4 | 43 | 24 |
| Infants (31 days to 1 year) | |||||||
| BDCPA (bidirectional Glenn) | 2271 | 29 | 1.28 | 6 | 5 | 10 | 10.9 |
| Superior cavopulmonary anastomosis(es) + PA reconstruction | 807 | 19 | 2.35 | 7 | 5 | 13 | 14.7 |
| Bilateral BDCPA (bilateral bidirectional Glenn) | 314 | 7 | 2.23 | 7 | 5 | 12 | 13.9 |
| HemiFontan | 311 | 8 | 2.57 | 7 | 5 | 13 | 13.4 |
| TOF repair, Ventriculotomy, Transanular patch | 266 | 2 | 0.75 | 7 | 5 | 12 | 11.7 |
| VSD repair, Patch | 204 | 3 | 1.47 | 7 | 5 | 14 | 14.5 |
| PA, reconstruction (plasty), Branch, Central (within the hilar bifurcation) | 201 | 13 | 6.47 | 8 | 5 | 24 | 20.7 |
| Aortic arch repair | 198 | 9 | 4.55 | 10 | 6 | 22 | 21.2 |
| AVC (AVSD) repair, Complete (CAVSD) | 185 | 8 | 4.32 | 11 | 7 | 22 | 21.0 |
| Pulmonary venous stenosis repair | 185 | 22 | 11.89 | 12 | 7 | 25 | 20.8 |
| Children (>1 year to 18 years) | |||||||
| Fontan, TCPC, External conduit, Fenestrated | 1852 | 29 | 1.57 | 9 | 7 | 15 | 14.3 |
| Conduit reoperation | 1435 | 10 | 0.70 | 5 | 4 | 6 | 6.5 |
| Fontan, TCPC, External conduit, Nonfenestrated | 1375 | 20 | 1.45 | 9 | 7 | 14 | 13.3 |
| PVR | 1186 | 6 | 0.51 | 4 | 3 | 5 | 4.9 |
| Pacemaker procedure | 1173 | 2 | 0.17 | 1 | 0 | 2 | 2.7 |
| Fontan, TCPC, Lateral tunnel, Fenestrated | 887 | 6 | 0.68 | 9 | 7 | 13 | 12.3 |
| Pacemaker implantation, Permanent | 690 | 4 | 0.58 | 2 | 1 | 4 | 3.8 |
| Aortic stenosis, Subvalvar, Repair | 678 | 2 | 0.29 | 4 | 3 | 5 | 4.9 |
| Valvuloplasty, Mitral | 635 | 6 | 0.94 | 5 | 4 | 7 | 8.2 |
| Conduit placement, RV to PA | 527 | 4 | 0.76 | 5 | 4 | 6 | 6.6 |
| Adults (>18 years) | |||||||
| PVR | 764 | 2 | 0.26 | 5 | 4 | 6 | 5.4 |
| Pacemaker procedure | 427 | 1 | 0.23 | 1 | 0 | 1 | 1.6 |
| Conduit reoperation | 185 | 3 | 1.62 | 5 | 4 | 6 | 6 |
| Arrhythmia surgery - atrial, Surgical Ablation | 176 | 6 | 3.41 | 7 | 6 | 11 | 10.8 |
| Fontan revision or conversion (Re-do Fontan) | 149 | 15 | 10.07 | 9 | 7 | 13 | 12.2 |
| Pacemaker implantation, Permanent | 148 | 3 | 2.03 | 3 | 1 | 4 | 4.2 |
| Conduit placement, RV to PA | 121 | 4 | 3.31 | 5 | 4 | 6 | 6.3 |
| Aortic aneurysm repair | 97 | 3 | 3.09 | 7 | 5 | 9 | 9.7 |
| ICD (AICD) implantation | 94 | 1 | 1.06 | 1 | 1 | 4 | 3.0 |
| PA, reconstruction (plasty), Branch, Central (within the hilar bifurcation) | 91 | 1 | 1.10 | 5 | 4 | 6 | 6.0 |
Abbreviations: BDCPA, bidirectional cavopulmonary anastomosis; PVR, valve replacement, pulmonic; TCPC, total cavopulmonary connection; ASO, arterial switch operation; MBTS, modified Blalock-Taussig shunt; TAPVC, total anomalous pulmonary venous connection; VSD, ventricular septal defect; PAB, PA banding; PDA, patent ductus arteriosus; AVSD, atrioventricular septal defect; CAVSD, complete atrioventricular septal defect; ICD, implantable cardioverter defibrillator; AICD, automatic implantable cardioverter defibrillator.
Discussion
Since the 1980s, advances in operative techniques and peri-operative critical care of neonates and infants with congenital cardiac disease have resulted in the vast majority of these patients living into the adult years. One result of this success is that an increasing number of congenital cardiac operations are now reoperations. A second result of this success is that as of 2000, more adults than children are alive with congenital heart disease.7 The need for reoperation(s) for residual or recurrent hemodynamic burdens in many of these patients, together with the established benefits of a staged approach to many common types of complex congenital cardiac disease, has resulted in the expectation that many patients undergoing surgical treatment of congenital heart disease will require multiple operations over their lifetime.
Overall, the most common operations performed with one or more previous CPB operations in the STS-CHSD are superior cavopulmonary anastomosis(es) and Fontan operations, reflecting the prevalence of staged reconstruction for anomalies that do not lend themselves to complete repair in one stage. Although readmission for reoperative cardiac surgery is rare in neonates, those neonates that do undergo reoperation in the neonatal period are at especially high risk. As shown in Table 1, the discharge mortality for modified Blalock-Taussig shunt, Norwood procedure, repair of total anomalous pulmonary venous connection, aortic arch repair, and pulmonary artery banding when these operations are performed in neonates with one or more previous CPB operations is 13.16%, 21.62%, 55.56%, 18.18%, and 36.36%, respectively. Although these operations are rarely performed in neonates with one or more previous CPB operations, they appear to be especially high risk.
Single institutional data from the Mayo clinic has documented that increased early mortality after reoperation is associated with an increase in number of reoperative sternotomies (Fig. 3).1 Multi-institutional data from the STS-CHSD supports this concept. The value of multi-institutional data is exemplified by outcomes after Fontan revision or conversion (re-do Fontan). Fontan conversion as initially developed was associated with an early mortality of 0.9% (one out of 111 patients with a mean age 22.5 ± 7.9 years) in a single institutional experience.8 These excellent results have not been replicated in the STS-CHSD, where the early mortality after Re-do Fontan is 10.07% (15 out of 149 patients).
Figure 3.
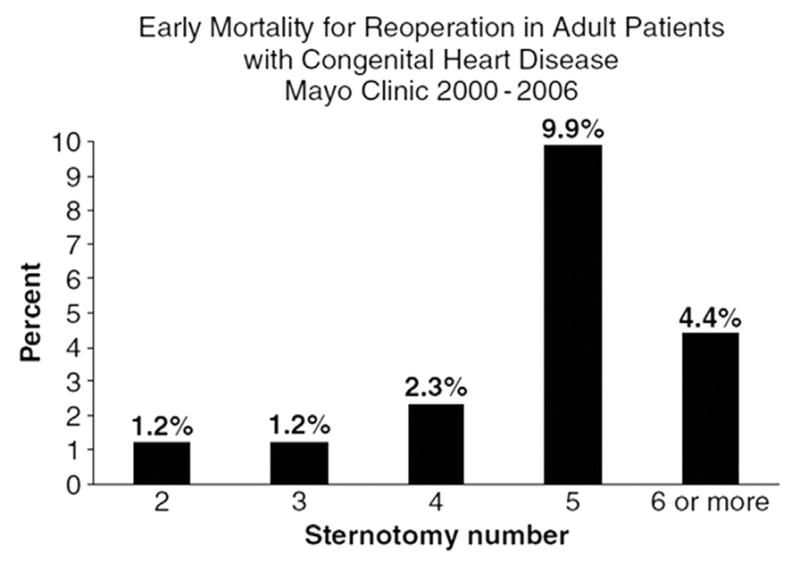
Single institutional data from the Mayo clinic which documents that increased early mortality after reoperation is associated with an increase in number of reoperative sternotomies.1
This manuscript confirms the results of two seemingly conflicting reports.1,9 On the one hand, re-sternotomy in and of itself, and reoperative surgery in general, has been shown to be well-tolerated and not to be a significant risk factor for operative mortality.9 On the other hand, by the time a patient reaches four or five re-sternotomies, a greater risk of mortality clearly exists.1 It is not known whether this increased risk is related to the technical challenges of safe entry and dissection after so many previous sternotomies, or whether it is more the case that four or five previous operations is a marker for complex heart disease with residual hemodynamic burdens that may affect myocardial function and even other organ systems. Meanwhile, a third analysis of 1,000 repeat sternotomies for congenital cardiac surgery was published in 200910 with results that are seemingly between the experience from the Mayo Clinic1 and Texas Children’s Hospital.9 In the Emory experience,10 the risk of injury at re-entry went up with increased number of prior sternotomies, but the risk of mortality did not increase with injury at re-entry. In the series from Emory, risk factors for injury were presence of a right ventricle-pulmonary artery conduit and sternotomy number; furthermore, operative mortality was associated with sternotomy number but not re-entry injury.
The increasing number of resternotomies has led to the development of a variety of surgical strategies designed to minimize the risk of re-entrant sternotomy. The use of pericardial substitutes to aid in future re-entrant median sternotomy is believed to minimize the risk of catastrophic cardiac injury.11 Similarly, novel technical strategies such as the use of anterior sternal retraction with the Rultract retractor (Cleveland, OH) have been developed to minimize the risk of re-entrant median sternotomy (Figs. 4 and 5).12
Figure 4.
Use of the anterior sternal retraction of the lower sternum with the Rultract retractor at the time of re-entrant median sternotomy.
Figure 5.
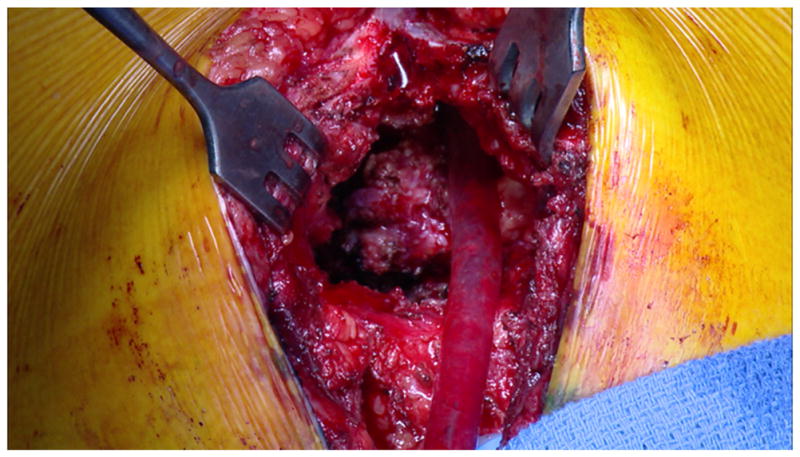
Use of the anterior sternal retraction of the lower sternum with the Rultract retractor at the time of re-entrant median sternotomy, demonstrating how the heart can be separated from the sternum under direct vision.
Limitations of this analysis include the retrospective nature of this review and the lack of risk adjustment. Future analyses that incorporate risk adjustment represent a potential future direction of research. An additional limitation relates to the fact that as a surrogate for reoperation, operations in the STS-CHSD were stratified by the variable “Number of prior CPB cardiothoracic operations.” This strategy overlooks patients who underwent non-bypass procedures via sternotomy, such as systemic-to –pulmonary artery shunts, pulmonary artery band(s), hybrid procedures (such as pulmonary artery bands with ductal stents), and off-pump pulmonary outflow patches. This strategy also overlooks patients with previous thoracotomies (such as repair of coarctation, repair of coarctation combined with pulmonary artery band, etc.), which do not necessitate re-sternotomy but may represent a higher degree of complexity. This limitation is unavoidable using the strategy used in this analysis.
Another limitation is that because this analysis is based on the index operation of a surgical admission, reoperations performed during the same hospitalization are excluded. This fact may partially explain the relatively small number of reoperations on neonates detected in this analysis.
This analysis does not allow determination as to whether increased risk is associated with re-operation itself or whether risk is inherent to the operation that is being performed and would not necessarily be different whether the patient had a previous operation or not. Because this analysis does not include adjustment for case complexity, it may just be that those patients in the categories with more previous operations are undergoing a current procedure in a higher risk category. This report is intended to be descriptive. In the absence of risk adjustment and without details of some intra-operative events, it is not possible to ascertain how much risk, if any, is associated with sternal re-entry itself, and how much is related to severity of patient disease and overall procedural complexity. These patients are challenging for many reasons, only some of which are related to the technical challenges of re-entry. Nevertheless, this multi-institutional descriptive analysis does provide useful data about patterns of practice and outcomes associated with reoperative cardiac surgery in patients with pediatric and congenital cardiac disease.
Conclusion
This descriptive analysis of patients in the STS-CHSD who underwent reoperative cardiac surgery after prior CPB cardiothoracic operations provides previously unavailable data concerning practice patterns and outcomes. Reoperative cardiac surgery for congenital heart disease is common, representing one third of all index cardiac operations in the STS-CHSD. This analysis suggests that although a history of previous cardiac surgery does not independently confer a significant incremental risk of operative mortality, patients with the greatest number of previous operations appear to be a higher risk group.
Acknowledgments
Dr. Kevin Hill reports receiving grant support from Gilead Scholars in CV Diseas, Mend A Heart Foundation, and the US FDA.
References
- 1.Dearani JA, Connolly HM, Martinez R, et al. Caring for adults with congenital cardiac disease: successes and challenges for 2007 and beyond. Cardiol Young. 2007;17(suppl 2):87–96. doi: 10.1017/S1047951107001199. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs JP, Jacobs ML, Mavroudis C, et al. Executive summary: The Society of Thoracic Surgeons Congenital Heart Surgery Database – Nineteenth Harvest – (July 1, 2009 – June 30, 2013) The Society of Thoracic Surgeons (STS) and Duke Clinical Research Institute (DCRI), Duke University Medical Center; Durham, North Carolina, United States: Fall. 2013. Harvest. [Google Scholar]
- 3.Jacobs ML, Daniel M, Mavroudis C, et al. Report of the 2010 Society of Thoracic Surgeons Congenital Heart Surgery Practice and Manpower Survey. Ann Thorac Surg. 2011;92:762–769. doi: 10.1016/j.athoracsur.2011.03.133. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs JP, Jacobs ML, Maruszewski B, et al. Initial application in the EACTS and STS Congenital Heart Surgery Databases of an empirically derived methodology of complexity adjustment to evaluate surgical case mix and results. Eur J Cardiothorac Surg. 2012;42:775–780. doi: 10.1093/ejcts/ezs026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dokholyan RS, Muhlbaier LH, Falletta J, et al. Regulatory and ethical considerations for linking clinical and administrative databases. Am Heart J. 2009;157:971–982. doi: 10.1016/j.ahj.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Dearani JA, Mavroudis C, Quintessenza JA, et al. Surgical advances in the treatment of adults with congenital heart disease. Curr Opin Pediatr. 2009;21(5):565–572. doi: 10.1097/MOP.0b013e3283303fa7. [DOI] [PubMed] [Google Scholar]
- 8.Mavroudis C, Deal BJ, Backer CL, et al. J. Maxwell Chamberlain Memorial Paper for congenital heart surgery. 111 Fontan conversions with arrhythmia surgery: surgical lessons and outcomes. Ann Thorac Surg. 2007;84:1457–1465. doi: 10.1016/j.athoracsur.2007.06.079. [discussion, 1465–1466] [DOI] [PubMed] [Google Scholar]
- 9.Morales DL, Zafar F, Arrington KA, et al. Repeat sternotomy in congenital heart surgery: no longer a risk factor. Ann Thorac Surg. 2008;86:897–902. doi: 10.1016/j.athoracsur.2008.04.044. discussion, 897–902. [DOI] [PubMed] [Google Scholar]
- 10.Kirshbom PM, Myung RJ, Simsic JM, et al. One thousand repeat sternotomies for congenital cardiac surgery: risk factors for reentry injury. Ann Thorac Surg. 2009;88:158–161. doi: 10.1016/j.athoracsur.2009.03.082. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs JP, Iyer RS, Weston JS, et al. Expanded PTFE membrane to prevent cardiac injury during resternotomy for congenital heart disease. Ann Thorac Surg. 1996;62:1778–1782. doi: 10.1016/s0003-4975(96)00610-8. [DOI] [PubMed] [Google Scholar]
- 12.Mavroudis C, Deal BJ, Backer CL, Stewart RD. Operative techniques in association with arrhythmia surgery in patients with congenital heart disease. World J Pediatr Cong Heart Surg. 2013;4:85–97. doi: 10.1177/2150135112449842. [DOI] [PubMed] [Google Scholar]