Abstract
Objective: Certolizumab pegol (CZP) is a novel anti-TNF agent that is used for patients with moderate to severe active rheumatoid arthritis (RA). However, the efficacy of CZP in RA remains controversial. Thus, we performed this meta-analysis to assess the efficacy and safety of CZP in the treatment of RA patients. Methods: Eligible studies were randomized controlled trials (RCTs) that evaluated the efficacy and safe of CZP in the patients with active RA. The primary outcome was American College of Rheumatology 20% (ACR20), and secondary outcome were ACR50, ACR70, disease activity, patient-reported outcomes (PROs), and adverse events. A fixed-effect model or random-effect model was used to pool the estimates, depending on the absence or presence of heterogeneity among the included studies. Results: Nine RCTs with a total of 5228 patients were included in this meta-analysis, and all of the patients were administered CZP or placebo. The pooled results showed that CZP significantly improved the ACR20, ACR50, ACR70 response rates, and physical function. CZP was associated with a statistically significant reduction in Disease Activity Score in 28 joints-Erythrocyte sedimentation rate, arthritis pain, and fatigue. Patients who received CZP treatment did not have a higher incidence of treatment-related adverse events, no matter in any intensity. Conclusions: CZP 200 or 400mg in the treatment of active RA significantly reduced the RA signs and symptoms, and improved physical function as compared with the placebo. More large-scale RCTs are needed to evaluate the long-term efficacy and safety of CZP in the treatment of active RA.
Keywords: Certolizumab pegol, rheumatoid arthritis, meta-analysis
Introduction
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease characterized by synovial lining of joints, tendons, and periarticular structures [1]. The joint inflammation leads to tenderness and pain, which reduces the quality of life of patients. Proinflammatory cytokine, produced by a variety of immune cells, would prolong the joint inflammation if patients were untreated. Among these cytokines, tumor necrosis factor-α (TNFα) plays an important role in the inflammatory process, which can activate the immune cell and cause chronic inflammation [2]. Thus, TNFα became a fundamental target of anti-RA therapy. TNF inhibitors, which were first developed at the begging of the 2000s, have shown significant benefits in the treatment of RA. These agents can significantly improve the signs and symptoms of RA [3,4], inhibit the progression of joint destruction [5,6], and improve physical function and health-related quality of life (HEQ-oL) [5,6], when used in combination with methotrexate (MTX).
Certolizumab pegol (CZP) is a relatively new member of the TNFα inhibitor family, which composed of a humanized Fab’ fragment combined with polyethylene glycol (PEG) to increase its half-life [7]. As a Fc-free TNFα inhibitor, CZP does not lead to the formation of immune complexes, activation of complement, and the produce of antibody-dependent cytotoxicity [7]. The efficacy and safety of CZP in the treatment of active RA have been demonstrated in several studies [8,9]. CZP, is clinically used in combination with MTX for the treatment of patients with active RA, who failed to response to disease-modifying antirheumatic drug (DMARD). CZP can also be given as monotherapy in those who cannot tolerate MTX [10].
An early meta-analysis published in 2010 [11] compared the clinical efficacy of CZP with that of other anti-cytokine agents for the treatment of RA, and the authors found that CZP had a highest OR for ACR20 response, among these anti-cytokines agents. This meta-analysis confirmed the effects of CZP in the treatment of RA, however, the efficacy assessment of CZP was performed based on only two randomized controlled trials (RCTs), which gave a less statistical power to pool the estimates. And with the widely used of CZP around the world, more and more large-scale RCTs that evaluating the efficacy and safety of CZP have been conducted, thus, it is necessary to update the meta-analysis and provide a quantitative assessment of CZP in the treatment of RA. Moreover, in this updated meta-analysis, we also assess the effect of CZP in disease activity and patient-reported outcomes (PROs), as well as treatment-related adverse events, which had not been discussed in previous meta-analysis.
Methods
Literature search
Pubmed, Embase, and Web of Science databases (up to June 14, 2014) were searched to identify relevant studies, which assessed the efficacy and safety of CZP in the treatment of RA patients. The search items were generated as: (“certolizumab pegol” [Supplementary Concept] or “certolizumab pegol” [All Fields]) and (“arthritis, rheumatoid” [MeSH Terms] or (“arthritis” [All Fields] and “rheumatoid” [All Fields]) or “rheumatoid arthritis” [All Fields] or (“rheumatoid” [All Fields] and “arthritis” [All Fields]). The search was limited to human and RCTs, and no language limitation was imposed. We also reviewed the references cited in the included studies, to identify additional articles. When several trials with the same population were reported, we only chose the study with most informative or the latest data.
Study inclusion and exclusive criteria
All the published studies were included if they met the following inclusion criteria: (1) used a randomized controlled study design; (2) patients were ≥ 18 years old, and had a diagnosis of RA; (3) patients in the study arm were administered CZP or CZP-based therapy, while those in the control arm were administered placebo therapy; (4) evaluated the efficacy and safety of CZP in the treatment of AS; (5) provided interest data of American College of Rheumatology 20% (ACR20), ACR50, ACR70 response rates, disease activity and PROs, and the adverse events. Any studies that met the following exclusion criteria were excluded: (1) non-RCTs; (2) patients in the study group did not receive CZP or CZP-based therapy; (3) patients were aged less than 18 years old, or diagnosed with any other disease; (4) the study did not provide data of interest.
Data extraction and quality assessment
Two investigators (Qing Zhou and Yaodong Zhou) independently reviewed the eligible study and extracted the information as follows: the first author’s name, year of publication, mean age, sample size (number of patients in the study arm and control arm), treatment regimen, ACR20/ACR50/ACR70 response rate, Health Assessment Questionnaire disability index (HAQ-DI) rate, mean and SE of Disease Activity Score in 28 joints-Erythrocyte sedimentation (DAS28-ESR) rate, and adverse events. The disagreements between two investigators were resolved by discussion and consensus.
To assess the methodological quality of each study, Jadad scale [12] was used in this meta-analysis. This scale consists of three items, including randomization (0-2 points), blinding (0-2 points), and dropouts and withdraws (0-1 point) to describe the quality of a RCT [12]. The full score is 5 and high score indicates better reporting. Studies with a score ≥ 3 points are considered as high quality [13].
Statistical analysis
We assessed the efficacy and safety of CZP in the treatment of AS based on the data from included studies. Cochran’s Q statistic and the I2 statistic were used to test the heterogeneity among the included studies, in which I2 > 50% or chi-squared test P < 0.1 were considered as the existence of substantial heterogeneity. The meta-analysis was carried out to pool the estimates of the risk ratio (RR), or Weighted Mean Difference (WMD) and 95% confidence intervals (CIs), using a fixed-effect model [14] when there is no existence of substantial heterogeneity. Otherwise, a random-effect model [15] was used to calculate the estimates. We also intended to perform subgroup analysis according to the dosage of CZP. Publication bias was assessed by using the Begg [16] and Egger test [17]. A P value less than 0.05 was judged as statistically significant. All statistical analyses were conducted by using of STATA software version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Identification of eligible studies
A total of 226 studies were identified in the literature search, including 145 articles in Pubmed, 65 articles in Embase, and 16 articles in Web of Science. After a check for duplicates, 64 articles were removed, leaving 162 articles for abstract/title and full-text review. Of these, 145 were excluded after the abstract and title review, leaving 17 for full text review. Among the remaining 17 studies, 8 were excluded for the following reasons: five studies were single-arm study design [18-22]; two studies reported outcomes unrelated with our topics [23,24]; one study were excluded for no data available [25]. Finally, nine studies with a total of 5228 patients were included in this meta-analysis [8,9,26-32] (Figure 1). Of these included studies, the RA Prevention of Structural Damage 1 (RAPID1) [8,30] trial and RAPID2 [9,31] trial present two publications, respectively. However, both of the two publications reported different variables, thus, we included both of these publications.
Figure 1.
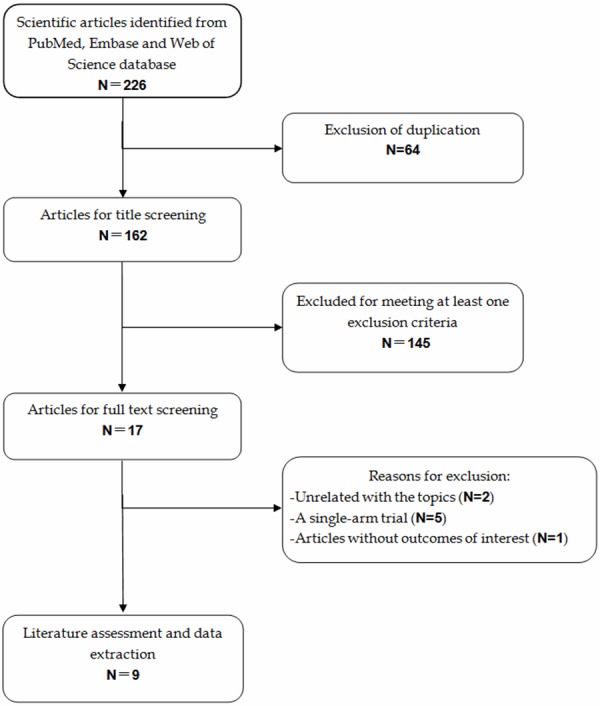
Identification of eligible randomized-controlled trials.
Characteristics of eligible studies and quality assessment
Table 1 illustrated the baseline characteristics of the included studies. All studies for inclusion were selected strictly according to prior inclusion criteria. The sample size of included studies ranged from 194 to 1063. There were no statistical differences in the patients’ baseline characteristics or disease activity status between the study group and control group. Among the 5228 eligible patients, 4122 (78.8%) of them were female, and 1106 (21.2%) were male. Patients in three trials [27,29,32] were administered CZP 400mg plus MTX or placebo, while patients in the remaining six trials [8,9,26,28,30,31] received additional treatment regimens of CZP 200 mg plus MTX. Rheumatoid factor (RF) (≥ 14 IU/ml) positive were diagnosed in approximately 77.7% of the patients. According to the quality criteria, all of the nine studies had a high quality, and the median Jadad score was 4 (range from 4 to 5).
Table 1.
Baseline characteristics of the trials included in the meta-analysis
| Study | Treatment | No. of patients | Age (mean ± SD, year) | Male/Female | Duration of disease (mean ± SD, year) | RF positive (%) (≥ 14 IU/ml) | HAQ-DI, mean ± SD | DAS28-ESR, mean ± SD | Jadad scale |
|---|---|---|---|---|---|---|---|---|---|
| Keystone E [8] | Placebo + MTX | 199 | 52.2 ± 11.2 | 32/167 | 6.2 ± 4.4 | 82.8 | 1.7 ± 0.6 | 7.0 (4.9, 8.7)* | 5 |
| CZP 200 mg + MTX | 393 | 51.4 ± 11.6 | 69/324 | 6.1 ± 4.2 | 79.6 | 1.7 ± 0.6 | 6.9 (4.3, 8.9)* | ||
| CZP 400 mg +MTX | 390 | 52.4 ± 11.7 | 64/326 | 6.2 ± 4.4 | 83.6 | 1.7 ± 0.6 | 6.9 (4.8, 9.1)* | ||
| Yamamoto K [26] | Placebo + MTX | 77 | 51.9 ± 11.1 | 11/66 | 5.8 ± 4.1 | 85.7 | 1.2 ± 0.7 | 6.5 ± 0.9 | 5 |
| CZP 200 mg +MTX | 82 | 50.6 ± 11.4 | 13/69 | 5.6 ± 4.2 | 86.6 | 1.1 ± 0.7 | 6.2 ± 0.8 | ||
| CZP 400 mg + MTX | 85 | 55.4 ± 10.3 | 16/69 | 6.0 ± 3.9 | 89.4 | 1.1 ± 0.6 | 6.3 ± 0.8 | ||
| Fleischmann R [27] | Placebo | 109 | 54.9 ± 11.6 | 12/97 | 10.4 ± 9.6 | 100.0 | 1.6 ± 0.65 | 6.3 ± 0.9 | 4 |
| CZP 400 mg | 111 | 52.7 ± 12.7 | 24/87 | 8.7 ± 8.2 | 100.0 | 1.4 ± 0.63 | 6.3 ± 1.1 | ||
| Smolen J [9] | Placebo + MTX | 127 | 51.5 ± 11.8 | 20/107 | 5.6 ± 3.9 | 78.2 | 1.6 ± 0.6 | 6.83 ± 0.87 | 4 |
| CZP 200 mg + MTX | 246 | 52.2 ± 11.1 | 40/206 | 6.1 ± 4.1 | 77.5 | 1.6 ± 0.6 | 6.85 ± 0.84 | ||
| CZP 400 mg + MTX | 246 | 51.9 ± 11.8 | 54/192 | 6.5 ± 4.3 | 75.5 | 1.6 ± 0.6 | 6.80 ± 0.79 | ||
| Landewé R [28] | Placebo + MTX | 107 | 39.9 ± 12.4 | 65/42 | 7.7 (0.3-50.9)* | NR | NR | NR | 4 |
| CZP 200 mg + MTX | 111 | 39.1 ± 11.9 | 67/44 | 6.9 (0.3-34.2)* | NR | NR | NR | ||
| CZP 400 mg + MTX | 107 | 39.8 ± 11.3 | 68/39 | 7.9 (0.3-44.8)* | NR | NR | NR | ||
| Smolen JS [29] | Placebo | 98 | 54 ± 12.4 | 23/75 | 4.7 ± 3.3 | 67.3 | 1.0 ± 0.6 | NR | 5 |
| CZP 400 mg | 96 | 53.6 ± 11.9 | 15/81 | 4.5 ± 3.5 | 74.0 | 1.1 ± 0.6 | NR | ||
| Strand V [30] | CZP 200 mg + MTX | 393 | 51.4 ± 11.6 | 69/324 | 6.1 ± 4.2 | 79.6 | 1.7 ± 0.6 | 6.9 (4.3, 8.9)* | 5 |
| CZP 400 mg + MTX | 390 | 52.4 ± 11.7 | 64/326 | 6.2 ± 4.4 | 83.6 | 1.7 ± 0.6 | 6.9 (4.8, 9.1)* | ||
| Placebo + MTX | 199 | 52.2 ± 11.2 | 32/167 | 6.2 ± 4.4 | 82.8 | 1.7 ± 0.6 | 7.0 (4.9, 8.7)* | ||
| Strand V [31] | Placebo + MTX | 127 | 51.5 ± 11.8 | 20/107 | 5.6 ± 3.9 | 78.2 | 1.6 ± 0.6 | 6.83 ± 0.87 | 4 |
| CZP 200 mg + MTX | 246 | 52.2 ± 11.1 | 40/206 | 6.1 ± 4.1 | 77.5 | 1.6 ± 0.6 | 6.85 ± 0.84 | ||
| CZP 400 mg + MTX | 246 | 51.9 ± 11.8 | 54/192 | 6.5 ± 4.3 | 75.5 | 1.6 ± 0.6 | 6.80 ± 0.79 | ||
| Weinblatt ME [32] | Placebo | 212 | 53.9 ± 12.7 | 43/169 | 8.9 ± 9.1 | 76.5 | 1.6 ± 0.6 | 5.7 ± 0.9 | 4 |
| CZP 400 mg | 851 | 55.4 ± 12.4 | 191/660 | 8.6 ± 8.8 | 73.9 | 1.5 ± 0.6 | 5.7 ± 0.9 |
data are expressed with median (minimum, maximum).
MTX, methotrexate; CZP, certolizumab pegol; RF, rheumatoid factor; HAQ, Health Assessment Questionnaire; DI, disability index; DAS28, Disease Activity Score 28-joint assessment; ESR, erythrocyte sedimentation rate.
Primary endpoint: ACR20
Seven studies reported the data of ACR20 response rate [8,9,26-29,32]. The aggregated results showed that CZP plus MTX significantly improved ACR20 response rate in the treatment of RA (RR = 3.05, 95% CI: 2.33, 3.99; P = 0.000) (Figure 2). The test for heterogeneity was significant (P = 0.000, I2 =84.6%). Thus, we performed subgroup analysis to identify the potential sources of heterogeneity.
Figure 2.

Comparison of certolizumab pegol (CZP) with placebo for active ankylosing spondylitis (AS) patients in terms of ACR20 response rate.
Subgroup analysis based on CZP dosage showed that, RA patients who were treated with 200 mg or 400 mg of CZP plus MTX had a higher ACR20 response rate, compared with those treated with placebo (for CZP 200 mg: RR = 3.14, 95% CI: 1.87, 5.27, P = 0.000; for CZP 400 mg: RR = 3.01, 95% CI: 2.16, 4.19, P = 0.000) (Figure 2).
Subgroup analysis based on the different time-endpoints suggested that ACR20 response rates at either week 12 or 24 were superior in the CZP plus MTX group than that in the placebo group (at week 12: RR = 2.00, 95% CI: 1.60, 2.48, P = 0.000; at week 24: RR = 4.04, 95% CI: 3.16, 5.18, P = 0.000) (Figure 3). The Begg’s and Egger’s test suggested that there were no publication bias (for Begg’s test, Z = 0.99, P = 0.322; for Egger’s test, t = 0.12, P = 0.907).
Figure 3.
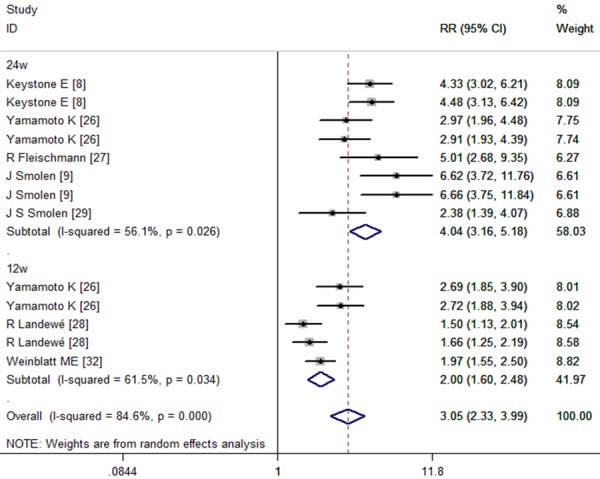
Subgroup analysis of ACR20 response rate for the treatment of certolizumab pegol (CZP) versus placebo.
Secondary endpoints: ACR50, ACR70
Seven of the included studies provided data of ACR50 response rate [8,9,26-29,32]. The pooled estimates using random-effect model showed that CZP plus MTX significantly increase ACR50 response rate in the treatment of active RA patients (RR=4.03, 95% CI: 3.15, 5.16; P = 0.000) (Figure 4). According to the subgroup analysis based on different CZP dosage and time end-points, we found that, at any time point examined, ACR50 response rates for those receiving 200 mg or 400 mg of CZP plus MTX were significant higher than those treated with placebo alone (for CZP 200 mg: RR = 4.19, 95% CI: 2.68, 6.54, P = 0.000; for CZP 400 mg: RR = 3.99, 95% CI: 2.90, 5.49, P = 0.000; at week 12: RR = 3.13, 95% CI: 2.47, 3.95, P = 0.000; at week 24: RR = 5.04, 95% CI: 4.00, 6.36, P = 0.000) (Figures 4, 5).
Figure 4.
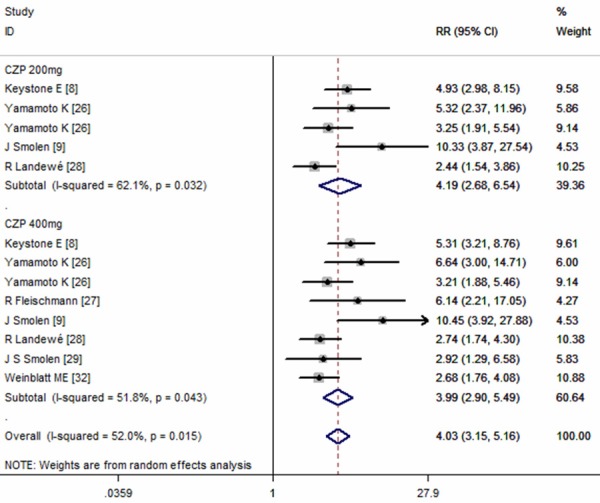
Comparison of certolizumab pegol (CZP) with placebo for active ankylosing spondylitis (AS) patients in terms of ACR50 response rate.
Figure 5.
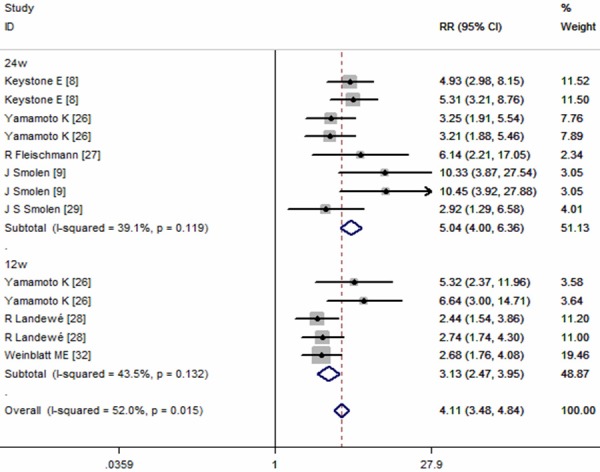
Subgroup analysis of ACR50 response rate for the treatment of certolizumab pegol (CZP) versus placebo.
Six studies reported the data in ACR70 [8,9,26,27,29,32]. The pooled results using a fixed-effect model indicated that, RA patients treated with CZP plus MTX had benefit in ACR70, compared to those treated with placebo (RR = 8.66, 95% CI: 5.93, 12.65; P = 0.000) (Figure 6). Subgroup analysis based on different CZP dosage and time end-points were performed, and the results showed that, CZP plus MTX significantly improved ACR70 response rate in the treatment of RA patients, irrespective of the CZP dosage or time point examined (for CZP 200 mg: RR = 10.08, 95% CI: 5.11, 19.89, P = 0.000; for CZP 400 mg: RR = 6.52, 95% CI: 3.90, 10.88, P = 0.000; at week 12: RR = 7.76, 95% CI: 3.85, 15.65, P = 0.000; at week 24: RR = 9.06, 95% CI: 5.77, 14.22, P = 0.000) (Figures 6, 7).
Figure 6.

Comparison of certolizumab pegol (CZP) with placebo for active ankylosing spondylitis (AS) patients in terms of ACR70 response rate.
Figure 7.
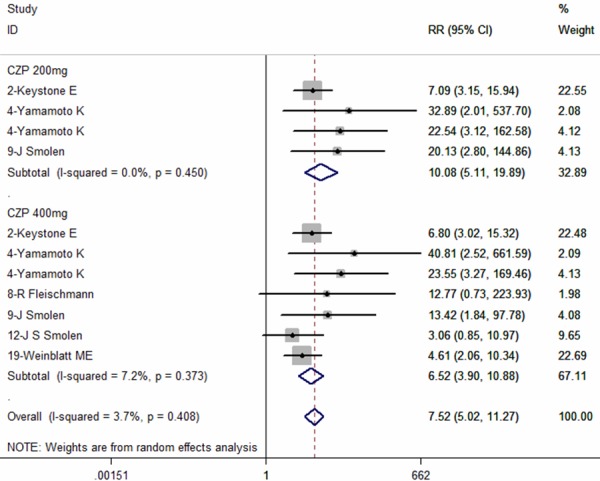
Subgroup analysis of ACR70 response rate for the treatment of certolizumab pegol (CZP) versus placebo.
We performed the Begg’s and Egger’s test for ACR50 and ACR70 to assess the presence of any publication bias. No evidence of obvious publication bias was found (for ACR50: Begg’s test, Z = 1.50, P = 0.133, Egger’s test, t = 1.22, P = 0.276; for ACR70: Begg’s test, Z = 0.75, P = 0.452, Egger’s test, t = 2.31, P = 0.082).
Disease activity and patient-reported outcomes
Four studies reported the results of DAS28-ESR [8,9,27,32]. The pooled analysis using random-effect model showed a significantly reduction in DAS28-ESR (WMD = -1.25, 95% CI:-1.60, -0.89; P = 0.000) (Figure 8), with significant heterogeneity between the studies (I2 = 97.8%, P = 0.000).
Figure 8.
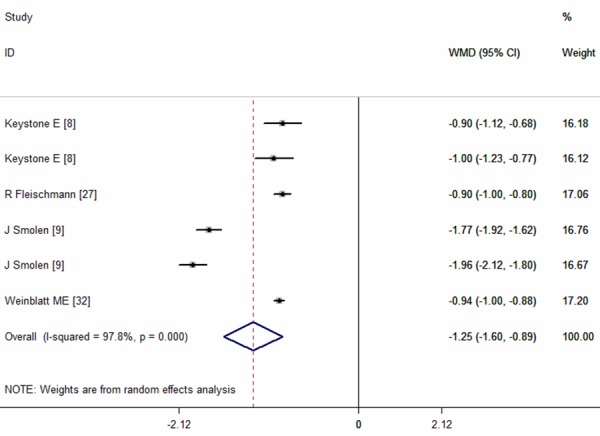
Disease Activity Score 28-joint assessment erythrocyte sedimentation rate (DAS28-ESR) for the treatment of certolizumab pegol (CZP) versus placebo.
Four studies provided the data of PROs, including HAQ-DI, arthritis pain, and fatigue. The pooled results showed that CZP plus MTX significantly reduced the incidence of arthritis pain (RR = 4.00, 95% CI: 3.14, 5.09; P = 0.000), fatigue (RR = 3.79, 95% CI: 2.90, 4.95; P = 0.000) and improved the physical function (RR = 4.57, 95% CI: 3.84, 5.44; P = 0.000), compared with controls (Figure 9).
Figure 9.

Patients-reported outcomes (PROs) for the treatment of certolizumab pegol (CZP) versus placebo.
Adverse events
Six studies reported the data of adverse events (AEs) [8,9,26-28,32]. The most frequent noninfectious treatment-related AEs were headache, hypertension, and back pain. And the most frequent reported infectious AEs were urinary tract infections, nasopharyngitis, and upper respiratory tract infections. The pooled analysis using a random-effect model suggested that, the incidence of AEs in the CZP plus MTX group and control group was not statistically significant differences, no matter in mild adverse events (RR = 1.12, 95% CI: 0.81, 1.55; P = 0.000), moderate adverse events (RR = 1.01, 95% CI: 0.82, 1.25; P = 0.000), or severe adverse events (RR = 0.93, 95% CI: 0.70, 1.25; P = 0.000) (Figure 10).
Figure 10.
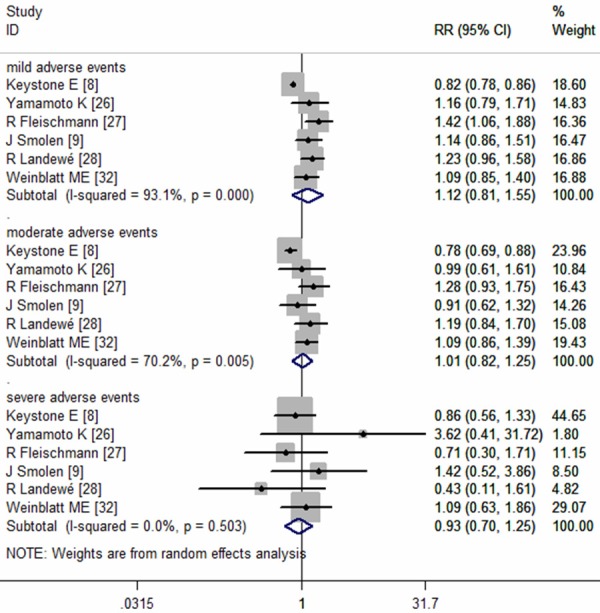
The risk ratio of treatment-related adverse events (AEs) in AS patients with certolizumab pegol (CZP) versus placebo.
Discussion
This meta-analysis demonstrated that CZP (200 or 400 mg) combined with MTX was associated with significantly higher ACR20, ACR50 and ACR70 response rates at week 12 and 24, and had amelioration in PROs, including HAQ-DI, arthritis pain, and fatigue, in the treatment of patients with active RA. The incidence of AEs (any intensity) between the CZP group and control group was not statistically significant difference. This study confirmed the clinical benefits of CZP in the treatment of active RA, with rapid, significant and clinical meaningful improvements in multiple PROs, and ACR20/ACR50/ACR70 response rates, which was observed at week 12, and maintained until at week 24.
In a previously published meta-analysis [11], another three licensed anti-TNF agents, including adaliumab, infliximab, and etanercept, showed significantly efficacy in the treatment of RA, when compared with placebo. Interestingly, all these agents contain an immunoglobulin G Fc portion, which is lacking in CZP. This suggests that the immunoglobulin G Fc portion may not be necessary for TNF inhibitors to be active in the treatment of RA. Therefore, the primary mode of action of TNF inhibitors in RA may not be associated with Fc mediated effects, but the blockage of TNF by reversing signaling [33,34]. This meditation process could also be activated by Fc free Fab’ molecule such as CZP.
According to this meta-analysis, we found that the combination treatment of CZP with MTX significantly improved the ACR20/50/70 response rates in RA patients. And these clinical benefits were observed from 12 week until 24 week. Moreover, we also found that both dose of CZP significantly inhibited the progression of joint damage, increased physical function and quality of life, compared with placebo, which suggested that CZP 200 mg every 2 week could be recommended as an optimal treatment.
The treatment of CZP had an acceptable safety profile in that the incidence of adverse events (any intensity) was not significantly difference between the CZP and placebo group. However, more serious adverse events were observed in the RAPID2 [9], JRAPID [26], FAST4WARD [27], and REALISTIC [32] trials. A possible explanation is that the patients in the CZP group had longer treatment exposure compared with that in the control group, since those patients in the latter withdrew from the trials because of inefficacy treatment. In our study, the safety profile assessment was based on a short-term analysis with the event end-point at 24 week. Thus, whether CZP would bring additional treatment-related events after a few years’ administration remains unclear. In a recently published integrated analysis study, Bykerk VP et al. [35] assessed the long-term safety profile of CZP, in which a total of 4049 patients were exposed to the CZP treatment up to 7.6 years [35]. He found no new safety events reported in the long-term data. And the incidence of adverse events in the CZP group was similar to that in the control group.
Our meta-analysis has several strengths and limitations which should be considered when interpreting our results. First, a major strength of our study is that all of the available data were obtained from RCTs, and most of the studies were well performed, high-quality (Jadad scale ≥ 3). Second, all the included studies have enlarged sample size, which enhanced statistical power to provide reliable effect estimates. Although our meta-analysis represents a complete summary of the efficacy of CZP for RA patients, it also serves to note the potential limitations. First, the variety of criteria was used to define RA. In the majority of the studies, the definition of RA was based on American College of Rheumatology (ACR) 1987 criteria. Second, there was considerable heterogeneity among the included studies, including different population, duration of disease, disease activity status, prior disease-modifying antirheumatic drug (DMARDs). These factors would have potential impacts on our results. Third, in this meta-analysis, we only assessed the efficacy and safety of CZP at week 12 and 24. And we also tried to assess the long-term effect and safety of CZP in the treatment of RA. However, there was insufficient data for us to pool the estimates. Thus, a further long-term evaluation for the effect of CZP is urgently needed.
In summary, this meta-analysis demonstrated that CZP 200 or 400 mg was clinically effective in the treatment of active RA patients, with an improvement in ACR20/ACR50/ACR70 response rates, and amelioration in PROs. The incidence of AEs in the CZP group was not statistically significant different with that in the control group. However, considering the heterogeneity among the included studies, more large-scale, well-designed RCTs are urgently needed.
Disclosure of conflict of interest
None.
References
- 1.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–64. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maini RN, Brennan FM, Williams R, Chu CQ, Cope AP, Gibbons D, Elliott M, Feldmann M. TNF-alpha in rheumatoid arthritis and prospects of anti-TNF therapy. Clin Exp Rheumatol. 1993;11:S173–5. [PubMed] [Google Scholar]
- 3.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor-monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 4.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, St Clair EW, Keenan GF, van der Heijde D, Marsters PA, Lipsky PE Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–65. doi: 10.1002/art.20159. [DOI] [PubMed] [Google Scholar]
- 5.Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Cannon GW, Spencer-Green G, Finck BK. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443–50. doi: 10.1002/art.10308. [DOI] [PubMed] [Google Scholar]
- 6.Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, Fischkoff SA, Chartash EK. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 7.Nesbitt A, Fossati G, Bergin M, Stephens P, Stephens S, Foulkes R, Brown D, Robinson M, Bourne T. Mechanism of action of certolizumab pegol (CDP870): In vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007;13:1323–32. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
- 8.Keystone E, Heijde Dv, Mason D Jr, Landewé R, Vollenhoven RV, Combe B, Emery P, Strand V, Mease P, Desai C, Pavelka K. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–29. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- 9.Smolen J, Landewé RB, Mease P, Brzezicki J, Mason D, Luijtens K, van Vollenhoven RF, Kavanaugh A, Schiff M, Burmester GR, Strand V, Vencovsky J, van der Heijde D. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomized controlled trial. Ann Rheum Dis. 2009;68:797–804. doi: 10.1136/ard.2008.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CimziaR summary of products characteristics. [Internet. Accessed Nov 25, 2010]
- 11.Launois R, Avouac B, Berenbaum F, Blin O, Bru I, Fautrel B, Joubert JM, Sibilia J, Combe B. Comparison of certolizumab pegol with other anticytokine agents for treatment of rheumatoid arthritis: a multiple-treatment bayesian metaanalysis. J Rheumatol. 2011;38:835–845. doi: 10.3899/jrheum.100665. [DOI] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 13.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in metaanalyses. Ann Intern Med. 2001;135:982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 14.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeuchi T, Yamamoto K, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, Watanabe A, Origasa H, Shoji T, Miyasaka N, Koike T. Long-term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: 52-week results from an open-label extension of the J-RAPID study. Mod Rheumatol. 2014;20:1–10. doi: 10.3109/14397595.2014.881709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka Y, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Eguchi K, Watanabe A, Origasa H, Shoji T, Miyasaka N, Koike T. Long-term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients who could not receive methotrexate: 52-week results from an open-label extension of the HIKARI study. Mod Rheumatol. 2014;24:725–33. doi: 10.3109/14397595.2013.865822. [DOI] [PubMed] [Google Scholar]
- 20.Keystone E, Landewé R, van Vollenhoven R, Combe B, Strand V, Mease P, Shaughnessy L, Vanlunen B, van der Heijde D. Long-term safety and efficacy of certolizumab pegol in combination with methotrexate in the treatment of rheumatoid arthritis: 5-year results from the RAPID 1 trial and open-label extension. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203695. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keystone EC, Curtis JR, Fleischmann RM, Furst DE, Khanna D, Smolen JS, Mease PJ, Schiff MH, Coteur G, Davies O, Combe B. Rapid improvement in the signs and symptoms of rheumatoid arthritis following certolizumab pegol treatment predicts better longterm outcomes: post-hoc analysis of a randomized controlled trial. J Rheumatol. 2011;38:990–996. doi: 10.3899/jrheum.100935. [DOI] [PubMed] [Google Scholar]
- 22.Keystone EC, Combe B, Smolen J, Strand V, Goel N, van Vollenhoven R, Mease P, Landewé R, Fleischmann R, Luijtens K, van der Heijde D. Sustained efficacy of certolizumab pegol added to methotrexate in the treatment of rheumatoid arthritis: 2-year results from the RAPID 1 trial. Rheumatology. 2012;51:1628–1638. doi: 10.1093/rheumatology/kes082. [DOI] [PubMed] [Google Scholar]
- 23.Kavanaugh A, Smolen JS, Eemery P, Purcaru O, Keystone E, Richard L, Strand V, van Vollenhoven RF. Effect of certolizumab pegol with methotrexate on home and work place productivity and social activities in patients with active rheumatoid arthritis. Arthritis Rheum. 2009;61:1592–600. doi: 10.1002/art.24828. [DOI] [PubMed] [Google Scholar]
- 24.van Vollenhoven RF, Felson D, Strand V, Weinblatt ME, Luijtens K, Keystone EC. American College of Rheumatology hybrid analysis of certolizumab pegol plus methotrexate in patients with active rheumatoid arthritis: data from a 52-week phase iii trial. Arthritis Care Res (Hoboken) 2011;63:128–34. doi: 10.1002/acr.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivitz AJ, Schechtman J, Texter M, Fichtner A, de Longueville M, Chartash EK. Vaccine responses in patients with rheumatoid arthritis treated with certolizumab pegol: results from a single-blind randomized phase iv trial. J Rheumatol. 2014;41:648–57. doi: 10.3899/jrheum.130945. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, Watanabe A, Origasa H, Shoji T, Sakamaki Y, van der Heijde D, Miyasaka N, Koike T. Efficacy and safety of certolizumab pegol plus methotrexate in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: the J-RAPID randomized, placebo-controlled trial. Mod Rheumatol. 2014;24:715–24. doi: 10.3109/14397595.2013.864224. [DOI] [PubMed] [Google Scholar]
- 27.Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, Goel N, Brezinschek HP, Innes A, Strand V. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying an-tirheumatic therapy: the FAST4WARD Study. Ann Rheum Dis. 2009;68:805–811. doi: 10.1136/ard.2008.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landewé R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, Reveille JD, Rudwaleit M, van der Heijde D, Stach C, Hoepken B, Fichtner A, Coteur G, de Longueville M, Sieper J. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a doubleblind randomised placebo-controlled Phase 3 study. Ann Rheum Dis. 2014;73:39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smolen JS, Emery P, Ferraccioli GF, Samborski W, Berenbaum F, Davies OR, Koetse W, Purcaru O, Bennett B, Burkhardt H. Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double-blind, randomised, placebo-controlled trial. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204632. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strand V, Mease P, Burmester GR, Nikaï E, Coteur G, van Vollenhoven R, Combe B, Keystone EC, Kavanaugh A. Rapid and sustained improvements in health-related quality of life, fatigue, and other patient-reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Res Ther. 2009;11:R170. doi: 10.1186/ar2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strand V, Smolen JS, van Vollenhoven RF, Mease P, Burmester GR, Hiepe F, Khanna D, Nikaï E, Coteur G, Schiff M. Certolizumab pegol plus methotrexate provide broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trial. Ann Rheum Dis. 2011;70:996–1002. doi: 10.1136/ard.2010.143586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinblatt ME, Fleischmann R, Huizinga TW, Emery P, Pope J, Massarotti EM, van Vollenhoven RF, Wollenhaupt J, Bingham CO 3rd, Duncan B, Goel N, Davies OR, Dougados M. Efficacy and safety of certolizumab pegol in a broad population of patients with active rheumatoid arthritis: results from the REALISTIC phase IIIb study. Rheumatology. 2012;51:2204–2214. doi: 10.1093/rheumatology/kes150. [DOI] [PubMed] [Google Scholar]
- 33.Rigby WF. Drug insight: different mechanisms of action of tumor necrosis factor antagonists-passive-aggressive behavior? Nat Clin Pract Rheumatol. 2007;3:227–33. doi: 10.1038/ncprheum0438. [DOI] [PubMed] [Google Scholar]
- 34.Mitoma H, Horiuchi T, Hatta N, Tsukamoto H, Harashima S, Kikuchi Y, Otsuka J, Okamura S, Fujita S, Harada M. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-alpha. Gastroenterology. 2005;128:376–92. doi: 10.1053/j.gastro.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 35.Bykerk VP, Cush J, Winthrop K, Calabrese L, Lortholary O, de Longueville M, van Vollenhoven R, Mariette X. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203660. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


