Abstract
Objective: We conducted a meta-analysis to evaluate the efficacy and toxicity of DEB-TACE in the treatment of patients with intermediate-stage HCC. Methods: Studies published in PubMed, Embase and Web of Science, were systematically reviewed to identify those that assessed the efficacy and toxicity of DEB-TACE in the treatment of patients with HCC. Hazard ratio, risk ratioand 95% confidence intervalswere calculated, using a fixed-effects model or a random-effects model. Results: Nine studies with a total of 830 patients met the inclusion criteria were included in this study. DEB-TACE significantly improved overall survivaland progression free survival, and also increased objective response rateand disease control rate. However, in subgroup analyses, pooled results showed that, the survival benefits of DEB-TACE were not found in the randomized controlled trials, but were observed in Non-RCTs. The incidence of most common adverse events, including nausea, pain, fever, and fatigue, was not significant difference between the DEB-TACE group and conventional TACEgroup. Conclusions: Despite DEB-TACE significantly prolonged the survival and response rate in the patients with HCC, the conclusion about the survival benefits should be interpreted with caution, since these findings were only found in retrospective Non-RCTs, and not in prospective RCTs.
Keywords: DEB-TACE, hepatocellular carcinoma, efficacy, toxicity, meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer with an increasing incidence worldwide, and is the third most common cause of cancer-related death worldwide [1,2]. Despite curative therapies, such as liver transplantation, surgical resection, and radiofrequency ablation (RFA) are applied in patients with HCC, only less than 20% of HCC patients are eligible for these treatment options [3,4].
Transarterial chemoembolization (TACE) is widely used in the patients who are not suitable for curative treatments [5,6]. It involves the injection of chemotherapeutic agent, mixed with selective vascular embolization, both of which are delivered to the arterial of tumor. The slow release of these agents would result in a higher intratumor concentrations, and then occludethe blood vessel causing infarction and necrosis [7]. In the previous studies, TACE has been reported to have an improvement in partial response, as well as a delay in tumor progression and vascular invasion [8-11]. However, the post-TACE complications, suchasacute liver or renal failure, encephalopathy, and upper gastrointestinalbleeding, seems to be severe [12]. Therefore, there is a requirement for treatment regimens, which would improve the response rate and survival, as well as reduce the TACE-associated complications.
The drug-eluting beads (DC bead, Biocompatibles UK Ltd) is a novel drug delivery embolization system, which has been developed to deliver higher dose of chemotherapeutic agent and to prolong the time of contact time with tumor [13,14]. Results from the preclinical and clinical trials have shown that in the HCC patients, TACE with DC Bead has a higher intratumor concentration and lower systemic concentration of doxorubicin, compared with intra-arterial doxorubicin and conventional TACE (cTACE) [15,16]. PhaseI/II studies also have indicated that patients treated with doxorubicin-eluting beads TACE (DEB-TACE) have a prolonged survival and low toxicity [15-18].However, in a recently published meta-analysis [19], the pooled results showed that DEB-TACE did not increase the response rate in the HCC patients, when compared with cTACE. This controversial result raised the concern that DEB-TACE may not be so promising in improving patients’ response rate. Thus, we conduct an updated meta-analysis to re-evaluate the efficacy and safety of DEB-TACE in the treatment of HCC.
Methods
Literature search and inclusion criteria
Pubmed, Embase, and Web of Science databases (up to May 4, 2014) were searched to identifyrelevant studies which assessed the efficacy and safety of DEB-TACE in the treatment of HCC. The following search terms were used: Transarterial chemoembolization, TACE, drug-eluting bead, hepatocellular carcinoma, HCC, liver cell carcinoma. Results were limited to human subjects. We also manually searched the reference lists of included studies and related publications, until no potential studies could be found. For thesame trial that presented duplicated data in several studies, only the recent, or most complete study was included.
We included studies in all languages when the following inclusion criteria were met: (1) eligible patients were ≥ 18 years older with a diagnosis of HCC; (2) patients in the experimental group treated by TACE with DC Bead loaded with doxorubicin, while patients in the control group received other type of therapy; (3) the studies provided data of interest, including overall survival (OS), progression free survival (PFS), objective response rate (ORR), disease control rate (DCR) and adverse events.
Data extraction and quality assessment
Two reviewers (Xueping Zhou and Zhaohui Tang) independently extracteddata from the studies included using a standardized Excel file. Data as follows were recorded: first author, year of publication, number of patients, the mean age, treatment regimen, performance status, OS, PFS, and adverse events. Any disagreement was resolved by discussion and consensus.
The methodological quality of randomized controlled trials (RCTs) was assessed by the Jadad scale [20]. The scale consists of three items, which describe randomization (0-2 points), blinding (0-2 points), and dropouts and withdraws (0-1 point). The scale ranges from 0 to 5 points, and higher scores indicate better reporting. A score of 1 is obtained when each of points described is met. And another point is given when the method of randomization and/or blinding is given and appropriate; whereas one point is deducted when it is not appropriate. Any studies with a score ≥ 3 points are considered to be of high quality [21].
Statistical analyses
We assessed the efficacy of DEB-TACE in the treatment of HCC based on the data from the studies included. For time-to-event variable, such as OS and OS, hazard ratio (HR) with 95% confidence intervals (CIs) were directly extracted or calculated by a calculation sheet as previously described [22]. For dichotomous variables, such as ORR and adverse events, the number of patientswith the events of interestoccurredand the total number of patients were extracted. And the risk ratio (RR) with 95% CI was calculated. Statistical heterogeneity was assessed using Cochran Q test and I2 statistics [23]. The P value of Q test < 0.1 or I2 > 50% are considered to have heterogeneity among the included studies. A random-effects model (DerSimonian and Laird method) [24] was applied to pool the estimates when the heterogeneity existed, otherwise, a fixed-effects model (Mantel-Haenszel method) [25] was used. In the presence of heterogeneity, sensitivity analyses based on study design, and sample size were conducted to explore the potential sources. Publication bias was assessed by Begg and Egger’s test [26,27]. A P value less than 0.05 was judged as statistically significant, except where otherwise specified. Statistical analyses were performed by using STATA version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Identification of eligible studies
The search strategy identified 767 potential studies from PubMed, Embase, and Web of Science. Of these, 167 were excluded because they were duplicate records, 483 were excluded after the titles and abstracts review, mainly because they were reviews, comment, academic meeting abstracts, or un-related with our topics, leaving 117 for full text screening. In the review, four trials were excluded for the following reasons: three studies with unavailable data for analysis [28-30], and one study with a single-arm design [31]. Finally, nine studies [32-40] with a total of 830 patients were included in this meta-analysis. The detailed flowchart of search strategy was shown in Figure 1.
Figure 1.
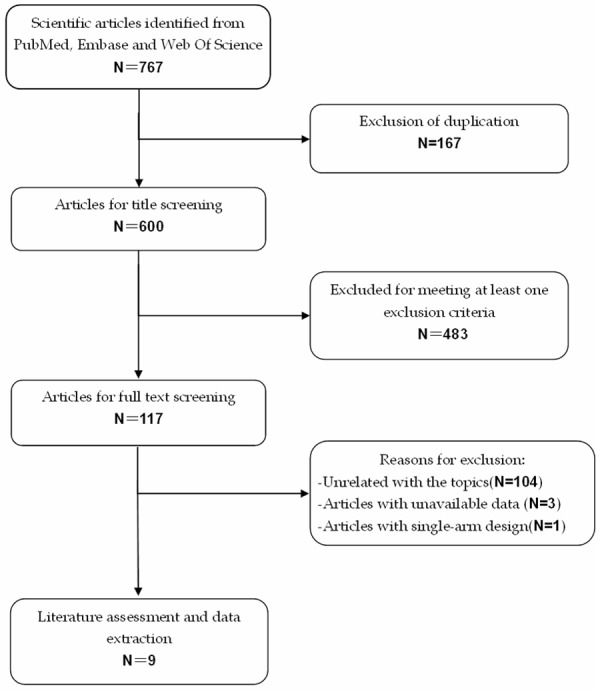
Eligibility of studies for inclusion in meta-analysis.
Study characteristics and quality assessment
The main characteristics of five RCTs and four Non-RCTs were presented in Table 1. These studies were published from 2008 to 2012. The sample size ranged from 41 to 201 (total 830). Five studies were prospective RCTs and four were prospective or retrospective Non-RCTs. The predominant reasons for etiology of cirrhosis were hepatitis C virus infection (HCV) (36.3%), hepatitis B virus infection (HBV) (27.1%), and alcohol consumption (25.9%). Approximately 82.2% of patients were rated as Child-Pugh Class A or B, indicating intermediate-stage HCC. The ORR was defined as complete response (CR) plus partial response (PR), and DCR as ORR plus stable disease (SD). Among the patients in the DEB-TACE group, DEB chemoembolization was performed using DC beads loaded with 50-75 mg of doxorubicin in each trial. The median Jadad scale of the included studies was 3 (range from 3 to 4).
Table 1.
Baseline Characteristics of the included studies
| Total (830) | Treatment | Median age (range) | Male/Female | Child-Pugh Class | ECOG PS | Etiology of cirrhosis | Studydesign | Jada scale |
|---|---|---|---|---|---|---|---|---|
| Lammer J [32] (201) | DEB-TACE | 67.3 ± 9.1 | 79/14 | A/B: 77/16 | PS 0/1: 74/19 | HCV/HBV/alcohol: 22/16/43 | RCT | 4 |
| cTACE | 67.4 ± 8.8 | 95/13 | A/B: 89/19 | PS 0/1: 80/28 | HCV/HBV/alcohol: 18/18/57 | |||
| MABED M [33] (100) | DEB-TACE | 52 (36-60) | 32/18 | A/B: 34/16 | PS 0/1-2: 13/37 | HBV/HCV: 6/37 | RCT | 3 |
| Intravenous doxrubicin | 51 (34-60) | 33/17 | A/B: 35/15 | PS 0/1-2: 15/35 | HBV/HCV: 8/35 | |||
| Recchia F [34] (105) | DEB-TACE | 72 (53-80) | 25/10 | NR | NR | NR | Prospective case-control | None |
| cTACE | 70 0 (47-80) | 50/20 | NR | NR | NR | |||
| Malagari K [35] (41) | DEB-TACE | 70.7 (6.9) | 31/10 | A/B: 23/18 | PS 0/1: 26/15 | NR | RCT | 3 |
| BLAND-embo | 70 (7.9) | 34/9 | A/B: 26/17 | PS 0/1: 28/15 | NR | |||
| Ferrer Puchol M.D. [36] (72) | DEB-TACE | 68.4 ± 8.54 | NR | NR | NR | NR | RCT | 3 |
| cTACE | 69.26 ± 11.80 | NR | NR | NR | NR | |||
| Dhanasekaran R [37] (71) | DEB-TACE | 59.96 (11.45) | 35/10 | A/B/C: 22/11/12 | NR | HCV/HBV/alcohol: 20/5/7 | Retrospective case-control | None |
| cTACE | 58.96 (13.3) | 19/7 | A/B/C: 11/11/4 | NR | HCV/HBV/alcohol: 11/3/3 | |||
| Sacco R [38] (67) | DEB-TACE | 71.3 ± 7.2 | 23/10 | A/B: 29/4 | NR | HCV/HBV/other: 22/4/7 | RCT | 4 |
| cTACE | 68.7 ± 8.1 | 22/12 | A/B: 25/9 | NR | HCV/HBV/other: 25/4/5 | |||
| Song MJ [39] (129) | DEB-TACE | 61.7 ± 9.8 | 42/18 | A/B: 56/4 | NR | HCV/HBV/alcohol: 8/44/4 | Retrospective cohort | None |
| cTACE | 59.4 ± 11.2 | 48/21 | A/B: 62/6 | NR | HCV/HBV/alcohol: 8/46/12 | |||
| Wiggermann P [40] (44) | DEB-TACE | 70.32 ± 7.06 | 18/4 | A: 22 | NR | Hepatitis/alcohol/other: 6/2/14 | Retrospective case-control | None |
| cTACE | 67.72 ± 9.02 | 19/3 | A: 22 | NR | Hepatitis/alcohol/other: 3/7/12 |
Abbreviations: DEB-TACE, doxorubicin-eluting bead transarterial chemoembolization; cTACE, conventional TACE; NR, not report.
OS
Seven studies reported data of OS [33,34,36-40]. The aggregated results showed that DEB-TACE significantly improved OS in the treatment of HCC patients when compared with cTACE (HR = 0.67, 95% CI: 0.49, 0.91; Z = 2.56, P = 0.010) (Figure 2). The test for heterogeneity was not significant (Z = 0.00, P = 1.000).
Figure 2.
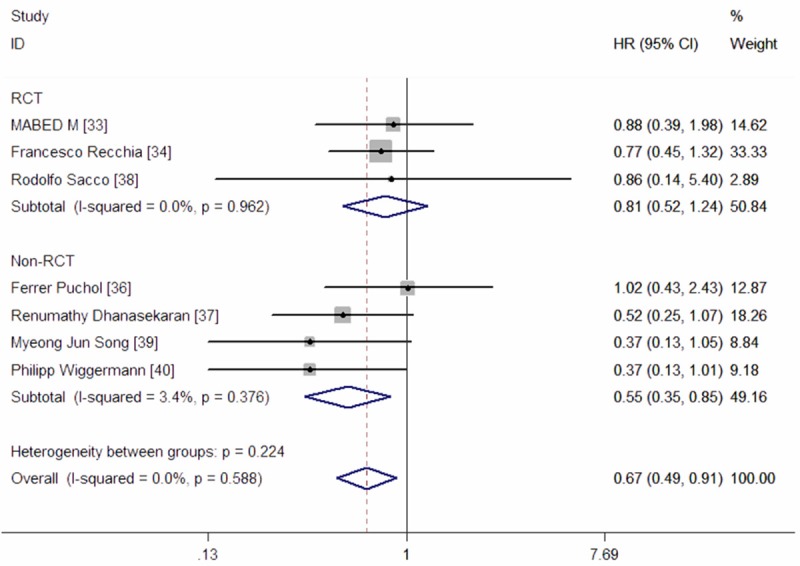
Meta-analysis exploring the effect of DEB-TACE on overall survival.
We also performed subgroup analyses based on different study design. Pooled estimates from five prospective RCTs showed that, DEB-TACE did not significantly prolong OS when compared with cTACE (HR = 0.81, 95% CI: 0.52, 1.24; Z = 0.97, P = 0.330) (Figure 2). While, in the three Non-RCTs, the pooled results suggested that DEB-TACE had an improvement in OS (HR = 0.55, 95% CI: 0.35, 0.85; Z = 2.66, P = 0.008) (Figure 2). The Begg’s and Egger’s test indicated no existence of publication bias (for Begg’s test, Z = 0.30, P = 0.764; for Egger’s test, t = -0.60, P = 0.575) (Figure 3).
Figure 3.
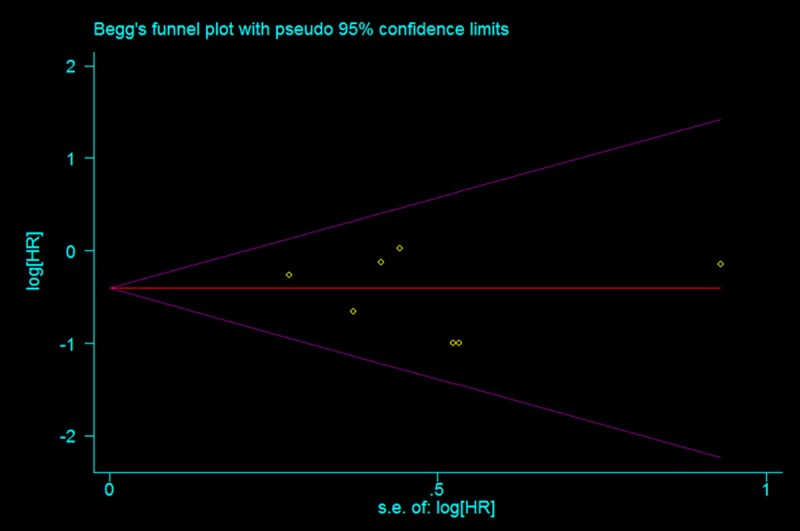
Test for publication bias for HR of overall survival.
PFS
Three RCTs and one Non-RCT reported data of PFS [33,34,38,39]. The pooled results of these studies indicated that DEB-TACE significantly prolonged PFS when compared with cTACE (HR = 0.64, 95% CI: 0.47, 0.86; Z = 2.97, P = 0.003) (Figure 4). The test for heterogeneity was not significant (P = 0.588, I2 = 0.0%).
Figure 4.
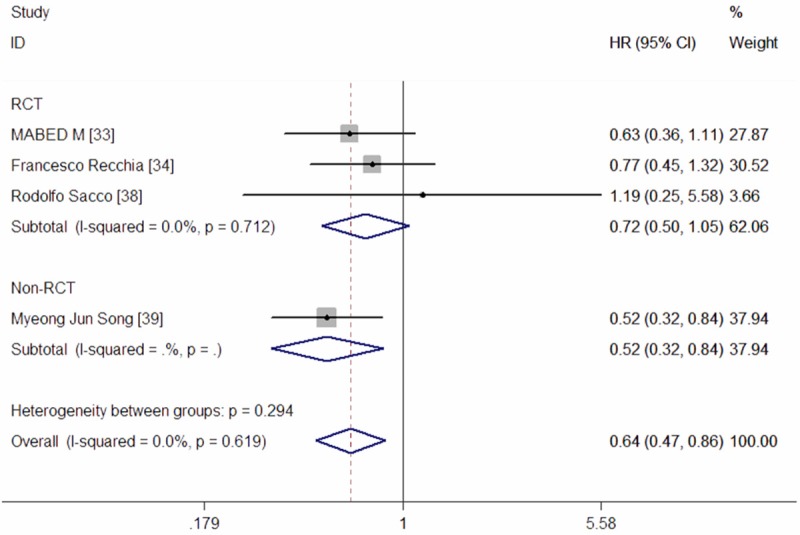
Meta-analysis exploring the effect of DEB-TACE on progression-free survival.
In the subgroup analyses, pooled results from three RCTs showed that no significant benefit in PFS was observed for patients with HCC (HR = 0.72, 95% CI: 0.50, 1.05; Z = 1.69, P = 0.091) (Figure 4), following treatment with DEB-TACE. Since only one Non-RCT reported data of PFS, we did not perform the subgroup analysesof Non-RCT. The Begg’s and Egger’s test indicated no existence of publication bias (for Begg’s test, Z = 0.34, P = 0.734; for Egger’s test, t = 1.35, P = 0.310).
ORR
Six studies reported data of ORR [32,33,35,36,39,40]. The pooled results suggested that patients with HCC under the treatment of DEB-TACE had a higher ORR when compared with those treated with cTACE (RR = 1.43, 95% CI: 1.20, 1.72; Z = 3.92, P = 0.000) (Figure 5). The test for heterogeneity was not significant (P = 0.275, I2 = 21.1%).
Figure 5.

Meta-analysis exploring the effect of DEB-TACE on objective response rate.
In the subgroup analyses, a significantly high ORR was observed in patients in both of the RCTs (RR = 1.39, 95% CI: 1.06, 1.82; Z = 2.40, P = 0.016) and Non-RCTs (RR = 1.48, 95% CI: 1.17, 1.88; Z = 3.24, P = 0.001).
DCR
Five studies reported data of DCR [32,33,35,39,40]. The pooled results showed that patients with HCC under the treatment of DEB-TACE had a higher DCR when compared with those treated with cTACE (RR = 1.29, 95% CI: 1.14, 1.46; Z = 4.05, P = 0.000) (Figure 6). The test for heterogeneity was not significant (P = 0.379, I2 = 4.9%).
Figure 6.
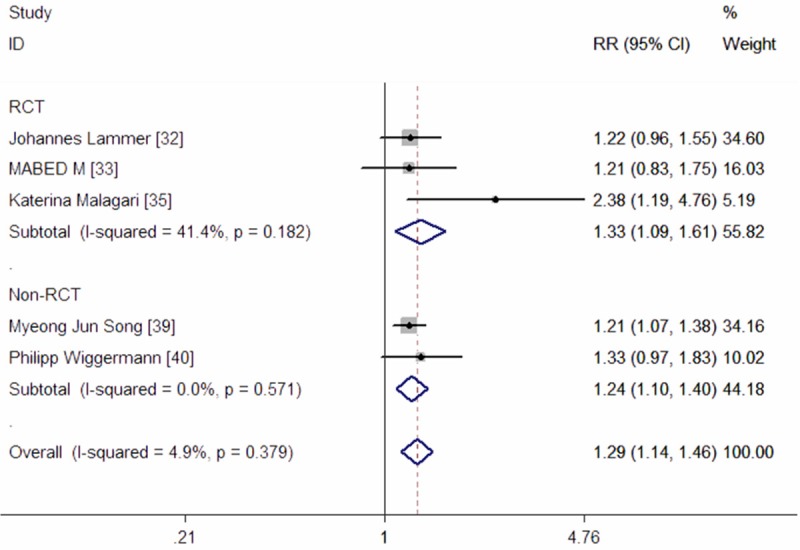
Meta-analysis exploring the effect of DEB-TACE on disease control rate.
In the subgroup analyses, a significantly high DCR was observed in patients in both of the RCTs (RR = 1.33, 95% CI: 1.09, 1.61; Z = 2.84, P = 0.004) and Non-RCTs (RR = 1.24, 95% CI: 1.10, 1.40; Z = 3.44, P = 0.001) (Figure 6).
Adverse events
Eight prospective RCTs and Non-RCTs reportedadverse events [32-38,39,40], but only six studies provided available data for analysis [32,35-37,39,40]. The most common adverse events were post-embolization complications, including nausea, pain, fever, and fatigue. The pooled results showed that the incidence of adverse events were not significantly difference between the two groups (RR = 0.73, 95% CI: 0.37, 1.46; Z = 0.89, P = 0.374) (Figure 7).
Figure 7.
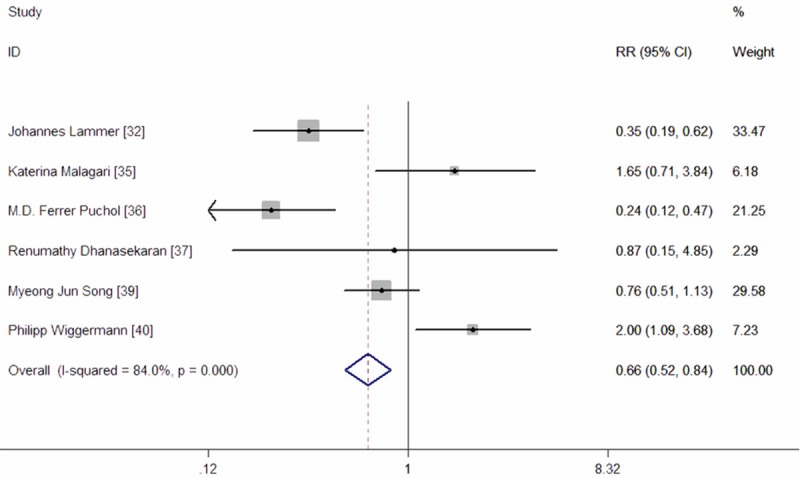
Meta-analysis exploring the risk ratio of adverse events in patients with HCC.
Discussion
The major purpose of this meta-analysis was to update and re-evaluate the efficacy and safety of DEB-TACE in patients with HCC. This meta-analysis was performed based on five RCTs and four retrospective cohort or case-control studies. Our results suggest that DEB-TACE significantly improved the OS (HR = 0.67, 95% CI: 0.49, 0.91; Z = 2.56, P = 0.010) and PFS (HR = 0.64, 95% CI: 0.47, 0.86; Z = 2.97, P = 0.330), and also increased ORR (RR = 1.39, 95% CI: 1.06, 1.82; Z = 3.92, P = 0.000) and DCR (RR = 1.33, 95% CI: 1.09, 1.61; Z = 4.05, P = 0.000), compared with cTACE. However, in the subgroup analysis based on different study design, the survival benefit of DEB-TACE was not observed in the prospective RCTs, whereas was found in the retrospective studies. The adverse events in the two groups were not significantly difference.
Two meta-analysis exploring the effect of DEB-TACE in patients with HCC were published in 2013 [19] and 2014 [41]. In the study of Sheng Gao [19], the pooled estimates of odds ratios (OR) showed that there were no significantly difference in the CR, PR, SD, and OR between the DEB-TACE group and cTACE group. In the study of Kaijun Huang [41], DEB-TACE provides significantly better tumor response compared with cTACE, in terms of one-year and 2-year survival. However, all the two published studies had limitations. First, Sheng Gao et al. [19] did not perform the quality assessment of the included studies, which were of low quality, resulting in bias. Second, both of the studies [19,41] used OR instead of HR to estimate the pooled effect. HR, which takes into account the patient numbers, time of events and censored data, was the most appropriate parameter for time-to-event analysis [42].
cTACE is regarded as the first-line treatment for patients with inoperable and intermediate HCC. Although it has been used for several years, the response rates vary greatly between different trials. The DC Bead is a novel precise drug delivery embolization system. It has been designed to load drugonto microspheres. And then microspheres are injected into the tumor, slowly releasing drug over 14 days. The higher and more sustained release of drug would maximize the drug’s effectiveness in terms of response [43]. In addition, the limited release of drug in the systemic circulation, would reduce the drug’s systemic toxicity [43]. Preclinical and clinical trials have indicated that doxorubicin reserved a higher and prolonged concentration within the tumor, and a lower concentration in the systemic circulation, after TACE with DC Bead was performed [44-46].Thus, it is assumed that TACE with doxorubicin-loaded would have promising efficacy.
In this meta-analysis, the results suggested that HCC patients treated with DEB-TACE had significant improvement survival benefits in OS and PFS, compared with those treated with cTACE. However, these benefits wereonly found in patients from retrospective cohort or case-control studies, while patients from prospective RCTs did not seem to obtain these benefits. Despite the patients in the retrospective studies were stringently selected according rigorous criteria, and their baseline characteristics were well matched between the two groups, the selection bias was unavoidable. Moreover, RCTs are scientifically the most rigorous method for evaluation of effectiveness of medical interventions, and their results were more reliable andvalid. Thus, the survival benefits of DEB-TACE may not be so promising as it had been thought.
Despite our results indicated that the DEB-TACE treatment had no survival benefit in patients with HCC, some studies have obtained interesting outcomes in special subgroup population. In the prospective study conducted by M. Mabed et al. [33], the authors found that patients with serum albumin > 3.3 g/dL in the experimental group had a prolonged OS than those in the control group; the median OS in the two groups were 60 weeks and 36 weeks, respectively [33]. Similar results were also observed in another two studies conducted by O’Suilleabhain et al. [47] and Wigmore et al. [48]. And the authors assumed that serum albumin may be an independent prognostic factor for the better OS in HCC patients treated with DEB-TACE.
However, this study has several potential limitations. First, we admit that our meta-analysis included some studies, which had a relatively small sample size. Overestimation of the treatment effect is more likely in smaller trials compared with larger trials. Second, some of the included studies did not provide sufficient data of time-to-event outcomes for meta-analysis directly. In order to obtain these data, we extracted the HRs with 95% CI from the Kaplan-Meier curves, using Engauge Digitizer, which may lead to inaccurate data. Third, the targeted population varied greatly, such as the gender, ethnicity, and disease status. These factors may have a potential impact on our results.
In conclusion, this meta-analysis suggested that the treatment of DEB-TACE significantlyimproved OS and PFS, and also increased ORR and DCR, in the patients with HCC. However, the pooled results from RCTs showed that DEB-TACE did not have beneficial survival in the treatment of HCC patients. Thus, these results should be interpreted with caution. Given the limited number of RCTs and small sample size, additional larger scale RCTs are needed to confirm the current findings and investigate the prognostic factors for the beneficial survival in HCC patients treated with DEB-TACE.
Disclosure of conflict of interest
None.
References
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 3.Kassahun WT, Fangmann J, Harms J, Hauss J, Bartels M. Liver resection and transplantation in the management of hepatocellular carcinoma: A review. Exp Clin Transplant. 2006;4:549–558. [PubMed] [Google Scholar]
- 4.Guan YS, Liu Y. Interventional treatments for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2006;5:495–500. [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 7.Raoul JL, Heresbach D, Bretagne JF, Ferrer DB, Duvauferrier R, Bourguet P, Messner M, Gosselin M. Chemoembolisation of hepatocellular carcinomas. A study of the biodistribution and pharmacokinetics of doxorubicin. Cancer. 1992;70:585–590. doi: 10.1002/1097-0142(19920801)70:3<585::aid-cncr2820700308>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolisation in patients with unresectable hepatocellular carcinoma-a randomised controlled trial. Gastroenterology. 1998;94:453–456. doi: 10.1016/0016-5085(88)90436-2. [DOI] [PubMed] [Google Scholar]
- 9.Group d’Etude et de Traitment du Carcinome He’patocellulaire. A comparison of lipiodol chemoembolisation and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995;332:1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 10.Raoul JL, Guyader D, Bretagne JF, Heautot JF, Duvauferrier R, Bourguet P, Bekhechi D, Deugnier YM, Gosselin M. Prospective randomized trial of chemoembolisation versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26:1156–1161. doi: 10.1002/hep.510260511. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, Vilana R, Rodés J. Transarterial embolisation versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomised, controlled trial in a single institution. Hepatology. 1998;27:1578–1583. doi: 10.1002/hep.510270617. [DOI] [PubMed] [Google Scholar]
- 12.Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: Which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Interv Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 13.Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, Leppard SW, Wolfenden LC, Palmer RR, Stratford PW. DC Bead: in vitro characterisation of a drug-delivery device for transarterial chemoembolisation. J Vasc Interv Radiol. 2006;17:335–342. doi: 10.1097/01.RVI.0000195323.46152.B3. [DOI] [PubMed] [Google Scholar]
- 14.Lewis AL, Gonzalez MV, Leppard SW, Brown JE, Stratford PW, Phillips GJ, Lloyd AW. Doxorubicin eluting beads. 1. Effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007;18:1691–1699. doi: 10.1007/s10856-007-3068-8. [DOI] [PubMed] [Google Scholar]
- 15.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, Bruix J. Chemoembolisation of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolisation for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100–1108. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Kettenbach J, Stadler A, Katzler IV, Schernthaner R, Blum M, Lammer J, Rand T. Drug-loaded microspheres for the treatment of liver cancer: review of current results. Cardiovasc Intervent Radiol. 2008;31:468–476. doi: 10.1007/s00270-007-9280-6. [DOI] [PubMed] [Google Scholar]
- 18.Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, Delis S, Gouliamos A, Kelekis D. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31:269–280. doi: 10.1007/s00270-007-9226-z. [DOI] [PubMed] [Google Scholar]
- 19.Gao S, Yang Z, Zheng Z, Yao J, Deng M, Xie H, Zheng S, Zhou L. Doxorubicin-Eluting Bead versus Conventional TACE for Unresectable Hepatocellular Carcinoma: A Meta-Analysis. Hepatogastroenterology. 2013;60:813–20. doi: 10.5754/hge121025. [DOI] [PubMed] [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 22.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Alexander J, Sutton KRA, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Wiley Chichester. 2000 [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolini D, Svegliati-Baroni G, Candelari R, Mincarelli C, Mandolesi A, Bearzi I, Mocchegiani F, Vecchi A, Montalti R, Benedetti A, Risaliti A, Vivarelli M. Doxorubicin-eluting bead vs conventional transcatheter arterial chemoembolization for hepatocellular carcinoma before liver transplantation. World J Gastroenterol. 2013;19:5622–5632. doi: 10.3748/wjg.v19.i34.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lencioni R, Malagari K, Vogl T. Reduced liver toxicity in intermediate-stage hepatocellular carcinoma patients treated by chemoembolization with drug eluting beads: results from the PRECISION V randomized trial. Hepatology. 2009;50:1079A. doi: 10.2214/AJR.10.4379. [DOI] [PubMed] [Google Scholar]
- 30.Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, Denys A, Lee C. Liver, Gastrointestinal, and Cardiac Toxicity in Intermediate Hepatocellular Carcinoma Treated With PRECISION TACE With Drug-Eluting Beads: Results From the PRECISION V Randomized Trial. AJR Am J Roentgenol. 2011;197:W562–70. doi: 10.2214/AJR.10.4379. [DOI] [PubMed] [Google Scholar]
- 31.Malagari K, Alexopoulou E, Chatzimichail K, Hall B, Koskinas J, Ryan S, Gallardo E, Kelekis A, Gouliamos A, Kelekis D. Transcatheter chemoembolization in the treatment of HCC in patients not eligible for curative treatments: midterm results of doxorubicin-loaded DC bead. Abdom Imaging. 2008;33:512–9. doi: 10.1007/s00261-007-9334-x. [DOI] [PubMed] [Google Scholar]
- 32.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y, Avajon Y, Gruenberger T, Pomoni M, Langenberger H, Schuchmann M, Dumortier J, Mueller C, Chevallier P, Lencioni R PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mabed M, Esmaeel M, El-Khodary T, Awad M, Amer T. A randomized controlled trial of transcatheter arterial chemoembolization with lipiodol, doxorubicin and cisplatin versus intravenous doxorubicin for patients with unresectable hepatocellular carcinoma. Eur J Cancer Care (Engl) 2009;18:492–9. doi: 10.1111/j.1365-2354.2008.00984.x. [DOI] [PubMed] [Google Scholar]
- 34.Recchia F, Passalacqua G, Filauri P, Doddi M, Boscarato P, Candeloro G, Necozione S, Desideri G, Rea S. Chemoembolization of unresectable hepatocellular carcinoma: Decreased toxicity with slow-release doxorubicin‑eluting beads compared with lipiodol. Oncol Rep. 2012;27:1377–83. doi: 10.3892/or.2012.1651. [DOI] [PubMed] [Google Scholar]
- 35.Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, Moschouris H, Emmanouil E, Rizos S, Kelekis D. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541–51. doi: 10.1007/s00270-009-9750-0. [DOI] [PubMed] [Google Scholar]
- 36.Ferrer Puchol MD, la Parra C, Esteban E, Vaño M, Forment M, Vera A, Cosín O. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma. Radiologia. 2011;53:246–53. doi: 10.1016/j.rx.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC) J Surg Oncol. 2010;101:476–80. doi: 10.1002/jso.21522. [DOI] [PubMed] [Google Scholar]
- 38.Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, Tumino E, Ginanni B, Federici G, Cioni R, Metrangolo S, Bertoni M, Bresci G, Parisi G, Altomare E, Capria A, Bartolozzi C. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545–1552. doi: 10.1016/j.jvir.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Song MJ, Chun HJ, Song do S, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM, Lee HG, Yoon SK. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–50. doi: 10.1016/j.jhep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Wiggermann P, Sieron D, Brosche C, Brauer T, Scheer F, Platzek I, Wawrzynek W, Stroszczynski C. Transarterial Chemoembolization of Child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE) Med Sci Monit. 2011;17:CR189–95. doi: 10.12659/MSM.881714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang K, Zhou Q, Wang R, Cheng D, Ma Y. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. Journal of Gastroenterology and Hepatology. 2014;29:920–925. doi: 10.1111/jgh.12439. [DOI] [PubMed] [Google Scholar]
- 42.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12:2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 44.Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, Leppard SW, Wolfenden LC, Palmer RR, Stratford PW. DC bead: in vitro characterisation of a drug-delivery device for transarterial chemoembolisation. J Vasc Interv Radiol. 2006;17:335–342. doi: 10.1097/01.RVI.0000195323.46152.B3. [DOI] [PubMed] [Google Scholar]
- 45.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolisation for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100–1108. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 46.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, Bruix J. Chemoembolisation of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 47.O’Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325–331. doi: 10.1002/bjs.4045. [DOI] [PubMed] [Google Scholar]
- 48.Wigmore SJ, Redhead DN, Thomson BN, Park RW, Garden OJ. Predicting survival in patients with liver cancer considered for transarterial chemoembolization. Eur J Surg Oncol. 2004;30:41–45. doi: 10.1016/j.ejso.2003.10.007. [DOI] [PubMed] [Google Scholar]


