Abstract
Objective: A meta-analysis was performed to comprehensively evaluate the correlations between the C3435T polymorphism of ABCB1 (the ATP-binding cassette, subfamily B, member 1 transporter gene) and drug resistance in epilepsy. Methods: Inclusion and exclusion criteria and a strategy for searching original literature were developed and utilized to search Chinese and non-Chinese databases. Research reports discussing correlations between the ABCB1 C3435T polymorphism and patient responses to anti-epileptic drug (AED) therapy were collected. Comparisons and comprehensive quantitative analyses were conducted using an allele model (C vs. T), and a genotype model (CC vs. CT+TT). In addition, subgroup analyses were performed that divided the included studies according to the race of the study subjects (Asian or Caucasian), based on the geographical region in which each study was conducted. Results: The meta-analysis included a total of 23 publications that examined a total of 3,912 drug-resistant epileptic patients and 4,419 epileptic patients for whom drug treatment was effective. The included studies did not exhibit publication bias. Statistical analyses revealed that the C3435T polymorphism was not significantly correlated with drug resistance in epilepsy. The random-effects model comparing the C and T alleles produced an odds ratio (OR) of 1.10 with a 95% confidence interval (CI) of 0.98-1.25 and P = 0.46. Subgroup analyses suggested that in Caucasian population there are significant differences between resistance group (RN) and control group (N) in both allele model (C vsT: OR = 1.13; 95% CI: 1.03-1.25) and genotype model (CC vsCT+TT: OR = 1.27; 95% CI: 1.08-1.50). Conclusion: The ABCB1 C3435T polymorphism is associated with drug resistance in epilepsy in Caucasian population.
Keywords: Epilepsy, gene polymorphism, ATP-binding cassette, subfamily B, member 1 transporter gene (ABCB1), drug resistance, meta-analysis
Introduction
Epilepsy is one of the most common chronic diseases of the nervous system (1). After treatment with anti-epileptic drugs (AEDs), most epileptic patients show excellent prognoses. However, among 20-30% of epileptic patients, the disease is not effectively controlled after drug therapy because these patients exhibit drug resistance to AEDs (2). P-glycoprotein (P-gp) is the expression product of ABCB1 (the ATP-binding cassette, subfamily B, member 1 transporter gene), which is also known as MDR1 (multi-drug resistance gene 1). P-gp is capable of removing drugs from brain tissues, and studies have demonstrated that patients with drug-resistant epilepsy have elevated levels of ABCB1 expression in the brain. It has therefore been suggested that elevated ABCB1 expression in the brain leads to decreased AED concentrations in the brain, thereby reducing the clinical efficacy of AEDs. Siddiqui et al. (3) first reported that among Caucasians, the C3435T single nucleotide polymorphism (SNP) of ABCB1 was correlated with drug resistance in epilepsy. In particular, it was observed that relative to other individuals, individuals with the 3435CC genotype had significantly increased P-gp expression levels and activity as well as a significantly higher risk for drug resistance. These findings opened new avenues for the genetic diagnosis of intractable epilepsy. Subsequently, many scholars have conducted similar studies on epileptic patients from different regions and various ethnic groups; however, the findings of these investigations are not consistent. To compensate for the deficiencies of each individual investigation, the current study performed a meta-analysis of the results of prior investigations to quantitatively combine and comprehensively evaluate these previous findings. Using this approach, this study sought to further explore the correlations between the ABCB1 C3435T polymorphism and drug resistance in epilepsy and thereby provide a reference for early predictions of the risk of drug resistance in epilepsy.
Materials and methods
Sources of data
The keywords “polymorphism”, “multi-drug resistance gene 1”, “C3435T”, “epilepsy”, “intractable epilepsy”, “antiepileptic drugs”, and “drug-resistant” were used to search the China National Knowledge Infrastructure (CNKI) database, the China Biomedical Literature (CBM) database, the Wanfang Data Resource System, the MedCH international medical abstract database, the PubMed database, the ScienceDirect database, and other databases. These searches were supplemented by retrospective and manual searches of the literature, among other methods. The first report on the relationship between the ABCB1 C3435T polymorphism and drug resistance in epilepsy appeared in 2003, and the end date for the retrieval process was March 31, 2014.
Literature inclusion and exclusion criteria
Literature inclusion criteria: (1) Each publication was required to be a domestic or international study published in Chinese or English that addressed the correlation between the ABCB1 C3435T polymorphism and drug resistance in epilepsy. (2) Each publication was required to report complete data, the number of examined individuals in the drug-resistant group and the therapeutically effective group, the frequency of the CC, CT, and TT genotypes at the 3435 locus of the ABCB1 gene, and data that could be used to calculate odds ratio (OR) values with 95% confidence intervals (CIs) for the combined effects of C3435T polymorphism status on drug resistance. (3) Each publication was also required to include study subjects who were epileptic patients and to utilize drug therapy as the intervention measure.
Exclusion criteria: Studies were excluded if they featured [1] duplicate findings from the same population examined in another publication, in which case only the publication with the largest sample size was retained, or [2] little data, poor-quality data, or incomplete data that could not be utilized.
Data extraction
Data extraction was performed independently by two researchers, and the extracted data were subsequently verified. Ultimately, consensus was reached regarding all of the examined items. The retrieved data included the author names, the date of publication, the nationality of the study population, allele frequency distributions, and genotype frequency distributions. If genotype frequency distributions were expressed as percentages, then data were entered after converting these percentages into numbers of cases. If allele distributions were not provided, then these distributions were calculated from genotype distributions.
Data analysis
Heterogeneity testing
The Q test was utilized to evaluate the consistency of the results of the included studies. Heterogeneity was assessed using the value of I2. In particular, if I2 < 50%, a fixed-effects model was used to pool study data; if I2 ≥ 50%, a random-effects model was used to pool study data. Pooled OR values and 95% CIs were calculated.
Meta-analysis
RevMan 5.0 software was used to compile and analyze the raw data from all included studies. The OR and 95% CI values of an allele model (C3435T), a genotype model (CC vs. CT+TT) and a allele model were used as effect indicators. The drug-resistant group (RN) and the therapeutically effective group (N) were compared to determine differences in allele distribution frequencies and genotype distribution frequencies between these two groups. In addition, subgroup analyses were performed that divided the included studies according to the race of the study subjects (Asian or Caucasian), based on the geographical region in which each study was conducted.
Results
Search results and literature inclusion conditions
After screening, 23 publications [3-25] were included in the meta-analysis for this study. These publications examined a total of 8,331 cases, including 3,912 drug-resistant epileptic patients and 4,419 in the therapeutically effective group. There were 6 studies that indicated that the ABCB1 C3435T polymorphism was statistically correlated with drug resistance in epilepsy [3-8]; in particular, 4 studies indicated that the homozygous CC genotype was correlated with a higher risk of drug resistance [3,4,6,8], whereas 2 studies indicated that the homozygous TT genotype was correlated with a higher risk for drug resistance [5,7]. The remaining 17 studies [9-25] indicated that there was no significant correlation between the C3435T polymorphism and drug resistance in epilepsy. The included studies examined different Asian populations, such as the Chinese Han [7,11,17,24,25], Taiwanese Han [6], Indian [16,19-22,24], Japanese [5], Korean [15], Turkish [13,14], Iranian [8], and Malaysian [24] populations, and various Caucasian populations, such as the British [3,4], Australian [9], Scottish [10], Irish [12], German [18], and Spanish [21] populations. There was a multi-center study that included both Asians (from Hong Kong, China) and Caucasians (Australians and Scots) [17]. In the subgroup analysis, patients from Hong Kong, China were included in the Asian population, whereas patients from Australia and Scotland were included in the Caucasian population. Therefore, there were effectively a total of 13 studies examining Asian populations and a total of 12 studies that examined Caucasian populations (Table 1).
Table 1.
The characteristics of included studies
| Authors | Publication year | Country | NR | R | NR | R | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| CC | CT | TT | CC | CT | TT | C | T | |||
| Chen L et al. | 2007 | China | 15 | 25 | 10 | 63 | 79 | 22 | 50 | 164 |
| Dericiogl et al. | 2008 | Turkey | 26 | 34 | 29 | 25 | 49 | 26 | 89 | 100 |
| Di Q et al. | 2011 | China | 44 | 37 | 10 | 32 | 30 | 17 | 91 | 79 |
| Dong et al. | 2011 | China | 64 | 75 | 18 | 82 | 83 | 28 | 157 | 193 |
| Grover et al. | 2010 | India | 13 | 44 | 30 | 14 | 55 | 56 | 87 | 125 |
| Haerian et al. | 2011 | Asian | 109 | 158 | 56 | 110 | 180 | 72 | 323 | 362 |
| Hung et al. | 2007 | China | 40 | 55 | 19 | 39 | 107 | 67 | 114 | 213 |
| Kim et al. | 2009 | Korea | 73 | 97 | 28 | 81 | 90 | 22 | 198 | 193 |
| Kumaril et al. | 2011 | India | 12 | 67 | 46 | 42 | 120 | 98 | 125 | 260 |
| Kwan et al. | 2007 | China | 80 | 104 | 37 | 114 | 161 | 22 | 221 | 297 |
| Ozgon et al. | 2008 | Turkey | 13 | 26 | 5 | 16 | 29 | 8 | 44 | 53 |
| Sanchez et al. | 2010 | Spain | 40 | 49 | 22 | 52 | 81 | 45 | 111 | 178 |
| Sayyah et al. | 2011 | Iran | 34 | 55 | 43 | 32 | 80 | 88 | 132 | 200 |
| Seo et al. | 2006 | Japan | 34 | 58 | 34 | 36 | 34 | 14 | 126 | 84 |
| Shahwan et al. | 2007 | Ireland | 20 | 64 | 38 | 37 | 119 | 77 | 122 | 233 |
| Siddiqui et al. | 2003 | UK | 55 | 106 | 39 | 18 | 63 | 34 | 200 | 115 |
| Sills et al. | 2005 | Scotland | 41 | 112 | 77 | 32 | 82 | 56 | 230 | 170 |
| Soranzo et al. | 2004 | UK | 73 | 145 | 62 | 20 | 80 | 36 | 280 | 136 |
| Szoeke et al. | 2009 | Australia | 21 | 27 | 16 | 34 | 67 | 47 | 64 | 148 |
| Szoeke et al. | 2009 | Hong Kong | 1 | 8 | 2 | 13 | 20 | 1 | 11 | 34 |
| Szoeke et al. | 2009 | Scotland | 20 | 69 | 44 | 34 | 72 | 46 | 133 | 152 |
| takhan et al. | 2009 | India | 9 | 52 | 33 | 38 | 104 | 89 | 94 | 231 |
| Tan et al. | 2004 | Australia | 75 | 193 | 133 | 37 | 115 | 56 | 401 | 208 |
| Ufer et al. | 2009 | Germany | 44 | 85 | 59 | 20 | 46 | 37 | 188 | 103 |
| Vahab et al. | 2009 | India | 3 | 61 | 49 | 3 | 8 | 43 | 113 | 54 |
Note: NR: Anti-epileptic drug no response (Case group); R: effective group (Control group).
Meta-analysis results
Analysis of the allele contrast model (C vs. T) for the overall population revealed that there was high heterogeneity among the included studies (I2 = 69%, P < 0.001); therefore, a random-effects model was used to pool the OR values for the frequency of the 3435C allele. The pooled OR value was 1.10 (95% CI: 0.98-1.25, P = 0.11) in allele model and 1.06 (95% CI: 0.90-1.26, P = 0.46) in genotype model, indicating that the 3435C allele was not significantly correlated with drug resistance in epilepsy (Figures 1, 2). Subgrouping in accordance with the race of the study subjects was conducted based on the regions where the studies were conducted. There was significant heterogeneity among the studies examining Asian populations (I2 = -61%, P < 0.001); therefore, a random-effects model was used to pool OR values, producing a pooled OR value of 1.00 (95% CI: 0.82-1.21, P = -0.960) in allele model and 0.90 (95% CI: 0.70-1.17, P = -0.43) in genotype model (Figures 3, 4). There was no heterogeneity among studies examining Caucasian populations (I2 = 32%, P = 0.14); therefore a fixed-effect was utilized to merge the OR values. We found in Caucasian population there are significant differences between resistance group and control group in both allele model (C vs T: OR = 1.13; 95% CI: 1.03-1.25) and genotype model (CC vs CT+TT: OR = 1.27; 95% CI: 1.08-1.50) (Figures 5, 6).
Figure 1.
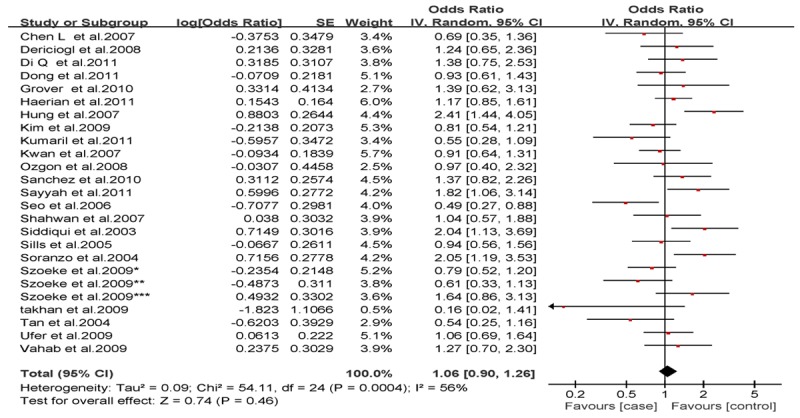
Forest plot of C3435T polymorphism of the ABCB1 gene and drug resistance in epilepsy in total, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI (CC vs. CT+TT).
Figure 2.
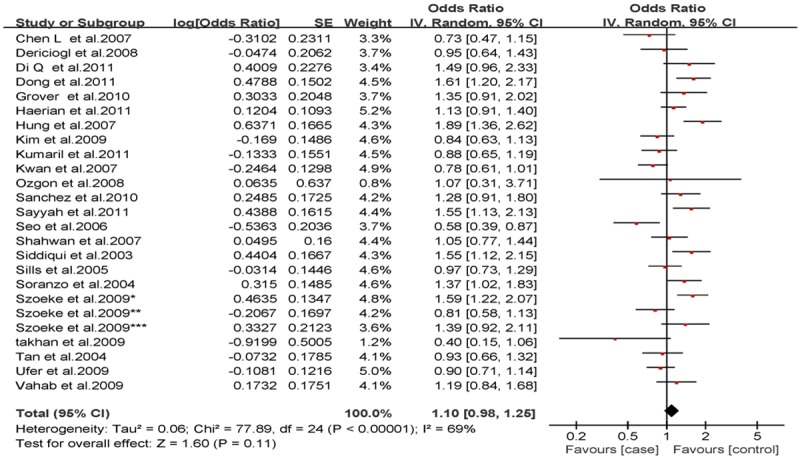
Forest plot of C3435T polymorphism of the ABCB1 gene and drug resistance in epilepsy in total, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI (C vs. T).
Figure 3.
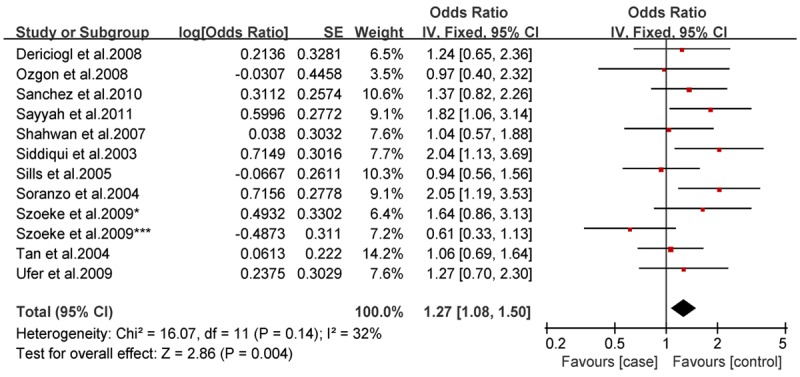
Forest plot of C3435T polymorphism of the ABCB1 gene and drug resistance in epilepsy in Caucasian population, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI (CC vs. CT+TT).
Figure 4.
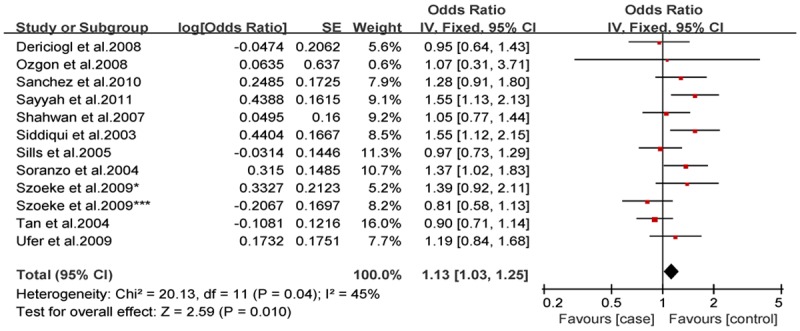
Forest plot of C3435T polymorphism of the ABCB1 gene and drug resistance in epilepsy in Caucasian population, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI (C vs. T).
Figure 5.
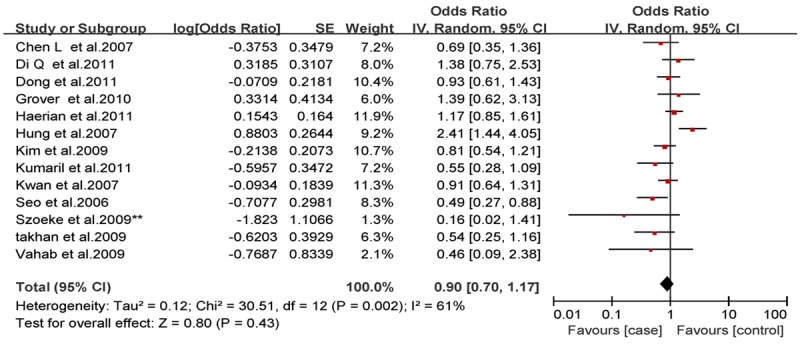
Forest plot of C3435T polymorphism of the ABCB1 gene and drug resistance in epilepsy in Asian population, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI (CC vs. CT+TT).
Figure 6.
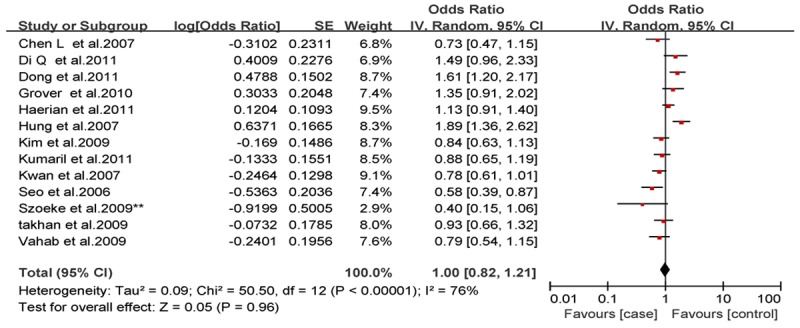
Forest plot of C3435T polymorphism of the ABCB1 gene and drug resistance in epilepsy in Asian population, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI (C vs. T).
Quality analyses of the included studies
Sensitivity analysis
Sensitivity analysis was performed on the included literature using the method of one-by-one elimination. The appropriate effects models after any one study was excluded indicated that the OR values for each altered group were close to the corresponding original OR values and that the overall OR value remained similar and did not significantly change from the original overall OR value. These results indicated that this study exhibited relatively good stability.
Analysis of publication bias
Bias analysis for the allele contrast model (C vs. T) produced a funnel plot that was essentially symmetric, with most points lying within the 95% CI. Validation using Egger’s linear regression method demonstrated that, the bias coefficient, was -0.37 (95% CI: -0.34-2.33), with P = 0.717. Therefore, the included studies could not be regarded as having publication bias (Figure 7). Similarly, additional analyses of the studies included in the examined genetic models and subgroups revealed no significant publication bias, indicating that the study results were relatively creditable.
Figure 7.
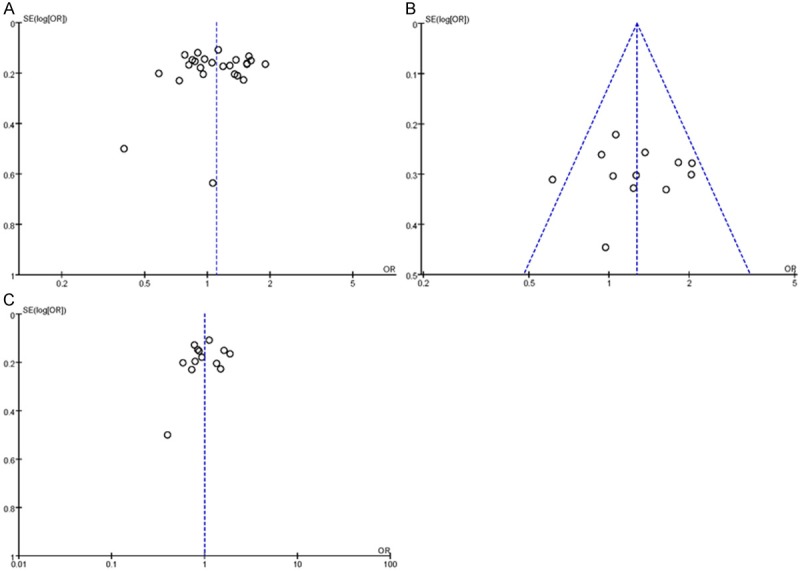
Begg’s funnel plot for publication bias tests. Each point represents a separate study for the indicated association. Log or represents natural logarithm of OR. Vertical line represents the mean effects size. A: In total; B: In Caucasian population; C: In Asian population
Discussion
ABCB1 is located on the long arm of chromosome 7 (at 7q21.1). The full length of the complementary DNA (cDNA) for this gene is 4,669 bp, and the gene contains 28 exons and encodes 1,280 amino acids. P-gp, the expression product of ABCB1, can protect cells from damage induced by toxins or metabolites. However, P-gp overexpression in the brain can reduce AED concentrations in brain tissues, which constitutes an important underlying mechanism for drug resistance in epilepsy [7]. This meta-analysis collected 23 publications addressing the relationship between the ABCB1 C3435T polymorphism and drug resistance in epilepsy. A total of 6 studies produced positive results [3-8]; among these studies, 4 investigations suggested that the 3435C allele is a risk factor for drug resistance in epilepsy [3,4,6,8], and 2 investigations suggested that the 3435T allele is a risk factor for drug resistance in epilepsy [5,7]. In the remaining 17 studies, no correlation was found between the C3435T polymorphism and drug resistance in epilepsy. In the current study, no statistically significant correlation between the ABCB1 C3435T polymorphism and drug resistance in epilepsy was found in analyses of either the allele model or genetic model in total population. Furthermore, subgroup analyses organized in accordance with subjects’ racial groups (Asian or Caucasian), as determined using the geographic regions of the included studies, revealed positive correlations between this polymorphism and drug resistance in epilepsy in Caucasian population but not in Asian population.
The conclusions of the various studies included in the meta-analysis were inconsistent. This phenomenon may be correlated with the following factors. (1) The specific pathogenic gene loci that cause differences in ABCB1 function remain unclear. Thus, the possibility that many SNPs other than C3435T can concurrently affect ABCB1 gene function cannot be excluded. (2) Various included studies involved different uses of AEDs. For instance, certain included studies involved AED monotherapies, whereas others included investigations involved combination therapies. Among the currently known AEDs, phenytoin, levetiracetam, lamotrigine, and phenobarbital are all transported by P-gp in the human body. In contrast, valproic acid is not transported by P-gp; thus, if valproic acid was administered to many of the examined patients, it may be difficult to accurately determine whether the ABCB1 C3435T polymorphism is truly correlated with drug resistance in epilepsy. (3) Currently, there is no universally accepted definition of drug resistance in epilepsy. Siddiqui et al. (3) defined drug resistance in epilepsy as the occurrence of at least four seizures during the year prior to a subject’s enrollment despite the use of at least three appropriately selected AEDs at these drugs’ maximum tolerated doses. Because different researchers used different criteria, certain patients who would have been classified into the therapeutically effective group by the aforementioned definition were instead classified into the drug resistance group in certain studies. This difference in patient categorization is also an important reason for the different results of various studies.
In summary, various recent studies have produced different findings regarding the relationship between the ABCB1 C3435T polymorphism and drug resistance in epilepsy. The current meta-analysis only confirmed the existence of significant correlations between this polymorphism and drug resistance in epilepsy in Caucasian population. In the future, to further enhance the consistency and persuasiveness of studies addressing these correlations, a unified definition of drug resistance in epilepsy should be devised, larger sample sizes should be examined, and multi-center investigations should be conducted that assess multiple SNP loci.
Disclosure of conflict of interest
None.
References
- 1.Liu YM, Lowe H, Zak MM, Kobayashi J, Chan VW, Donner EJ. Can children with hyperlipidemia receive ketogenic diet for medication-resistant epilepsy? J Child Neurol. 2013;28:479–83. doi: 10.1177/0883073813476140. [DOI] [PubMed] [Google Scholar]
- 2.Braakman HM, Vaessen MJ, Hofman PA, Debeijvan Hall MH, Backes WH, Vles JS, Aldenkamp AP. Cognitive and behavioral complications of frontal lobe epilepsy in children: a review of the literature. Epilepsia. 2011;52:849–56. doi: 10.1111/j.1528-1167.2011.03057.x. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui A, Kerb R, Weale ME, Brinkmann U, Smith A, Goldstein DB, Wood NW, Sisodiya SM. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348:1442–1448. doi: 10.1056/NEJMoa021986. [DOI] [PubMed] [Google Scholar]
- 4.Soranzo N, Cavalleri GL, Weale ME, Wood NW, Depondt C, Marguerie R, Sisodiya SM, Goldstein DB. Identifying candidate causal variants responsible for altered activity of the ABCBl multidrug resistance gene. Genome Res. 2004;14:1333–1344. doi: 10.1101/gr.1965304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.See T, Ishitsu T, Ueda N, Nakada N, Yurube K, Ueda K, Nakagawa K. ABCB1 polymorphisms influence the response to antiepileptic drugs in Japanese epilepsy patients. Pharmacogenomics. 2006;7:551–561. doi: 10.2217/14622416.7.4.551. [DOI] [PubMed] [Google Scholar]
- 6.Hung CC, Jen Tai J, Kao PJ, Lin MS, Liou HH. Association of polymorphisms in NRI12 and ABCBl genes with epilepsy treatment responses. Pharmacogenomics. 2007;8:l151–l158. doi: 10.2217/14622416.8.9.1151. [DOI] [PubMed] [Google Scholar]
- 7.Kwan P, Baurn L, Wong V, Ng PW, Lui CH, Sin NC, Hui AC, Yu E, Wong LK. Association between ABCB1 C3435T polymorphism and drug-resistant epilepsy in Han Chinese. Epilepsy Behav. 2007;11:112–117. doi: 10.1016/j.yebeh.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Sayyah M, Kamgarpour F, Maleki M, Karimipoor M, Gharagozli K, Shamshiri AR. Association analysis of intractable epilepsy with C3435T and G2677T/A ABCB1 gene polymorphisms in Iranian patients. Epileptic Disord. 2011;13:155–165. doi: 10.1684/epd.2011.0443. [DOI] [PubMed] [Google Scholar]
- 9.Tan NC, Heron SE, Scheffer IE, Pelekanos JT, McMahon JM, Vears DF, Mulley JC, Berkovic SF. Failure to confirm association of a polymorphism in ABCBl with multidrug-resistant epilepsy. Neurology. 2004;63:1090–1092. doi: 10.1212/01.wnl.0000137051.33486.c7. [DOI] [PubMed] [Google Scholar]
- 10.Sills GJ, Mohanraj R, Butler E, McCrindle S, Collier L, Wilson EA, Brodie MJ. Lack of association between the C3435T polymorphism in the human multidrug resistance (MDRl) gene and response to antiepileptic drug treatment. Epilepsia. 2005;46:643–647. doi: 10.1111/j.1528-1167.2005.46304.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Liu CQ, Hu Y, et al. Research on the correlation between childhood epilepsy drug reactions of multidrug resistance gene MDRl C3435T polymorphism. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:11–14. [PubMed] [Google Scholar]
- 12.Shahwan A, Murphy K, Doherty C, Cavalleri GL, Muckian C, Dicker P, McCarthy M, Kinirons P, Goldstein D, Delanty N. The controversial association of ABCBl polymorphisms in refiactory epilepsy: an analysis of multiple SNPs in an Irish population. Epilepsy Res. 2007;73:192–198. doi: 10.1016/j.eplepsyres.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Dericioglu N, Babaoglu MO, Yasar U, Bal IB, Bozkurt A, Saygi S. Multidrug resistance in patients undergoing resective epilepsy surgery is not associated with C3435T polymorphism in the ABCBI (MDRI) gene. Epilepsy Res. 2008;80:42–46. doi: 10.1016/j.eplepsyres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Ozgon GO, Bebek N, GUl G, Cine N. Association of MDRl (C3435T) polymorphism and resistance to carbarnazepine in epileptic patients from Turkey. Eur Neurol. 2008;59:67–70. doi: 10.1159/000109264. [DOI] [PubMed] [Google Scholar]
- 15.Kim DW, Lee SK, Chu K, Jang IJ, Yu KS, Cho JY, Kim SJ. Lack of association between BCBI, ABCG2, and ABCC2 genetic polymorphisms and multidrug resistance in partial epilepsy. Epilepsy Res. 2009;84:86–90. doi: 10.1016/j.eplepsyres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Lakhan R, Misra UK, Kalita J, Pradhan S, Gogtay NJ, Singh MK, Mittal B. No association of ABCBl polymorphisms with drug-refractory epilepsy in a north Indian population. Epilepsy Behav. 2009;14:78–82. doi: 10.1016/j.yebeh.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Szoeke C, Sills GJ, Kwan P, Petrovski S, Newton M, Hitiris N, Baum L, Berkovic SF, Brodie MJ, Sheffield LJ, O’Brien TJ. Multidrug-resistant genotype (ABCBl) and seizure recurrence in newly treated epilepsy: data from intemational pharmacogenetic cohorts. Epilepsia. 2009;50:689–1696. doi: 10.1111/j.1528-1167.2009.02059.x. [DOI] [PubMed] [Google Scholar]
- 18.Ufer M, Mosyagin I, Muhle H, Jacobsen T, Haenisch S, Häsler R, Faltraco F, Remmler C, von Spiczak S, Kroemer HK, Runge U, Boor R, Stephani U, Cascorbi I. Non-response to antiepileptic pharmacotherapy is associated with the ABCC2-24C>T polymorphism in young and adult patients with epilepsy. Pharmacogenet Genomics. 2009;19:353–362. doi: 10.1097/fpc.0b013e328329940b. [DOI] [PubMed] [Google Scholar]
- 19.Vahab SA, Sen S, Ravindran N, Mony S, Mathew A, Vijayan N, Nayak G, Bhaskaranand N, Banerjee M, Satyamoorthy K. Analysis of genotype and haplotype effects of ABCBl (MDRl) polymorphisms in the risk of medically refractory epilepsy in an Indian population. Drug Metab Pharmacokinet. 2009;24:255–260. doi: 10.2133/dmpk.24.255. [DOI] [PubMed] [Google Scholar]
- 20.Grover S, Bala K, Sharma S, Gourie-Devi M, Baghel R, Kaur H, Gupta M, Talwar P, Kukreti R. Absence of a general association between ABCB1 Genetic variants and response to antiepileptic drugs in epilepsy patients. Biochimie. 2010;92:1207–1212. doi: 10.1016/j.biochi.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez MB, Herranz JL, Leno C, Arteaga R, Oterino A, Valdizán EM, Nicolás JM, Adín J, Armijo JA. Genetic factors associated with drug-resistance of epilepsy: Relevance of stratification by patient age and aetiology of epilepsy. Seizure. 2010;19:93–101. doi: 10.1016/j.seizure.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Kumari R, Lakhan R, Garg RK, Kalita J, Misra UK, Mittal B. Pharmacogenomic association study on the role of drug metabolizing. drug transporters and drug target gene polymorphisms in drug-resistant epilepsy in a north Indian population. Indian J Hum Genet. 2011;17:S32–40. doi: 10.4103/0971-6866.80357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong L, Luo K, Tong Y, Cai X, Mao M, Yu D. Lack of association between ABCBl gene polymorphisms and pharmacoresistant epilepsy: an analysis in a western Chinese pediatric population. Brain Res. 2011;1391:114–124. doi: 10.1016/j.brainres.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Haerian BS, Lim KS, Mohamed EH, Tan HJ, Tan CT, Raymond AA, Wong CP, Wong SW, Mohamed Z. Lack of association of ABCBl and PXR polymorphisms with response to treatment in epilepsy. Seizure. 2011;20:387–394. doi: 10.1016/j.seizure.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Di Q, Wang LL, Xu LG, et al. Refractory epilepsy and multidrug resistance gene 1 C3435T polymorphism correlation. Zhonghua Shen Jing Yi Xue Za Zhi. 10:127–131. 201. [Google Scholar]


