Abstract
Epidemiological data suggest there is a female sex advantage in melanoma skin cancer. Female sex hormones have been attributed to this protection. There has been no experimental evidence to link female sex hormones directly with melanoma protection until our recently published work on mouse melanoma (B16F10) cells. Our recently published work showed that progesterone significantly inhibited mouse melanoma cell growth in vitro. This study was extended to human melanoma (BLM) cells. Research work revealed that progesterone inhibited human melanoma cell growth also in vitro. The mechanism of inhibition was due to autophagy and this effect of progesterone was not mediated through progesterone receptor.
Keywords: Progesterone, human melanoma (BLM) cells, cell growth, autophagy, 3-methyladenine (3-MA) rescue, RU-486, progesterone receptor
Introduction
Melanoma is a dangerous form of skin cancer [4]. It is the only common cancer that has shown a gender difference in outcome. Epidemiological data indicate a female sex advantage in malignant melanoma. For years doctors have known that the overall survival outcomes for women with malignant melanoma were superior to the outcomes for men [5-8]. Data obtained from National Cancer Data Base (NCDB) of more than 23,000 melanoma patients were used to examine patterns of long-term survival by patient gender and age, stage of disease, disease morphology and anatomic sub-site. Data suggested a role for hormones in the improved survival of young women with melanoma [9]. The overall survival outcome for young women (45 years of age and under) was far superior to older women (55 years of age and older) and men of any age group [9]. One study, which analyzed survival data in 6383 patients, demonstrated a 22% survival advantage and 17% 5 year disease-free interval advantage for females [10]. In another 22 epidemiological studies, all but four studies female patients were found to have a significant survival advantage over males [10]. In fact, women survived longer than men after the development of stage III disease. There were instances of melanoma diagnosed in premenopausal women metastasizing many years later in the post-menopausal period possibly due to the loss of some protective effect existing in the younger patient [10]. It has been postulated that female hormones may exert a protective effect against melanoma metastases [10]. Since the databases were not designed with respect to patient sex steroid status, no correlation with sex steroid hormones could be made in these studies. Most of the evidences linking sex and cutaneous melanoma are epidemiological in nature. Direct experimental evidence is lacking.
Endocrinologically, the major difference between male and female is in the sex steroid hormones [11]. In fact, the difference between premenopausal and post-menopausal women is in the concentration of estrogen and progesterone in the blood [11]. These two hormones could be playing a role in offering protection to the young menstruating female. Literature search for the role of estrogen in melanoma revealed that most of the research on sex hormone therapy in melanoma was concerned with anti-neoplastic, anti-estrogen receptor drug tamoxifen (TAM) [9]. Studies have demonstrated negative results. A recent study which used monoclonal antibodies to detect high-affinity estrogen receptor (ER) failed to show receptors in wide range of melanocytic proliferations [9]. Moreover, in vitro dose-curve study of estrogen with human melanoma (BLM) cells did not show any inhibition (data not shown) in our hand. So, we turned our attention to progesterone and the question whether progesterone could offer protection to young females. Literature survey showed that information available on the role of progesterone in melanoma is inconclusive [12-14]. Moreover, Progesterone binding has been reported using biochemical assays [15], but the presence of progesterone receptors have not been confirmed by immunohiostochemical studies [16]. Since there is a gap in the information relating to the role of progesterone on melanoma growth, we decided to fill this gap by using human melanoma cell line and progesterone. At this junction, it is appropriate to point out our recently published work on mouse melanoma cell line B16F10 [1,2], which showed that progesterone significantly inhibited mouse melanoma cell growth in vitro. So, we extended our study to include human melanoma cells [3] to determine if there was a direct effect of progesterone on human melanoma cell growth.
Materials and methods
Progesterone, estrogen, RU-486, were all obtained from Sigma Chemical Company, St. Louis, MO. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide), DAPI (4’-6 diamidino-2-phenylindole), trypan blue, isopropanol, spermidine, 3-methyladenine, and agarose were also obtained from Sigma Chemical Company. Caspase-3 assay kit (EnzChek caspase-3 assay kit #1) was purchased from Molecular Probes, Eugene, Oregon. Fetal bovine serum (FBS), Trypsin-EDTA (1X), and PBS powder were purchased from Atlanta Biological, Lawrenceville, GA. RPMI and antibiotic/antimycotic solution 100X (10,000 I.U/ml penicillin, 10 mg/ml streptomycin, 25 μg/ml amphotericin B) were purchased from Fisher scientific, Houston, TX. SLT Spectra plate reader was used for quantitation of MTT assay.
Cell line
Human melanoma cell line BLM was obtained from a colleague (Dr. James L. Cox) in the department.
Growth medium (GM)
All cell culture works were carried out in RPMI 1640 medium containing 10% FBS +1X Pen/Strep/Ampho.
Preparation of steroid hormones, MTT and DAPI solutions
Initially, 10 mM stock solutions of steroid hormones in ethanol were prepared. One mM working stock solution was made by diluting one part of alcohol stock with nine parts of GM. Different concentrations of hormones were made from 1 mM working stock by serial dilution. MTT stock solution was made by dissolving 5 mg in one ml of PBS. DAPI at 5 mg/ml stock solution was made and was diluted 1:1000 in PBS before the experiment.
Cell growth assay
MTT proliferation assay [17] was used to quantitate cell growth in treated (progesterone) and in untreated (control) samples. In addition, microscopic pictures of cells treated with different concentrations of progesterone were taken to document hormonal effects on cell growth.
MTT proliferation assay
BLM cells were suspended in growth medium (GM) and plated at a density of 1 × 104 cells/ well in a 96 well plate. Cells were left overnight at 37°C to attach to the plate. Following day growth medium was replaced by GM containing hormones at different concentrations and incubated for 48 hrs. After 48 hrs medium was replaced by 100 μl of 1 in 10 diluted (in GM) MTT solution and incubated for another 4 hrs at 37°C. After 4 hrs MTT solution was removed. MTT was reduced by metabolically viable cells to a colored (purple) water insoluble formazan salt. The purple color precipitate was solubilized by adding 100 μl of isopropanol and shaken for 20-30 min at room temperature. Intensity of resultant purple color was measured at 570 nm in a SLT spectra plate reader.
Trypan blue staining [18]
Trypan blue is a dye that does not interact with cell unless the cell membrane is damaged. Healthy, undamaged cells exclude the dye, but it is readily absorbed by damaged cells and renders them clearly visible under microscope. After 48 hrs of incubation of BLM cells with hormone or hormone +3-MA, 100 ul of 0.4% trypan blue in PBS was added to the chamber slide containing 1 × 104 cells and incubated for 5 min at room temperature. Cells were washed with PBS to remove excess trypan blue dye and were then examined under a microscope. Only dead cells would take up the dye and appear as darkly stained cells under the microscope.
DAPI staining [19]
DAPI is a blue fluorescent DNA probe. DAPI stains nucleus specifically with little or no cytoplasmic labeling. After 48 hrs. of incubation of BLM cells with hormone or hormone +3-MA, cells in the chamber slide were fixed with 2% paraformaldehyde for 20 min. Cells were then washed with PBS to remove paraformaldehyde. One hundred ul of 1 in 1000 diluted DAPI solution was added to the cells and they were incubated in the dark for 5 min. Excess DAPI was removed by washing with PBS and was viewed under bright and fluorescent light microscope.
Agarose gel electrophoresis [20]
1.2% agarose gel was made by mixing agarose in 1X TAE buffer. After cooling the solution, ethidium bromide was added to the solution and poured into the gel apparatus. Forty microgram of DNA was loaded on the well and ran at 100 volts till the bromophenol dye moved ¾ of the gel. The gel was photographed under UV light.
Caspase-3 assay
Caspase-3 (CPP-32, Apoptain, Yama, SCA-1) is a critical executioner of apoptosis, as it is either partially or totally responsible for the proteolytic cleavage of many key proteins, such as the nuclear enzyme poly (ADP-ribose) polymerase (PARP) [21]. Assays were carried out as per the manufacturer’s protocol. Briefly, cells (25000 cell/well) were lysed with lysis buffer. Two times (2X) reaction buffer was added, followed by 2X substrate working solution was added. The plate was covered and incubated at room temperature for 30 min. The plate was read at an excitation wavelength of 340 nM and an emission wavelength of 440 nM.
Experiments
Initially dose response curve was carried out with progesterone starting from 100 nM to 200 μM. After dose-curve, time course assays were carried out by using MTT assays at the end of 12, 24, 48 and 72 hours of incubation with progesterone to determine the optimal incubation time with progesterone. Following time-course study, the mechanism of inhibition of cell growth was determined. First, for necrosis staining with trypan blue dye was carried out to find the number of dead cells in both progesterone treated and untreated control cells. For apoptosis DAPI staining was carried out to check for change in the shape of nucleus. In addition agarose gel electrophoresis was carried out with DNA harvested from control and progesterone treated cells to check for DNA ladder formation. Caspase-3 assay was carried out to check whether there was an increase in the effector caspase activity. Finally for autophagy, co-incubation of progesterone with 3-methyladenine (3-MA) was carried out to check if there was suppression of autophagy and rescue of cell growth. In order to check the mechanism of progesterone action, bioassays involving co-incubation of progesterone with RU-486 and pretreatment of cells with RU-486 followed by progesterone treatment were carried out.
Statistical analysis
All experimental points were carried out in triplicate (3 wells). Average of all the three values was taken as mean and standard error was calculated. Each experimental point was expressed as Mean ± SEM. Each experiment was repeated a minimum of two times to check for consistency in results. Significance between any two experimental conditions was decided using Student’s ‘t’ test. Unpaired t-test was used to determine the P value and a P value of 0.05 or less was considered significant.
Results
Dose and time course curves of progesterone with human melanoma (BLM) cells
Initially dose curve study was carried out with progesterone starting from 100 nM to 200 μM to find out progesterone’s effect on human melanoma (BLM) cell growth. There was a dose-dependent (sigmoidal) inhibition of human melanoma cell growth (Figure 1A). The dose-response curve was similar to the dose-response curve of mouse melanoma cell growth [2]. Secondly, to optimize the length of incubation time to effect significant inhibition of cell growth, dose-curves were carried out at 12, 24, 48 and 72 hrs. time points (Figure 1B). The dose-response curves of 48 and 72 hrs were very close, so we decided to incubate melanoma cells with progesterone for 48 hrs.
Figure 1.
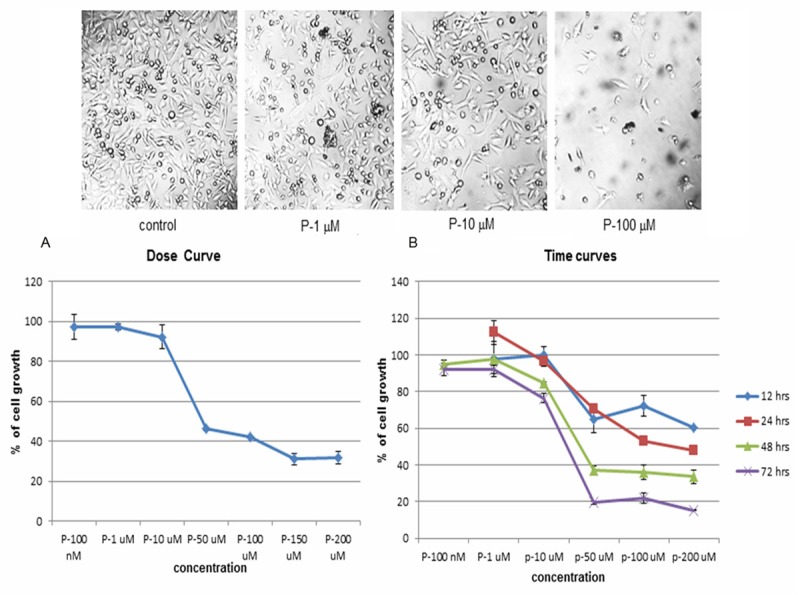
Dose and Time course studies with progesterone. A. To check the effect of progesterone on human melanoma (BLM) cell growth, dose-response study was carried out starting from a concentration of 100 nM to 200 μM. There was a sigmoidal dose response curve, indicating it was a genuine biological action. B. In order to find out the optimal time for incubation of BLM cells with progesterone to produce maximum inhibition, dose response curves were carried out at 12, 24, 48 and 72 hours. Dose response curves at 48 and 72 hrs. Yielded similar pattern of growth. So, it was decided to incubate BLM cells with progesterone for 48 hrs.
Mechanism of cell growth inhibition
There was a significant decrease in melanoma cell growth with increasing concentration of progesterone. There were also cells floating in the medium. So, cell growth inhibition was due to apparent cell death. Hence, we decided to investigate the mechanism of cell death.
Necrosis
Cell death by necrosis occurs when the cell membrane is ruptured. Necrosis is a process of cellular lysis with a corresponding release of cytoplasmic components into the surrounding tissue. Trypan blue dye exclusion test was used to check if necrosis had occurred after progesterone treatment. Trypan blue dye could get into the cell and stain the dead cell. Viable cells would exclude trypan blue from inside. Trypan blue staining did not show any significant increase in the number of dead cells between control and progesterone treated cells (Figure 2), indicating necrosis was not the mechanism by which cell growth was inhibited.
Figure 2.
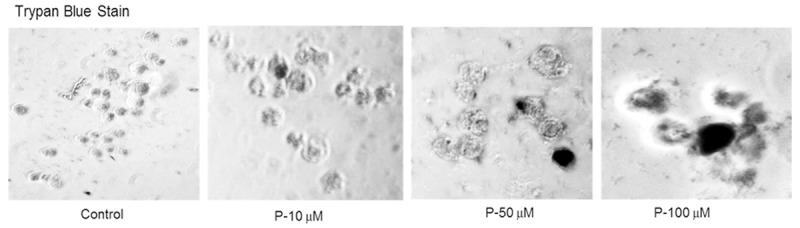
Mechanism of cell death-Necrosis. To find out whether the cell death was caused by necrosis, cells treated with progesterone were stained with equal volume of 0.4% trypan blue dye and were examined under a microscope. There was no significant difference in the number of stained cells between control and progesterone treated cells, indicating necrosis was not the cause of cell death.
Apoptosis
Apoptosis, also known as programed cell death proceeds systematically. Chromatin condensation followed by change in nuclear shape takes place. So DAPI was used to stain the nucleus to check for change in nuclear shape.
A and B-DAPI staining
DAPI staining did not show any change in the shape of nuclei between control and progesterone treated cells, as viewed by bright (Figure 3A) and fluorescent (Figure 3B) light microscope.
Figure 3.
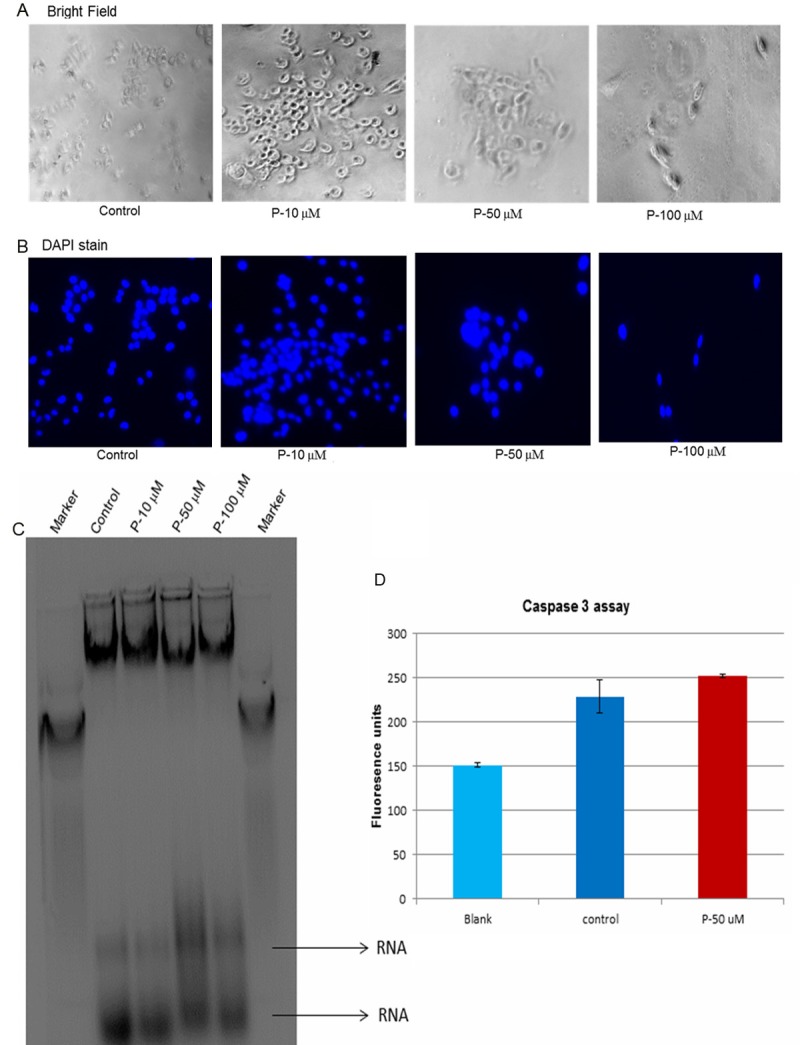
Mechanism of cell death-Apoptosis. In order to check whether the mechanism of cell death was due to apoptosis, cells after incubation with progesterone for 48 hrs. were subjected to DAPI staining, DNA analysis by agarose gel electrophoresis, and caspase-3 assay. (A and B) DAPI staining of the nucleus: As blebbing of the nucleus and chromatin condensation take place in apoptosis, DAPI staining of the nucleus was done to check for change in the shape of nucleus. DAPI staining did not show any change in the shape of the nuclei between control and progesterone treated cells. The cells were examined under bright light (A) and fluorescent light (B) microscope. (C) Agarose gel electrophoresis: In apoptotic cells, DNA breaks into smaller pieces. So analysis of DNA would give rise to DNA ladder formation. DNA was harvested from control and progesterone treated cells after 48 hrs. incubation. Forty micrograms of DNA was loaded in each lane and was analyzed by agarose gel (1.2%) electrophoresis. There was no DNA ladder formation in progesterone treated cells, indicating apoptosis was not the mechanism of cell death. (D) Caspase-3 assay: One important step in apoptosis is the activation of effector caspase, caspase-3. Caspase-3 activity was measured using a commercially available kit. There was no significant difference in caspase-3 activity between control and progesterone treated cells. Again indicating that apoptosis was not the mechanism of cell death.
C-Agarose gel electrophoresis
As a follow-up study after DAPI staining, DNA was harvested from control and progesterone (10, 50 and 100 μM) treated BLM cells and was analyzed by agarose gel electrophoresis. Ethidium bromide stained gel did not show any DNA ladder formation (Figure 3C), indicating apoptosis was not the mechanism of cell death.
D-Caspase-3 assay
Effector caspase-3 which brings about cleavage of proteins would be increased in apoptosis. So caspase-3 assay was carried out to determine if the activity of the enzyme was increased. Assay of caspase-3 activity did not show any significant increase between control and progesterone (50 μM) treated cells (Figure 3D), again indicating apoptosis was not the mechanism of cell death.
Autophagy
Having ruled out necrosis and apoptosis, finally autophagy as the mechanism of cell death was checked by using 3-methyladenine (3-MA). 3-MA appears to act specifically on autophagic/lysosomal pathway of degradation. 3-MA has been used in several studies to suppress autophagy [22,23] in the cell.
A-Control autophagy and 3-MA rescue experiment
It has been shown that 3-MA rescued cells from autophagy [22,23]. So trial experiment with spermidine, which induced autophagy [24,25] was set up. Spermidine at 100 μM decreased BLM cell growth to 30% compared to untreated control (100%). Addition of 1 mM of 3-MA along with spermidine to cells, rescued cell growth partially to 60% (P value was 0.02) (Figure 4A).
Figure 4.
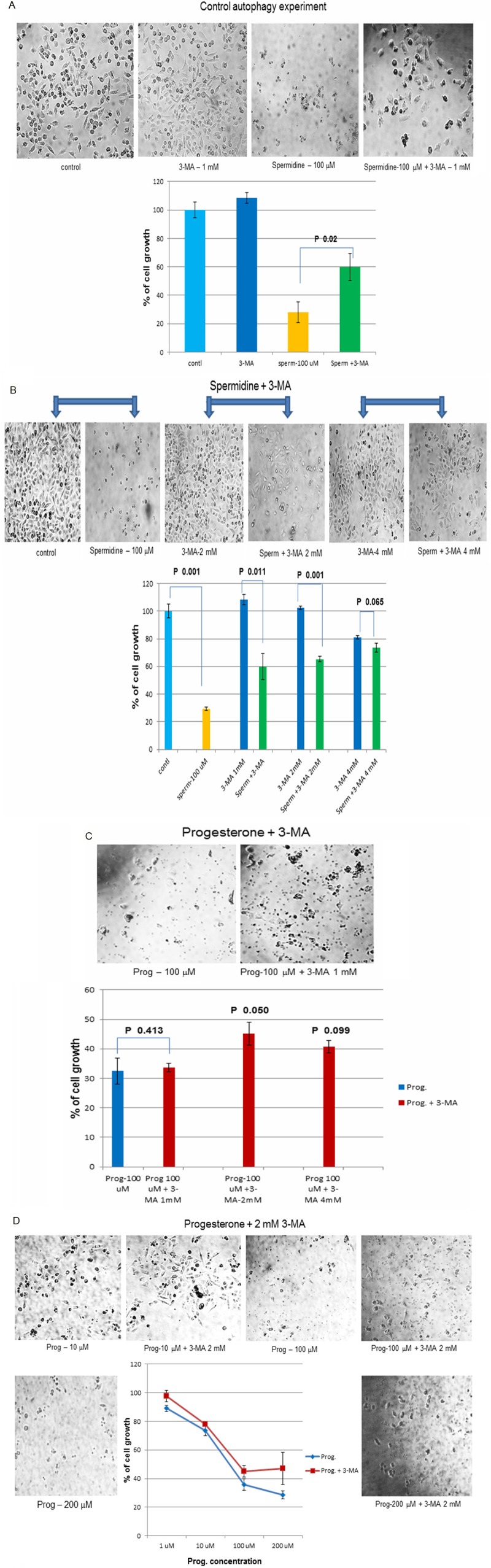
Mechanism of cell death-Autophagy: Finally autophagy as the cause of cell death was checked by co-incubating progesterone with 3-methyladenine (3-MA). 3-MA has been shown to suppress autophagic/lysosomal degradation of cellular proteins. 3-MA would suppress autophagy in cell and would rescue the cell growth. (A) Control autophagic experiment: In the control experiment, spermidine (100 μM) was used to induce autophagy in BLM cells and 3-MA (1 mM) was used to suppress autophagy. Spermidine decreased BLM cell growth to 30%. However, when 3-MA was added to the cell, it suppressed autophagic degradation and partially rescued cell growth to 60% (P value 0.02). (B) Trial experiments to determine the optimal concentration of 3-MA to be used: In order to check the optimal concentration of 3-MA to be used, 3-MA at 1, 2 and 4 mM were added to spermindine treated cells separately along with controls. 3-MA at 2 mM concentration showed maximum rescue in cell growth (65%, P=0.001) without causing any effect on the growth of control cells. (C) Also in the trial experiment, progesterone (100 μM) was co-incubated with 1, 2 and 4 mM concentrations of 3-MA separately and 3-MA at 2 mM showed maximum rescue of cell growth (45% with a P value of 0.050). It was decided to use 2 mM of 3-MA in subsequent experiments. (D) 3-MA rescue experiments: 2 mM of 3-MA was co-incubated with different concentrations of progesterone (1, 10, 100 and 200 μM). Addition of 2 mM of 3-MA was able to partially rescue cell growth at all concentrations of progesterone treated cells, compared to progesterone alone treated cells.
B-Standardization of 3-MA concentration to be used
In order to optimize the concentration of 3-MA which would rescue maximum cell growth, 3-MA at 1, 2 and 4 mM concentrations were added along with spermidine (100 μM) to control cells. Though, the rescue of cell growth (73.5%) was better with 4 mM concentration of 3-MA, it also inhibited control cell growth (80.9%). But, 3-MA at 2 mM concentration did not have any effect on control cell growth (100%) and was able to rescue cell growth to 65% (P value was 0.001) in spermidine treated cells (Figure 4B).
C-Standardization of 3-MA concentration with progesterone treated cells
After standardizing the concentration of 3-MA on control cells, standardization of 3-MA with progesterone (100 μM) treated cells were carried out. Again 3-MA at 2 mM concentration showed maximum rescue of progesterone treated cell growth (45%) with a P value of 0.05 (Figure 4C).
D-3-MA rescue of progesterone treated cells
Having found out that 2 mM of 3-MA was able to rescue cell growth, cells were incubated with different concentrations (1, 10, 100, 200 μM) of progesterone or progesterone plus 2 mM of 3-MA. There were partial rescue of cell growth at all concentrations of progesterone used (Figure 4D), indicating that the mechanism of cell death was due to autophagy.
Mechanism of progesterone action
Since progesterone is a steroid hormone, it is natural to expect a steroid hormone to act through steroid receptor. Hence, whether progesterone effect was mediated through progesterone receptor was a relevant question here. The following two bioassays were carried out to address that question.
5A-Progesterone and RU-486 coincubation
In this bioassay, progesterone was co-incubated with its receptor antagonist RU-486 to block the binding of progesterone to its receptors in BLM cells. A fixed concentration of progesterone (50 μM) was incubated with varying concentrations of RU-486 (10, 50 and 100 μM). If the inhibition was mediated through progesterone receptor, there would be a competition between progesterone and its receptor antagonist RU-486 for the limited number of progesterone receptors in cells. In this process they would nullify the effect of one another, allowing cells to grow instead of inhibiting cell growth. But, there was a synergistic effect on cell growth inhibition when progesterone and RU-486 were co-incubated (Figure 5A). This synergistic effect could be possible only if progesterone and RU-486 acted through different mechanism and not through progesterone receptor.
Figure 5.
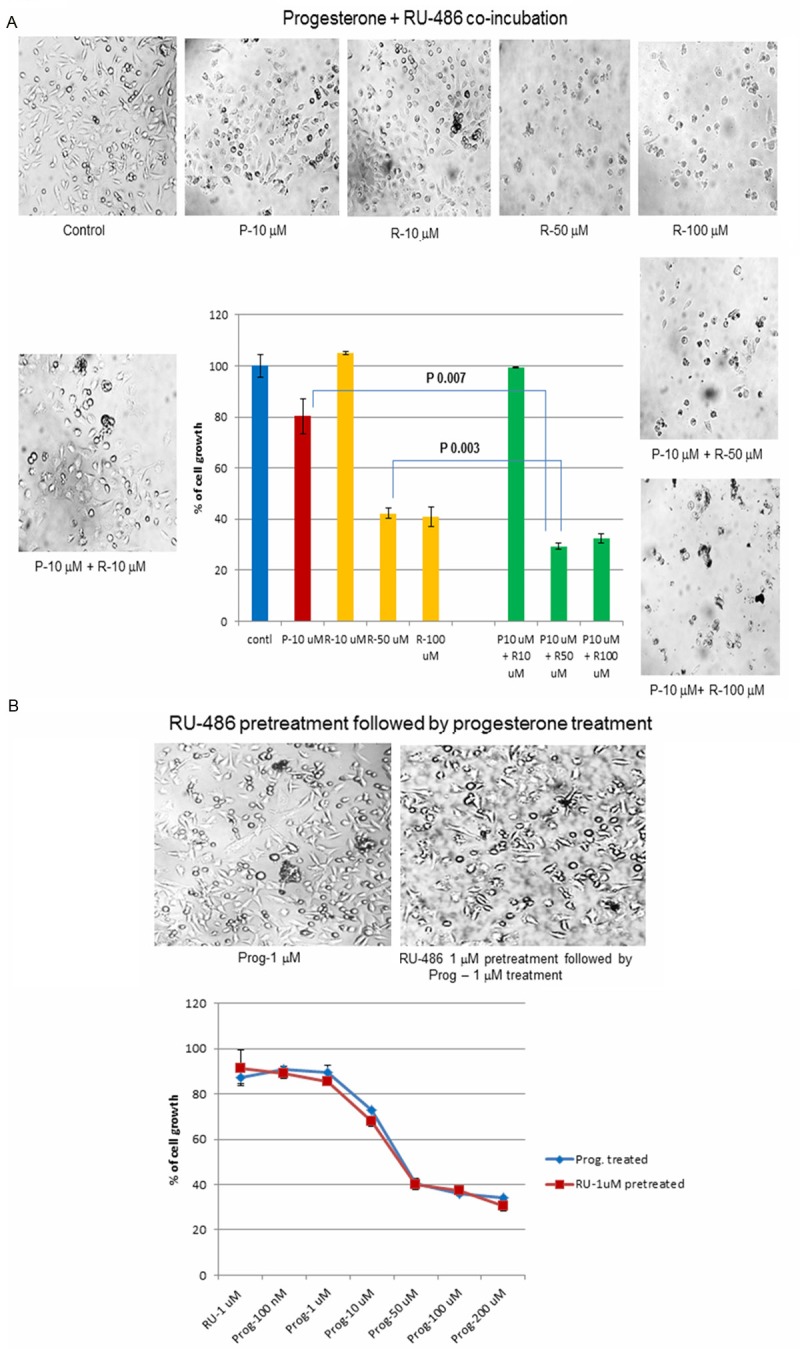
Mechanism of progesterone action: Since, progesterone induced autophagy, it was imperative to find out whether the action of progesterone was mediated through progesterone receptor. Bioassays were used to determine the involvement of progesterone receptor in mediating this action of progesterone. (A) Progesterone and RU-486 co-incubation: Ru-486 is a progesterone receptor antagonist. Co-incubation of fixed concentration of progesterone (10 μM) with varying concentrations of RU-486 (10, 50 and 100 μM) should set up a competition between progesterone and its receptor antagonist RU-486 resulting in an increase in cell growth, but not a decrease in cell growth. However, co-incubation experiment showed a synergistic effect between progesterone and RU-486 in decreasing the cell growth. This could be possible if progesterone and RU-486 were not acting through progesterone receptor but instead through a different mechanism. (B) Ru-486 pretreatment followed by progesterone treatment: Yet another way to find out if progesterone action was mediated through its receptor was to pre-incubate BLM cells with RU-486 (1 μM) so that progesterone receptors were blocked. After 2 to 3 hrs. cells were washed and incubated with progesterone at different concentrations to check its effect on cell growth. If the action of progesterone were mediated through progesterone receptor, progesterone could not bind to its receptors because they were blocked by RU-486. So progesterone could not exert its effect on cell growth, resulting in an increase in cell growth. However, in the experiment RU-486 pretreatment did not change the dose-response curve from the straight progesterone treated BLM cells dose curve, indicating that the effect of progesterone was not mediated through progesterone receptor.
5B-Preincubation with RU-486, followed by progesterone treatment
Yet another way to check the involvement of progesterone receptor was to incubate BLM cells initially with the receptor antagonist RU-486 (1 μM) and then followed by progesterone. RU-486 would bind to progesterone receptor and block subsequent binding of progesterone to the receptor, thereby blocking the action of progesterone. However the dose response curve of RU-486 pretreated cells followed by progesterone treatment was similar to the dose-response curve of straight progesterone treated cells (Figure 5B). This experiment indicated that progesterone receptor was not involved in the inhibition of melanoma cell growth.
Discussion
Epidemiological data indicate there is a female sex advantage in the outcome of melanoma skin cancer. Also, NCI SEER (Surveillance Epidemiology and End Results program) melanoma fact sheet shows difference between male and female in the incidence of melanoma (http://seer.cancer.gov/statfacts/html/melan.html). The incidence rate in men is 26.7 per 100,000 compared to females where it is 16.7 per 100,000. Life time risk for males is 0.96, whereas for females it is 0.57. The risk is cut roughly by half for females. Similarly the mortality rate for male is 4.0 per 100,000, whereas for females it is 1.7 per 100,000. Again, the mortality rate is roughly cut into half in females. Female sex hormones have been attributed to this protection. However, there has been no direct experimental evidence to link female sex hormones with melanoma protection. Our recent work with mouse melanoma cell line (B16F10) indicated that female sex hormone progesterone inhibited mouse melanoma cell growth in vitro significantly [2]. It has also been shown in this study that this effect was not a toxic, spurious or non-specific action of progesterone on melanoma cell growth and this action of progesterone was not mediated through progesterone receptor [2]. This study was extended to human melanoma cells to check if the same effect occurred upon progesterone treatment. A dose dependent inhibition (sigmoidal response curve) of human melanoma cell growth was observed with progesterone. Time-course study with progesterone revealed that 48 and 72 hours of incubation yielded similar pattern. So we decided to stick with 48 hrs. of incubation in all experiments with progesterone. As there were lot of cells floating in the medium after progesterone addition, it was apparent that progesterone decreased cell growth by causing cell death. Hence, the mechanism of cell death was also investigated in this study. Cell death by necrosis was ruled out by trypan blue dye exclusion test as there was no significant difference in the number of necrotic cells between untreated and progesterone treated cells. Apoptosis was also ruled out by DAPI nuclear staining. DAPI nuclear staining did not reveal any change in the shape of nuclei between untreated and progesterone treated cells. DNA harvested from control and progesterone treated cells did not show any DNA ladder formation when analyzed by agarose gel electrophoresis. Also effector caspase, caspase-3 assay did not show any significant increase between treated and untreated cells. In view of these results apoptosis as the mechanism of cell death was also ruled out. Finally, autophagy as the cause of cell death was determined by co-incubating BLM cells with progesterone and 3-methyladenine (3-MA). The effect of 3-MA in rescuing cells from autophagy has been well documented [22,23] and 3-MA has been used in these studies to determine autophagy. Hence, the well-established 3-MA rescue experiment was used in this study to determine autophagic mechanism of cell death. Initially, in the trial experiment autophagy was induced in BLM cells by incubating with 100 μM of spermidine. Spermidine is known to induce autophagy in cells [24,25]. There was a partial rescue in cell growth when 3-MA 1 mM was added to the cells, which was shown both qualitatively by microscopic pictures and quantitatively by MTT growth assay. Optimal concentration of 3-MA to be used along with progesterone was determined by carrying out trial experiments with 1 mM, 2 mM and 4 mM concentrations of 3-MA. Having found out that 2 mM of 3-MA rescued cell growth in the treated cells, we carried out growth assays with various concentrations of progesterone ranging from 1 μM to 200 μM. There were partial rescue of cell growth at all concentrations of progesterone used, thus indicating that the mechanism of cell death was autophagy. Moreover, by deductive logic also, one would arrive at the same conclusion after having ruled out necrosis and apoptosis as the mechanism of cell death. Autophagy was the only other major mechanism responsible for cell death. The next obvious question was whether the effect of progesterone was mediated through progesterone receptor. Progesterone and its receptor antagonist RU-486 co-incubation experiment suggested that the effect of progesterone was not mediated through progesterone receptor as there was a synergistic effect on the inhibition of melanoma cell growth. This could be possible only if progesterone and RU-486 acted through different mechanisms and not through progesterone receptor. Similarly pre-incubation of cells with RU-486 followed by treatment with progesterone did not show any change in dose curve from the regular progesterone treated dose-curve pattern. Again suggesting that progesterone action was not mediated through its receptor. This type of non-receptor mediated action of progesterone has been reported in human neuroblastoma growth inhibition [26].
Hence, in conclusion, progesterone inhibited human melanoma (BLM) cell growth in vitro by inducing autophagy and this effect of progesterone was not mediated through progesterone receptor. Autophagy as a mechanism to regulate cell growth by steroid hormone has been reported in prostate cancer also [27].
Acknowledgements
This work was supported by a start-up research grant (grant # 261-200-889) from KCOM and Warner/Fermaturo intramural research grant (grant # 261-8530-501392) from A.T. Still University to P.R. Technical assistance in preparation of the manuscript by Sujatha Bhuvanaraj is duly acknowledged.
Disclosure of conflict of interest
None.
References
- 1.Ramaraj P, Cox JL. Effect of steroids on in vitro melanoma cell growth and viability. Endocrine Reviews. 2011;12:1–29. [Google Scholar]
- 2.Ramaraj P, Cox JL. In-Vitro Effect of sex steroids on mouse melanoma (B16F10) cell growth. Cell Bio. 2014;3:60–71. [Google Scholar]
- 3.Ramaraj P, Cox JL. Effect of Progesterone on melanoma cell growth. Endocrine Reviews. 2012;33:582. [Google Scholar]
- 4.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted Therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 5.Thorn M, Ponten F, Bergstrom R, Sparen P, Adami HO. Clinical and histopathologic predictors of survival in patients with malignant melanoma: a population-based study in Sweden. J Natl Cancer Inst. 1994;86:761–769. doi: 10.1093/jnci/86.10.761. [DOI] [PubMed] [Google Scholar]
- 6.Stidham KR, Johnson JL, Seigler HF. Survival superiority of females with melanoma. A multivariate analysis of 6383 patients exploring the significance of gender in prognostic outcome. Arch Surg. 1994;129:316–324. doi: 10.1001/archsurg.1994.01420270094020. [DOI] [PubMed] [Google Scholar]
- 7.Shaw HM, Milton GW, Farago G, Farago G, McCarthy WH. Endocrine influences on survival from malignant melanoma. Cancer. 1978;42:669–677. doi: 10.1002/1097-0142(197808)42:2<669::aid-cncr2820420238>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Shaw JH. Malignant melanoma in Auckland, New Zealand. Surg Gynecol Obstet. 1988;166:425–430. [PubMed] [Google Scholar]
- 9.Kemeny MM, Busch E, Stewart AK, Menck HR. Superior survival of young women with malignant melanoma. Am J Surg. 1998;175:437–445. doi: 10.1016/s0002-9610(98)00070-1. [DOI] [PubMed] [Google Scholar]
- 10.Miller JG, Mac Neil S. Gender and cutaneous melanoma. British J Dermat. 1997;136:657–665. [PubMed] [Google Scholar]
- 11.Smith EL, Hill RL, Lehman IR, Lefkowitz RJ, Handler P, White A. Mammalian Biochemistry. 7th edition. Singapore: Mcgraw-Hill; 1983. Principles of Biochemistry; pp. 519–524. [Google Scholar]
- 12.Mather JP, Sato GH. The growth of mouse melanoma cells in hormone-supplemented, serum-free medium. Exp Cell Res. 1979;120:191–200. doi: 10.1016/0014-4827(79)90549-4. [DOI] [PubMed] [Google Scholar]
- 13.Pavelic K, Petrusic L, Osmak M, Culo F. In Vivo and In Vitro effect of progesterone on the growth of some mouse and human tumors. Res Exp Med (Berl) 1983;183:183–191. doi: 10.1007/BF01855641. [DOI] [PubMed] [Google Scholar]
- 14.Kanda N, Watanabe S. 17-b-Estradiol, Progesterone and Dihydrotestosterone suppress the growth of human melanoma by inhibiting Interleukin-8 production. J Invest Dermatol. 2001;117:274–283. doi: 10.1046/j.1523-1747.2001.01422.x. [DOI] [PubMed] [Google Scholar]
- 15.Neifeld JP, Lippman ME, Fisher RI. Receptors for steroid hormones in human melanoma. Surg Forum. 1976;27:108–110. [PubMed] [Google Scholar]
- 16.Duncan LM, Travers RL, Koerner FC, Mihm MC Jr, Sober AJ. Estrogen and progesterone receptor analysis in pregnancy associated melanoma: Absence of immunohistochemically detectable hormone receptors. Hum Pathol. 1994;25:36–41. doi: 10.1016/0046-8177(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 17.Denzit F, Lang R. Rapid colorimetric assay for cell growth and survival, modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 18.Simon LP. In: Viable cell counting using trypan blue, Cancer cell culture methods and Protocols. Simon P, editor. Langdon: Humana press; 2004. p. 26. [Google Scholar]
- 19.Li X, Melmed MR, Darzynkiewiz Z. Detection of apoptosis and DNA replication by different labeling of DNA strands with fluorochromes of different cells. Exp Cell Res. 1996;222:28–37. doi: 10.1006/excr.1996.0004. [DOI] [PubMed] [Google Scholar]
- 20.Hughes D, Mehmet H. Cell proliferation & Apoptosis. Bios Scientific publishers Ltd; 2003. Protocol 11.9: Detection of high molecular weight DNA fragments by gel electrophoresis; p. 312. [Google Scholar]
- 21.Nicholson DW, Ali A, Thornberry NA, Vai- llancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 22.Seglen PO, Gordon PB. 3-Methyladenine: Specific inhibitior of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–76. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 24.Madeo F, Eisenberg T, Butner S, Ruckenstuhl C, Kroener G. Spermidine-A novel autophagy inducer and longevity elixir. Autophagy. 2010;6:160–162. doi: 10.4161/auto.6.1.10600. [DOI] [PubMed] [Google Scholar]
- 25.Morselli E, Marino G, Bennetzen MV, Eisenbeerg T, Megalou E, Schroeder S, Cabrera S, Benit P, Rustin P, Criollo A, Kepp O, Galluzzi L, Shen S, Malik SA, Maiuro MC, Horio Y, Lopez-Otin C, Andersen JS, Tavernarakin N, Madeo F, Kroemer G. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. JCB. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atif F, Sayeed I, Yousuf S, Ishrat T, Hua F, Wang J, Brat DJ, Stein DG. Progesterone inhibits the growth of human neuroblastoma: in Vitro and In Vivo evidence. Mol Med. 2011;17:1084–1094. doi: 10.2119/molmed.2010.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y, Han JJ, Tennakoon JB, Mehta FF, Merchant FA, Burns AR, Howe MK, McDonnell DP, Frigo DE. Androgens promote prostate cancer cell growth through induction of autophagy. Mol Endocirinol. 2013;27:280–295. doi: 10.1210/me.2012-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]


