Abstract
Previous studies have investigated the relationship between oral contraceptives (OCs) use and kidney cancer risk. However, they yielded inconsistent results. To our knowledge, a comprehensive assessment of the association between OC and kidney cancer risk has not been reported. Hence, we conducted a meta-analysis to quantify the association. We identified all relevant studies up to July 2014 through a literature search of using PubMed and EMBASE, and by reviewing the references from the retrieved articles. Fixed-effect and random-effect models were used to estimate summary relative risks (SRRs) and the corresponding 95% confidence intervals (CIs). A total of 12 studies were eligible and included in this meta-analysis, involving 4,206 kidney cancer cases and 638,677 participants. The SRR of kidney cancer for ever versus never OC use was 0.89 (95% CI: 0.82-0.98). The protection became stronger when compared the longest duration of OC use with never use (RR = 0.80; 95% CI: 0.68-0.94). In dose-response analysis, we found that the kidney cancer risk decreased by 2% for per 1 year increment in OC use (RR = 0.98; 95% CI: 0.96-0.99). No apparent heterogeneity was observed across studies included in this analysis. Egger’s and Begg’s test also indicated no publication bias. The present study suggested that OC may reduce the risk of kidney cancer, especially for long-term users. More well-conducted and large-scale prospective studies are warranted to confirm the effects of OC use on kidney cancer.
Keywords: Oral contraceptive, kidney cancer, risk, meta-analysis
Introduction
Kidney cancer is the 13th most common malignancy in the world and approximately 271,000 new cases were diagnosed annually [1]. Although smoking, obesity, and hypertension have been established as the most consistently risk factors, the etiology of kidney cancer remains elusive [2]. In the 27 European Union countries, the estimated age-standardized incidence rate of kidney cancer were 15.8 for men and 7.1 for women per 100,000 Europeans in 2008 [3], and in the United States, kidney cancer incidence rates were 20.7 per 100,000 in males and 10.5 per 100,000 in females according to the US Surveillance, Epidemiology, and End Results (SEER) program during the period 2005-2009 [4]. The observed 2-fold higher kidney cancer incidence rate among men versus women, coupled with experimental evidence of estrogen effects on renal cancer development [5,6], have motivated interest in the role of female hormonal and reproductive factors in kidney cancer development including oral contraceptives (OCs).
Several epidemiologic studies have investigated the potential association between OC and kidney cancer risk including large prospective cohort studies. However, the results are controversial. Although a recent meta-analysis founded that OC may influence certain cancers including breast, cervical, colorectal and endometrial cancers [7], no comprehensive and quantitative assessment has been reported of the association between OC and kidney cancer risk so far. Therefore, to synthesize the published data and present more exact results, we carried out a meta-analysis on all prospective and case-control studies.
Methods
Literature search
We conducted a comprehensive English literature search in PubMed and EMBASE database up to July 2014, using the following key words: (oral contraceptive OR oral birth control OR reproductive factors) and (kidney OR renal OR renal cell) and (cancer OR neoplasm OR carcinoma OR tumor). Reference lists of identified articles were also reviewed to obtain other pertinent publications.
Inclusion and exclusion criteria
Two researchers (Huan Liu, Xing-chun Wang) independently selected eligible studies. Any disagreement between the two reviewers was resolved by discussing with the third reviewer (Yun-fei Xu). Inclusion criteria were as follows: a prospective cohort or case-control design; the exposure of interest was OCs; the outcome of interest was kidney cancer risk; odds ratio (OR), relative risk (RR) or hazard ratio (HR) with corresponding 95% confident interval (CI) were reported, or information suitable for us to calculate them. Case reports, letters to editor, review articles and fundamental researches were excluded. For studies published multiply from the same population or studies published with the same results in different journals, we chose the most recent one for the meta-analysis.
Data extraction
The following data were extracted from each publication by two reviewers independently (Huan Liu, Guang-Hui Hu): first author, publication year, country, study design, study period, number of cases and controls or samples, OC use, RR or OR or HR with corresponding 95% CI for OC use and kidney cancer risk, and the adjusted factors.
Due to different exposure categories of duration time across studies included, we therefore performed a dose-response meta-analysis to assess the effect of per 1 year increment use of OC on kidney cancer risk. The number of cases and total person-time (or non-cases) for each category of duration time and the risk estimates with their variance estimates were extracted. For each study, the median or mean time of OC use of each category was assigned to each corresponding RR estimate. If data were not provided, the midpoint of the upper and lower boundaries in each category was assigned as average duration. If the lowest category was open-ended, the lower boundary was set to zero. When the highest category was open-ended, we assumed that it had the same amplitude as the closest category.
Statistical analysis
Since the absolute risk of kidney cancer is relatively rare, the three measures of association were considered approximations of RR [8]. The summary relative risks (SRRs) and 95% CIs for ever versus never use of OC were calculated, using the inverse of the corresponding variances as weights. To assess the effect of long-term OC use, we also collected the RRs and 95% CIs for the longest duration of OC use compared to never use. Statistical heterogeneity was evaluated through the Cochrane’s Q test and I2 statistic [9]. For the Cochrane’s Q statistic, a P value < 0.10 was considered statistically significant for heterogeneity; For the I2 statistic, heterogeneity was interpreted as absent (I2: 0%-25%), low (I2: 25.1%-50%), moderate (I2: 50.1%-75%), or high (I2: 75.1%-100%) [10]. Random-effect model was used when substantial heterogeneity was detected (I2 > 50%; P value < 0.10), assuming that the studies included in the meta-analysis had varying effect sizes [11]. Otherwise, fixed-effect model was performed which assumed that the studies included in the analysis had the same effect size [12]. To investigate the influence of various study characteristics on the summary risk estimates, we also conducted subgroup analyses based on study design, geographic location, publication year and adjustments for potential confounding factors. Sensitivity analyses were also conducted to test the robustness of our results by removing each study separately and calculating the effect size and homogeneity for all of the rest studies.
We used methods described by Greenland and Longnecker [13] to obtain an estimated dose-response trend and combined the trends by using random-effect model. For dose-response analysis, the distributions of cases and person-years (or non-cases) and the RRs with the variance estimates for at least three quantitative exposure categories were needed to generate a specific slope and obtain a linear dose-response curve. For studies [4,14] reported the cases and overall person-years, but not the distributions, we used the RRs of every category to estimate [15]. The dose-response result was shown for per 1 year increment in OC use.
Potential publication bias was detected by using Begg’s funnel plots and Egger’s regression asymmetry test [16,17]. All statistical analyses were conducted using STATA (version 12.0; StataCorp, College Station, TX, USA), and p < 0.05 was considered statistically significant.
Results
Literature search and study characteristics
Figure 1 shows the flow diagram for study selection. We identified and screened 492 potentially relevant articles. On the basis of the title and abstract, we identified 17 papers. After detailed evaluation, six studies were excluded for reasons described in Figure 1. One additional article was added from the reference lists [18]. Finally, 12 eligible articles [4,14,18-27] containing six cohort [4,14,23,24,26] and seven case-control studies [18-22,25,27] were used in our meta-analysis (one article contained two cohort studies [4]). The characteristics of the included cohort and case-control studies were summarized in Table 1. Among the included studies, eight was conducted in North America [4,14,18,21,23,24,26,27], two in Europe [20,25] and one in Oceania [19]. The remaining one was carried out at five centers in different countries: Australia, Denmark, Germany, Sweden and the United States.
Figure 1.
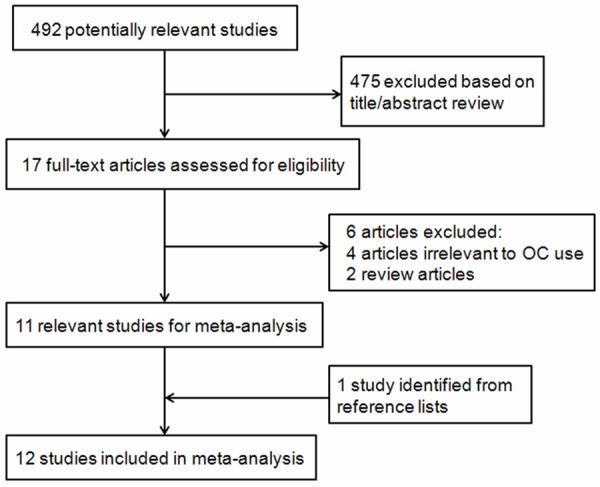
Flow diagram of the eligible study selection process.
Table 1.
Characteristics of the studies included in the meta-analysis
| References | Country | Study type | Study period | Cases/Size | OC use | RR(95% CI) | Data collection | Adjustment for confounders |
|---|---|---|---|---|---|---|---|---|
| McCredie M, 1992 | Australia | PCC | 1989-1990 | 268/292 | Ever vs. never | 0.7 (0.4-1.1) | Face to face interview using structured questionnaire | Age |
| Mellemgaard A, 1994 | Denmark | PCC | 1989-1992 | 368/396 | Ever vs. never ≥ 10 yr vs. never | 0.96 (0.42-2.2) 0.8 (0.3-2.2) | Face to face interview using structured questionnaire | Age, BMI, smoking, and socio-economic status |
| Chow HW, 1995 | USA | PCC | 1988-1990 | 165/227 | Ever vs. never ≥ 10 yr vs. never | 0.8 (0.4-1.3) 0.3 (0.1-1.0) | Face to face interview using structured questionnaire | Age, cigarette smoking and BMI |
| Lindblad P, 1995 | Multi- Centera | PCC | 1989-1991 | 608/766 | Ever vs. never ≥ 10 yr vs. never | 0.7 (0.5-0.9) 0.5 (0.3-0.9) | Face to face/telephone interview | Age, center, tobacco use and BMI |
| Gago-Dominguez M, 1999 | USA | PCC | 1986-1994 | 422/422 | Ever vs. never ≥ 120 movs. never | 1.0 (0.7-1.4) 1.3 (0.7-2.3) | Face to face interview using structured questionnaire | Level of education and history of hysterectomy |
| Kabat GC, 2007 | Canada | NBSS Cohort | 1980-2000 | 172/89, 835 | Ever vs. never ≥ 96 mo vs. never | 0.80 (0.58-1.09) 0.80 (0.48-1.31) | Self-administered questionnaires and face to face interview | Age, pack-years, BMI, menopausal status, education, study centre, randomisation group and the other variables |
| Molokwu JC, 2007 | USA | IWHS Cohort | 1986-2003 | 165/37440 | Ever vs. never | 0.96 (0.63-1.48) | Self-administered questionnaires | Age, BMI, WHR, alcohol use and history of hypertension |
| Zucchetto A, 2008 | Italy | HCC | 1992-2004 | 273/546 | Ever vs. never | 0.94 (0.51-1.72) | Face to face interview using structured questionnaire | Calendar year, education, smoking habits, BMI, family history and history of hypertension |
| Lee JE, 2009 | USA | NHS Cohort | 1976-2004 | 247/118, 219 | Ever vs. never ≥ 3 yr vs. never | 0.99 (0.75-1.32) 0.85 (0.58-1.24) | Biennial mailed questionnaires | History of hypertension, BMI, smoking status, fruit intake, vegetable intake and alcohol intake |
| Setiawan VW, 2009 | USA | Multiethnic bCohort | 1993-2005 | 229/106, 036 | Ever vs. never > 5 yr vs. never | 1.08 (0.75-1.55) 1.01 (0.60-1.68) | Self-administered questionnaires | Age, BMI, smoking status, hypertension, alcohol intake and diuretic use |
| Purdue MP, 2011 | USA | PCC | 2002-2007 | 497/546 | Ever vs. never > 15 yr vs. never | 1.1 (0.8-1.5) 1.1 (0.6-2.1) | Computer-assisted personal interviews | Sex, age, study center, education, smoking status, BMI, hypertension and family history of kidney cancer |
| Karami S, 2013 | USA | NIH-AARP Diet and Health Study and PLCO Cancer Screening Trial | 1995-2006 1993-2010 | 792/283, 952 | Ever vs. never ≥ 10 yr vs. never | 0.87 (0.75-1.02) 0.72 (0.55-0.96) | Self-administered questionnaires | BMI, educational level, race, study, and smoking status |
Abbreviations: OC = oral contraception; RR = relative risk; CI = confidence interval; PCC = population-based case-control; HCC = hospital-based case-control; yr = year; mo = month; BMI = body mass index; WHR = waist-to-hip ratio; NBSS = National Breast Screening Study; IWHS = Iowa Women’s Health Study; NHS = Nurses’ Health Study; NIH-AARP = National Institutes of Health-American Association of Retired Persons; PLCO = Prostate, Lung, Colorectal and Ovarian.
This study was carried out at five centers in different countries: Australia, Denmark, Germany, Sweden and the United States.
This study was carried out at Hawaii and Los Angeles including five racial/ethnic groups: African Americans, Japanese Americans, Latinos, Native Hawaiians and Whites.
Ever versus never use of OC
All the included studies reported the risk estimate between OC use and risk of kidney, involving 4,206 cases and 638,677 participants. The SRR was 0.89 (95% CI: 0.82-0.98) for ever versus never use of OCs, with no evidence of heterogeneity observed across studies (p = 0.707, I2 = 0.0%; Figure 2). In subgroup analyses, a borderline significant inverse association was observed in both cohort and case-control studies. The pooled RRs were 0.88 (95% CI: 0.75-1.02; p = 0.451, I2 = 0.0%) for case-control studies and 0.90 (95% CI: 0.81-1.01; p = 0.697, I2 = 0.0%) in cohort studies (Figure 2). Stratified by geographic location, no significant association was observed in Europe (RR = 0.95; 95% CI: 0.58-1.55; p = 0.968; I2 = 0.0%; N = 2), North America (RR = 0.93; 95% CI: 0.84-1.02; p = 0.783; I2 = 0.0%; N = 8) or Oceania (RR = 0.7; 95% CI: 0.4-1.1; N = 1) (Table 2). When we examined whether the associations differed by adjustment for potential confounders (hypertension, obesity or smoking status), the result was significantly altered by hypertension (P = 0.04). The SRRs were 1.03 (95% CI: 0.87-1.21) for studies adjusted for hypertension and 0.84 (95% CI: 0.75-0.94) for not adjusted (Table 2).
Figure 2.
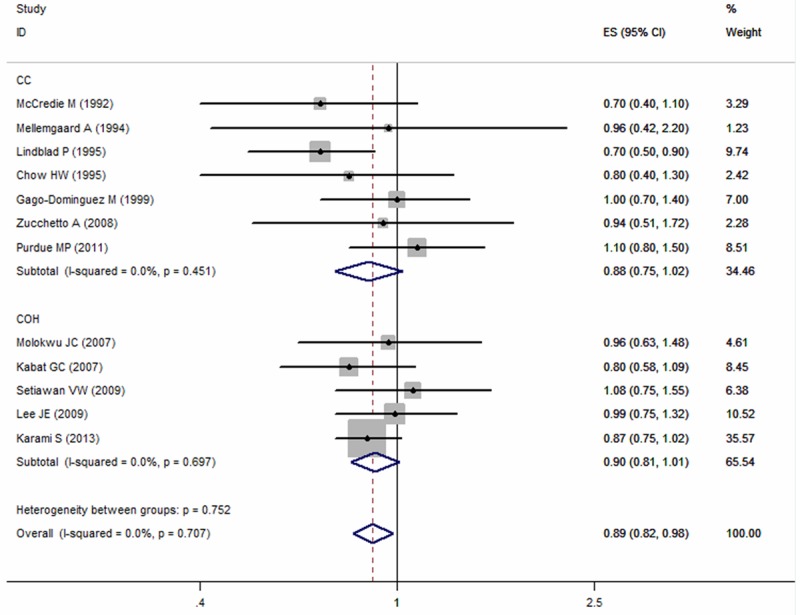
Summary relative risks of kidney cancer for ever versus never use of oral contraceptive from case-control and cohort studies.
Table 2.
Subgroup analyses of OC use and kidney cancer risk
| Subgroup | Ever use | Long-term use | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | RR (95% CI)a | I2 (%) | P-heterogeneity | N | RR (95% CI)a | I2 (%) | P-heterogeneity | |
| Total studies | 12 | 0.89 (0.82-0.98) | 0.0 | 0.707 | 9 | 0.80 (0.68-0.94) | 24.4 | 0.226 |
| Designs | ||||||||
| Case-control | 7 | 0.88 (0.75-1.02) | 0.0 | 0.451 | 5 | 0.76 (0.46-1.25) | 56.3 | 0.058 |
| Cohort | 5 | 0.90 (0.81-1.01) | 0.0 | 0.697 | 4 | 0.80 (0.66-0.97) | 0.0 | 0.697 |
| Geographic locations | ||||||||
| North America | 8 | 0.93 (0.84-1.02) | 0.0 | 0.783 | 7 | 0.83 (0.70-0.99) | 20.6 | 0.272 |
| Europe | 2 | 0.95 (0.58-1.55) | 0.0 | 0.968 | 1 | 0.80 (0.30-2.2) | NA | NA |
| Oceania | 1 | 0.70 (0.40-1.10) | NA | NA | - | - | - | - |
| Publication year | ||||||||
| Before 2000 | 5 | 0.80 (0.66-0.97) | 0.0 | 0.585 | 4 | 0.68 (0.36-1.25) | 61.3 | 0.052 |
| After 2000 | 7 | 0.92 (0.83-1.03) | 0.0 | 0.738 | 5 | 0.82 (0.68-0.98) | 0.0 | 0.671 |
| Adjusted factors Hypertension | ||||||||
| Yes | 5 | 1.03 (0.87-1.21) | 0.0 | 0.974 | 3 | 0.94 (0.71-1.23) | 0.0 | 0.746 |
| No | 7 | 0.84 (0.75-0.94) | 0.0 | 0.762 | 6 | 0.73 (0.54-0.97) | 36.7 | 0.162 |
| Smoking | ||||||||
| Yes | 9 | 0.91 (0.82-1.01) | 0.0 | 0.594 | 8 | 0.81 (0.64-1.02) | 33.9 | 0.158 |
| No | 3 | 0.82 (0.65-1.03) | 0.0 | 0.630 | 1 | 0.80 (0.48-1.31) | NA | NA |
| Obesity | ||||||||
| Yes | 10 | 0.89 (0.81-0.99) | 0.0 | 0.661 | 8 | 0.77 (0.65-0.91) | 9.9 | 0.353 |
| No | 2 | 0.89 (0.67-1.19) | 23.1 | 0.254 | 1 | 1.30 (0.72-2.36) | NA | NA |
Abbreviations: OC = oral contraception; RR = relative risk; CI = confidence interval; NA = not available.
Summary RRs (95% CIs) were calculated using the fixed or random effects model based on the results of I2-statistics.
In sensitivity analysis, results were significantly altered after removing Lindblad et al’s or Karami et al’s study. The SRRs were 0.92 (95% CI: 0.83-1.01) and 0.91 (95% CI: 0.81-1.02), respectively (Table S1).
Long-term versus never use of OC
Four prospective studies [4,14,23,26] and five case-control studies [18,20-22,27] investigated the association between long-term OC use and kidney cancer risk. The SRR indicated a stronger protection of OC on kidney cancer risk when compared the longest duration of OC use with never use (RR = 0.80; 95% CI: 0.68-0.94), with little evidence of heterogeneity observed (P = 0.226, I2 = 24.4%; Figure 3). Subgroup analyses showed significant inverse association among cohort studies (RR = 0.80; 95% CI: 0.66-0.97; p = 0.697; I2 = 0.0%), but not in case-control studies (RR = 0.76; 95% CI: 0.46-1.25; p = 0.058; I2 = 56.3%; Table 2). Most of the studies were conducted in North America (n = 7) and the RR was 0.83 (95% CI: 0.70-0.99; P = 0.272, I2 = 20.6%). When stratified by potential confounders, the estimated effect was also altered by hypertension. The SRRs were 0.94 (95% CI: 0.71-1.23) for hypertension-adjusted studies and 0.73 (95% CI: 0.54-0.97) for not adjusted (Table 2).
Figure 3.
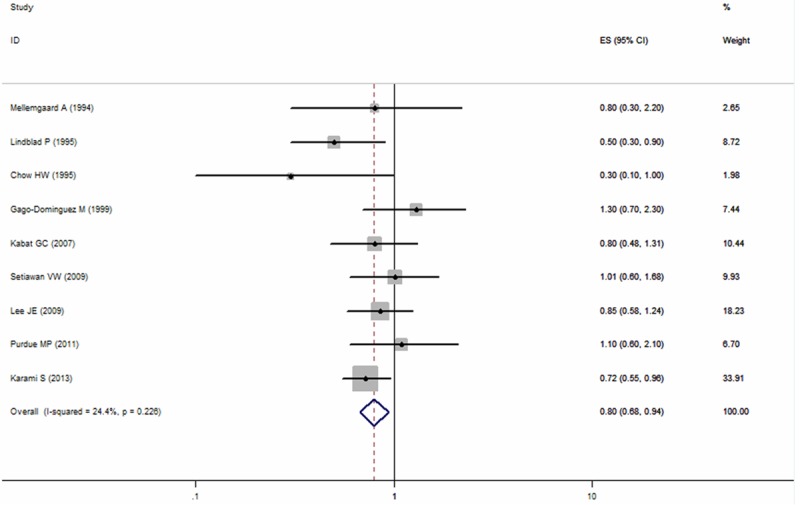
Summary relative risks of kidney cancer for the longest duration of oral contraceptives use versus never use from case-control and cohort studies.
Sensitivity analysis showed that Karami et al’s study [4] contributed significantly to the protective effect of OC on kidney cancer risk. After excluding this study, the SRR increased to 0.84 (95% CI: 0.69-1.02; P = 0.199, I2 = 28.7%) (Table S2).
Dose-response analysis
All the nine studies provided three or more categories of duration of OC use. We subsequently performed a dose-response analysis and found that the risk of kidney cancer reduced by 2% for per 1 year increment in OC use (RR = 0.98; 95% CI: 0.96-0.99; Figure 4).
Figure 4.
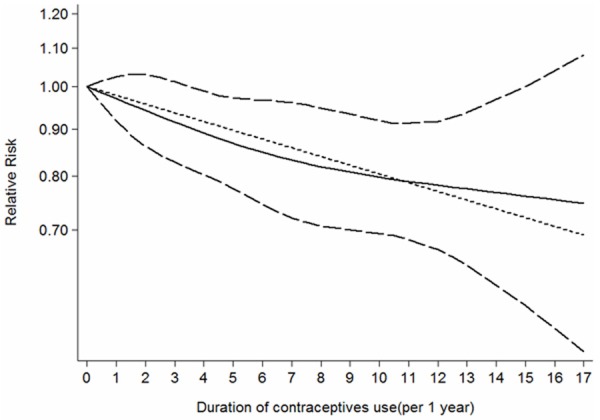
The does-response analysis between oral contraceptives use and kidney cancer risk. The solid line and long dash line represent the estimated RR and its 95%CI. Short dash line represents the linear relationship.
Publication bias
In the present meta-analysis, there was no indication of publication bias for studies on the association between OC use and kidney cancer risk. For studies focusing on ever versus never use of OC, the p value was 0.945 for Begg’s test and 0.806 for Egger’s test (Figure 5A). For studies on long-term use of OC versus never use, the p value was 1.000 for Begg’s test and 0.923 for Egger’s test (Figure 5B).
Figure 5.
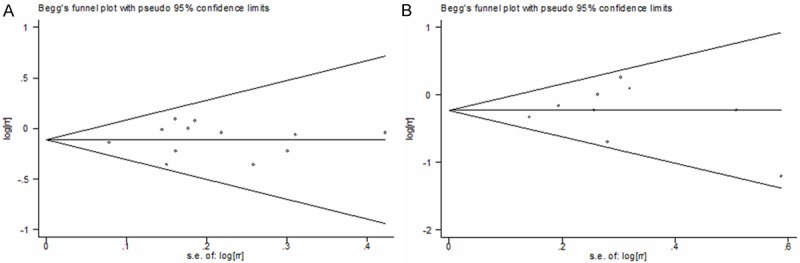
Begg’s funnel plot of oral contraceptives use on kidney cancer risk. A. Ever oral contraceptives use versus never use. B. Long-term oral contraceptives use versus never use.
Discussion
OC is the most effective and reversible method of contraception and is widely used every day around the world for preventing unintended pregnancies [28]. More than 300 million women are thought to have used OC since the introduction of it in the early 1960s [29]. In the present meta-analysis aimed to examine the role of OC use in the genesis of kidney cancer among women, we found a 11% reduction in kidney cancer risk among ever OC users and this effect was apparently stronger for long-term OC use (RR = 0.8). Furthermore, there was a dose-response relationship between the duration of OC use and kidney cancer risk. For per one year increment in OC use, the risk of kidney cancer decreased by 2%.
Despite the SRRs and dose-response analysis have shown consistent results of OC use (ever and long-term versus never use) and kidney cancer risk, the findings still should be treated with cautious. As in sensitivity analysis, results were significantly altered after removing Lindblad et al’s [22] or Karami et al’s study [4]. This two studies contained the largest samples and weighted nearly half (45.31%) of all the included studies. Lindblad and the colleagues reported an inverse association between OC use and kidney cancer risk based on a population-based case-control study conducted at five centers in Australia, Denmark, Germany, Sweden and the United States (Table 1). Karami et al’s prospective study was comprised of two cohort studies and also suggested that long-term OC use has a protective influence on kidney cancer risk (Table 1). Considering that these two studies were multi-center designed and contained larger cases and samples, we thought the results were more representative and reliable than other studies. More large-scale prospective studies are needed to affirm this association.
Although the exact biologic mechanisms underlying the association between OC and decreased risk of kidney cancer are not fully understood, several potential mechanisms might have been proposed. OCs on the market contained different types of estrogen and whether it can decrease the risk of kidney cancer may depend on the component of estrogen. Recent in vitro study has shown that estrogen can activate estrogen receptor β to act as a tumor suppressor and inhibit the proliferation of renal cell carcinoma cell lines, reduce migration and invasion, and enhance apoptosis [30]. Estradiol, one type of estrogen contained in OC, has also been shown to inhibit oxidative stress and lipid peroxidation [31], which has been hypothesized to be responsible for renal carcinogenesis [32]. Additionally, estradiol has the effect to lower blood pressure levels [31], thus it may inhibit kidney cancer because high blood pressure has been proved associated with increased risk of kidney cancer [33].
Subgroup analyses limited to prospective studies which are less vulnerable to recall and selection bias made our findings reliable, though the association was borderline significant for ever OC users. When we performed subgroup meta-analyses according to region, a significant protective effect was observed in North America for long-term OC use, while no significant association was found in Europe and Oceania. However, we were unable to rule out the possibility that the results were by chance because only two and one studies reported the association of OC use and kidney cancer risk in Europe and Oceania, respectively. Studies included in this meta-analysis were published in recent 30 years and we found that publication year significantly altered the associations between OC and kidney cancer risk (shown in Table 2). This may be explained by the temporal variations in OC formulations available on the market. For example, in 1980s, the 3rd generation contraceptive estrogen content was dropped to 30 μg and contraceptive progestin type has also been improved to play the role of anti-estrogen, and in the present OC, estrogen is reduced to 15-20 μg [34].
The strength of our study lies in a large sample size (638,677 participants and 4,206 renal cancer cases) and no significant evidence of heterogeneity and publication bias. Two investigators independently performed the article identification, data extraction, and verification and resolved all discrepancies. Most studies have adjusted for several important potential confounders. Furthermore, we have proved a dose-response relationship between OC use and kidney cancer risk. However, several limitations should also be noted in this meta-analysis. First, because all studies included in this analysis did not illustrate component and style (estrogen and progestin) of OCs, we could not examine the effects of different types of OCs on kidney cancer risk. Second, we were unable to perform subgroup analyses by dosage of OCs because none of the included studies reported the dosage of OC used. Third, although we have detected the influence of potential confounders in the present study and found that hypertension significantly altered the results, we still could not exclude the possibility that other unmeasured or inadequately measured factors have influenced the true association. Last, the possibility of recall and selection biases can’t be ruled out because more than half of the included studies were case-control studies, which were more susceptible to bias due to their nature.
In conclusion, we found a reduced risk of kidney cancer in ever OC users, and the protection is stronger for long-term OC use. Considering the limitations of our study, more well-designed and large-scale epidemiological studies which also report type and dosage of OCs, are needed to confirm the effect of OC use on kidney cancer.
Acknowledgements
This study was supported by grant No. 81370699 from National Natural Science Foundation of China.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lipworth L, Tarone RE, Lund L, McLaughlin JK. Epidemiologic characteristics and risk factors for renal cell cancer. Clin Epidemiol. 2009;1:33–43. doi: 10.2147/clep.s4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi F, Ferlay J, Galeone C, Lucchini F, Negri E, Boyle P, La Vecchia C. The changing pattern of kidney cancer incidence and mortality in Europe. BJU Int. 2008;101:949–958. doi: 10.1111/j.1464-410X.2008.07451.x. [DOI] [PubMed] [Google Scholar]
- 4.Karami S, Daugherty SE, Schonfeld SJ, Park Y, Hollenbeck AR, Grubb RL 3rd, Hofmann JN, Chow WH, Purdue MP. Reproductive factors and kidney cancer risk in 2 US cohort studies, 1993-2010. Am J Epidemiol. 2013;177:1368–1377. doi: 10.1093/aje/kws406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nissenkorn I, Servadio C, Avidor I. Oestrogen-induced renal carcinoma. Br J Urol. 1979;51:6–9. doi: 10.1111/j.1464-410x.1979.tb04235.x. [DOI] [PubMed] [Google Scholar]
- 6.Cavalieri EL, Kumar S, Todorovic R, Higginbotham S, Badawi AF, Rogan EG. Imbalance of estrogen homeostasis in kidney and liver of hamsters treated with estradiol: implications for estrogen-induced initiation of renal tumors. Chem Res Toxicol. 2001;14:1041–1050. doi: 10.1021/tx010042g. [DOI] [PubMed] [Google Scholar]
- 7.Gierisch JM, Coeytaux RR, Urrutia RP, Havrilesky LJ, Moorman PG, Lowery WJ, Dinan M, McBroom AJ, Hasselblad V, Sanders GD, Myers ER. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931–1943. doi: 10.1158/1055-9965.EPI-13-0298. [DOI] [PubMed] [Google Scholar]
- 8.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 10.Hedges LV, Pigott TD. The power of statistical tests for moderators in meta-analysis. Psychol Methods. 2004;9:426–445. doi: 10.1037/1082-989X.9.4.426. [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 13.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 14.Lee JE, Hankinson SE, Cho E. Reproductive factors and risk of renal cell cancer: the Nurses’ Health Study. Am J Epidemiol. 2009;169:1243–1250. doi: 10.1093/aje/kwp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ, Norat T. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 17.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gago-Dominguez M, Castelao JE, Yuan JM, Ross RK, Yu MC. Increased risk of renal cell carcinoma subsequent to hysterectomy. Cancer Epidemiol Biomarkers Prev. 1999;8:999–1003. [PubMed] [Google Scholar]
- 19.McCredie M, Stewart JH. Risk factors for kidney cancer in New South Wales, Australia. II. Urologic disease, hypertension, obesity, and hormonal factors. Cancer Causes Control. 1992;3:323–331. doi: 10.1007/BF00146885. [DOI] [PubMed] [Google Scholar]
- 20.Mellemgaard A, Engholm G, McLaughlin JK, Olsen JH. Risk factors for renal-cell carcinoma in Denmark. III. Role of weight, physical activity and reproductive factors. Int J Cancer. 1994;56:66–71. doi: 10.1002/ijc.2910560113. [DOI] [PubMed] [Google Scholar]
- 21.Chow WH, McLaughlin JK, Mandel JS, Blot WJ, Niwa S, Fraumeni JF Jr. Reproductive factors and the risk of renal cell cancer among women. Int J Cancer. 1995;60:321–324. doi: 10.1002/ijc.2910600307. [DOI] [PubMed] [Google Scholar]
- 22.Lindblad P, Mellemgaard A, Schlehofer B, Adami HO, McCredie M, McLaughlin JK, Mandel JS. International renal-cell cancer study. V. Reproductive factors, gynecologic operations and exogenous hormones. Int J Cancer. 1995;61:192–198. doi: 10.1002/ijc.2910610209. [DOI] [PubMed] [Google Scholar]
- 23.Kabat GC, Silvera SA, Miller AB, Rohan TE. A cohort study of reproductive and hormonal factors and renal cell cancer risk in women. Br J Cancer. 2007;96:845–849. doi: 10.1038/sj.bjc.6603629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molokwu JC, Prizment AE, Folsom AR. Reproductive characteristics and risk of kidney cancer: Iowa Women’s Health Study. Maturitas. 2007;58:156–163. doi: 10.1016/j.maturitas.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Zucchetto A, Talamini R, Dal Maso L, Negri E, Polesel J, Ramazzotti V, Montella M, Canzonieri V, Serraino D, La Vecchia C, Franceschi S. Reproductive, menstrual, and other hormone-related factors and risk of renal cell cancer. Int J Cancer. 2008;123:2213–2216. doi: 10.1002/ijc.23750. [DOI] [PubMed] [Google Scholar]
- 26.Setiawan VW, Kolonel LN, Henderson BE. Menstrual and reproductive factors and risk of renal cell cancer in the Multiethnic Cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:337–340. doi: 10.1158/1055-9965.EPI-08-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purdue MP, Colt JS, Graubard B, Davis F, Ruterbusch JJ, Digaetano R, Karami S, Wacholder S, Schwartz K, Chow WH. A case-control study of reproductive factors and renal cell carcinoma among black and white women in the United States. Cancer Causes Control. 2011;22:1537–1544. doi: 10.1007/s10552-011-9830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaisy A, Lotfinejad S, Zhian F. Risk of Cancer with Combined Oral Contraceptive Use among Iranian Women. Asian Pac J Cancer Prev. 2014;15:5517–5522. doi: 10.7314/apjcp.2014.15.14.5517. [DOI] [PubMed] [Google Scholar]
- 29.Cogliano V, Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F WHO International Agency for Research on Cancer. Carcinogenicity of combined oestrogen-progestagen contraceptives and menopausal treatment. Lancet Oncol. 2005;6:552–553. doi: 10.1016/s1470-2045(05)70273-4. [DOI] [PubMed] [Google Scholar]
- 30.Yu CP, Ho JY, Huang YT, Cha TL, Sun GH, Yu DS, Chang FW, Chen SP, Hsu RJ. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-beta activation. PLoS One. 2013;8:e56667. doi: 10.1371/journal.pone.0056667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gago-Dominguez M, Castelao JE. Lipid peroxidation and renal cell carcinoma: further supportive evidence and new mechanistic insights. Free Radic Biol Med. 2006;40:721–733. doi: 10.1016/j.freeradbiomed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Gago-Dominguez M, Castelao JE, Yuan JM, Ross RK, Yu MC. Lipid peroxidation: a novel and unifying concept of the etiology of renal cell carcinoma (United States) Cancer Causes Control. 2002;13:287–293. doi: 10.1023/a:1015044518505. [DOI] [PubMed] [Google Scholar]
- 33.Corrao G, Scotti L, Bagnardi V, Sega R. Hypertension, antihypertensive therapy and renal-cell cancer: a meta-analysis. Curr Drug Saf. 2007;2:125–133. doi: 10.2174/157488607780598296. [DOI] [PubMed] [Google Scholar]
- 34.Qin J, Yang T, Kong F, Zhou Q. Oral contraceptive use and uterine leiomyoma risk: a meta-analysis based on cohort and case-control studies. Arch Gynecol Obstet. 2013;288:139–148. doi: 10.1007/s00404-013-2797-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


