Abstract
Data indicates that some steroid derivatives may induce changes on glucose levels; nevertheless, data are very confusing. Therefore, more pharmacological data are needed to characterize the activity induced by the steroid derivatives on glucose levels. The aim of this study was to synthesize a new steroid derivative for evaluate its hypoglycemic activity. The effects of steroid derivative on glucose concentration were evaluated in a diabetic animal model using glibenclamide and metformin as controls. In addition, the pregnenolone-dihydrotestosterone conjugate was bound to Tc-99m using radioimmunoassay methods, to evaluate the pharmacokinetics of the steroid derivative over time. The results showed that the pregnenolone-dihydrotestosterone conjugate induces changes on the glucose levels in similar form than glibenclamide. Other data showed that the biodistribution of Tc-99m-steroid derivativein brain was higher in comparison with spleen, stomach, intestine liver and kidney. In conclusion, the pregnenolone-dihydrotestosterone conjugate exerts hypoglycemic activity and this phenomenon could depend of its physicochemical properties which could be related to the degree of lipophilicity of the steroidderivative.
Keywords: Steroid, dihydrotestosterone, biodistribution, glucose
Introduction
There are epidemiological and clinical studies which show that diabetes is a risk factor to develop cardiovascular diseases [1-3]. Several drugs have been used for treatment of diabetes; such as the biguanidines, sulfonylureas, α-glucosidase inhibitors and other drugs [4,5]. Nevertheless, some of these drugs can induce several adverse effects such as intrahepatic cholestasis and cutaneous bullae that are associated with glibenclamide therapy [6]. Other studies indicate that therapy with metformin can induce lactic acidosis in diabetic patients [7]. In addition, other data indicate that most frequent side-effects to acarbose treatment are flatulence and diarrhoea [8]. All these clinical data have opened the way for the developed of new drugs for treatment of diabetes; for example, the preparation of thiazolidinedionesthat partiallymimic certain effects of insulin on carbohydrate and lipidmetabolism in Type [9,10]. Other data [11] showed the synthesis of pyrazole-4-carboxylic Acidswith hypoglycemic activity in a diabetic animal model.Other reports showed that some steroid derivativesalso exert hypoglycemic activity; for example, the synthesis of two steroid-dihydropyrimidine derivatives as antidiabetic agents used in a diabetic animal model [12]. Additionally, recentlytwo glibenclamide-pregnenolone derivatives with hypoglycemic activity were prepared [13]. These data indicate that some steroid derivativesmay induce changes on glucose levels; nevertheless, data are very confusing, perhaps this phenomenon is due to differences in the chemical structure of the steroid derivatives or to the different pharmacological approaches used. Therefore, more pharmacological data are needed to characterize the activity induced by the steroid derivatives on glucose levels. To provide this information, the present study was designed to investigate the effects of new steroid derivative on glucose levels using a diabetic model.
Experimental
Chemical synthesis
The compounds N-(2-amino-ethyl)-succinamic acid 17-acetyl-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[á]phenanthren-3-yl-ester (1) and hemisuccinatedihydrotestosterone (2) were prepared with previously methods reported [14,15]. Other reagents were obtained from Sigma-Aldrich Chemical Co., Ltd. The melting point for the steroid derivative was determined on an Electrothermal (900 model). Infrared spectra (IR) were recorded using KBr pellets on a Perkin Elmer Lambda 40 spectrometer. 1H and 13C NMR (nuclear magnetic resonance) spectra were recorded on a Varian VXR-300/5 FT NMR spectrometer at 300 and 75.4 MHz (megahertz) in CDCl3 (deuterated chloform) using TMS (tetramethylsilane) as internal standard. EIMS (electron impact mass spectroscopy) spectra were obtained with a Finnigan Trace Gas Chromatography Polaris Q Spectrometer. Elementary analysis data were acquired from a Perkin Elmer Ser. II CHNS/0 2400 elemental analyzer.
Synthesis of N-{2-[3-(17-Acetyl-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yloxycarbonyl)propionylamino]-ethyl}-succinamic acid 10,13-dimethyl-3-oxo-hexadecahydro-cyclopenta[a]phenan- thren-17-yl-ester (compound 3)
A solution of 1 (120 mg, 0.26 mmol), 2 (100 mg, 0.26 mmol) and boric acid (60 µl, 0.43 mmol) in 10 mL of methanol was stirring for 72 h to room temperature. The reaction mixture was evaporated to a smaller volume. After the mixture was diluted with water and extracted with chloroform. The organic phase was evaporated to dryness under reduced pressure, the residue was purified by crystallization from methanol: water (1:3).
Biological activity
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal Care and Use Committee of University autonomous of Campeche (No. PI-420/12) and were in accordance with the Guide for the Care and Use of Laboratory Animals [16]. Female rats (Wistar; weighing 200-250 g) were obtained from UAC.
Reagents
Experimental induction of diabetes in rats: The rats were injected with alloxan monohydrate dissolved in sterile normal saline at a dose of 150 mg/kg body wt. intraperitoneally. After 2 weeks, rats with moderate diabetes having glycosuria (indicated by Benedict’s qualitative test) and hyperglycemia (i.e., with a blood glucose ≥ 200 mg/dl) were used for the experiment.
Experimental design and treatment
In the experiment (Table 1), a total of 78 rats were used. Diabetes was induced in rats, 2 weeks before starting the experiment. The rats were divided into thirteen groupsafter the induction of diabetes. Six rats were used in each group (72 diabetic surviving rats andsix normal rats).
Table 1.
Experimental design for evaluate the hypoglycemic activity of steroid derivatives using a diabetic rat male model. The dose administered daily had an intragastric tube for 30 days
| Group 1 (Normal rats) | 2 ml of normal saline |
| Group 2 (Diabetic control rats) | 2 ml of normal saline |
| Group 3 (Diabetic rats) | Glibenclamide (600 μg/kg body mass) |
| Group 4 (Diabetic rats) | Metformin (350 mg/kg body mass) |
| Group 5 (Diabetic rats) | Compound 1 (4 mg/kg body mass) |
| Group 6 (Diabetic rats) | Compound 1 (6 mg/kg body mass) |
| Group 7 (Diabetic rats) | Compound 1 (8 mg/kg body mass) |
| Group 8 (Diabetic rats) | Compound 2 (4 mg/kg body mass) |
| Group 9 (Diabetic rats) | Compound 2 (6 mg/kg body mass) |
| Group 10 (Diabetic rats) | Compound 2 (8 mg/kg body mass) |
| Group 11 (Diabetic rats) | Compound 3 (4 mg/kg body mass) |
| Group 12 (Diabetic rats) | Compound 3 (6 mg/kg body mass) |
| Group 13 (Diabetic rats) | Compound 3 (8 mg/kg body mass) |
Biochemical assays measured in acute form.
Blood glucose was determined from tail blood with a rapid glucose analyzer (Accutrend Sensor Comfort; Roche, U.S.A.) every 24 h.
Radiochemical study
The hemisuccinate of dihydrotestosterone (compound 1) and the pregnenolone-dihydrotestosterone conjugate (compound 3) were bound to Tc-99m using radioimmunoassay methods [17-19]. A solution of 20 mg of steroid derivative in 1.0 ml was adjusted to pH 7.0 with 0.1 M NaOH. The solution was then added to another freshly prepared solution (75 ml) of stannous chloride (2 mg/ml in 0.1 M HCl) and the pH was readjusted to 7.0. In addition, two ml of a Tc-99m pertechnetate solution eluted from a sterile 99 Mo-99m-Tc shielded generator was added to the mixture solution.
Quality control
Thin Layer Chromatography was used to quality control [20]. The labeling efficiencies with Tc-99m were evaluated chromatographically using a Silica-gel 60 F254 plate. To determinate the radiochemical purity of compound studied, a solvent system such as acetonitrile:water (4:1) was used. The plates were counted by images in a gamma camera equipped with a high resolution collimator with a digital computer (VP450). The Rf values were determined using as control Tc-99m pertechnetate and hydrolyzed Tc-99m colloid. The purities of Tc-99m-conjugate were determined by paper electrophoresis. The paper strips were run at a constant voltage of 600 V for 30 min using a buffer solution (0.1 M, pH 7.4). The paper strips were counted by images in a gamma camera equipped with a high resolution collimator with a digital computer. Movement was determined relative to Tc-99m pertechnetate and hydrolyzed Tc-99m colloid.
Biodistribution study
Six rats per group were used for each biodistribution study. Each diabetic rat received 0.3 ml (200 μCi) of Tc-99m-steroid derivative (compound 3) and Tc-99m-hemisuccinate of dihydrotesteosterone (compound 1) by tail vein administration. Sequential scintigrams were taken at predetermined intervals (15, 30, 45 and 60 min) with a gamma camera equipped with a high resolution collimator with a digital computer. The animals were sacrificed and the organs were removed and the radioactivity was counted by images in a gamma camera equipped with a high resolution collimator with a digital computer. The percentages of the injected dose per organ were determined by comparison of tissue radioactivity concentration with the total radioactivity. Additionally, the blood (ml) in the heart was collected to evaluate the radioactivity with the same equip.
Physicochemical parameters evaluation
In study, physicochemical descriptors such as LogP, π, Rm, Vm, Pc and St were evaluated using previously methods reported [21].
Statistical analysis
All the experimental data were statistically evaluated and all the results were expressed as mean ± S.E. The data obtained were put under Analysis of Variance with the Bonferroni correction factor [22] using the SPSS 12.0 program. The differences were considered significant when p was equal or smaller than 0.05.
Results
Chemical synthesis
The yieldof the reaction product (compound 3, Figure 1) was 68% with melting point of 190°C. In addition, the spectroscopic analyses show signals for IR (Vmax, cm-1) at 1744, 1720 and 1658. In addition, the chemical shifts of the spectroscopic analyses of 1H NMR and 13C NMR for the steroid derivative is showed in the Tables 2 and 3. Finally, the results of mass spectroscopy (MS) (70 electronvolts) shown; m/z 830.52. Additionally, the elementary analysis data for the steroid derivative (C50H74N2O8) were calculated (C, 72.26; H, 8.79; N, 3.37; O, 15.40) and found (C, 72.24; H, 8.76).
Figure 1.
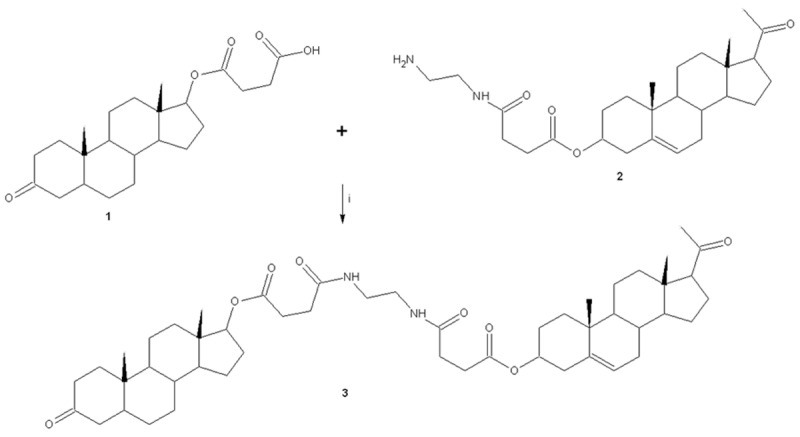
Synthesis of N-{2-[3-(17-Acetyl-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yloxycarbonyl)propionylamino]-ethyl}-succinamic acid 10,13-dimethyl-3-oxo-hexadecahydro-cyclopenta[a]phenanthren-17-yl-ester (3). Reaction of N-(2-amino-ethyl)-succinamic acid 17-acetyl-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[á]phenanthren-3-yl-ester (1) and hemisuccinate dihydrotestosterone (2) using boric acid as catalyst (i).
Table 2.
1H NMR (300 MHz, CDCl3) data for steroid derivative (compound 3)
| 0.62 (s, 3H), 0.78 (s, 3H), 0.90 (m, 1H), 0.98 (s, 3H), 1.01 (s, 3H), 1.04 (m, 1H), 1.05-1.15 (m, 4H), 1.16 (m, 1H), 1.19-1.21 (m, 3H), 1.20 (m, 1H), 1.32 (m, 1H), 1.35 (m, 1H), 1.36 (m, 1H), 1.39-1.46 (m, 3H), 1.51-1.56 (m, 3H), 1.61-1.70 (m, 5H), 1.73-1.88 (m, 4H), 1.89-1.96 (m, 2H), 2.06 (m, 1H), 2.07-2.08 (m, 2H), 2.13 (s, 3H), 2.14-2.23 (m, 5H), 2.31-2.38 (m, 2H), 2.42 (t, 2H, J=6.54), 2.49 (t, 2H, J=6.54), 2.51 (m, 1H), 2.54 (t, 2H, J=6.54 ), 2.56 (t, 2H, J=6.54), 3.32 (t, 2H, J=6.80), 3.44 (t, 2H, J=6.80 ), 4.53(m, 1H), 4.65 (m, 1H), 5.40 (d, 1H, J=6.80), 8.69 (broad, 2H) ppm. |
Table 3.
13C NMR (300 MHz, CDCl3) data for steroid derivative (compound 3)
| 12.06 (C-21), 13.10 (C-57), 17.06 (C-22), 19.58 (C-56), 20.75 (C-13), 21.90 (C-48), 22.90 (C-53), 23.50 (C-11), 23.58 (C-60), 27.00 (C-8), 27.60 (C-10), 27.76 (C-40), 28.84 (C-9), 29.42 (C-24, C-35), 30.43 (C-34), 31.40 (C-54), 31.78 (C-44, C-52), 31.90 (C-25), 35.16 (C-4), 35.30 (C-6), 36.92 (C-12), 37.02 (C-41), 37.37 (C-30), 37.50 (C-15), 37.78 (C-14), 37.94 (C-42), 38.20 (C-49), 38.80 (C-47), 42.40 (C-29), 42.66 (C-2), 43.40 (C-17), 43.90 (C-46), 45.40 (C-7), 47.20 (C-5), 50.02 (C-43), 50.65 (C-3), 56.78 (C-45), 63.60 (C-55), 73.95 (C-39), 82.40 (C-1), 122.70 (C-51), 139.60, 139.60 50), 169.81 (C-26), 171.28 (C-36), 171.40 (C-19), 171.66 (C-32), 209.20 (C-58), 212.36 (C-16) ppm. |
Biological activity
Glucose levels
The resultsobtained in an acute form on male rats, showed that Glibenclamide (470 to 112 mg/dl; p=0.05) and metformin (458 to 148 mg/dl; p=0.06) significantly reduced the bloodglucose levels in comparison with diabetic rats (without treatment) which were used as control (405 to 520 mg/dl). Additionally, other results showed thatthe compound 3 (Figure 2) significantly decrease the blood glucoselevels at doses of 4 mg/dl (430 to 134 mg/dl; p=0.05), 6.0 mg/dl (450 to 81 mg/dl; p=0.05) and 8.0 mg/dl (410 to 96 mg/d1; p=0.05).
Figure 2.
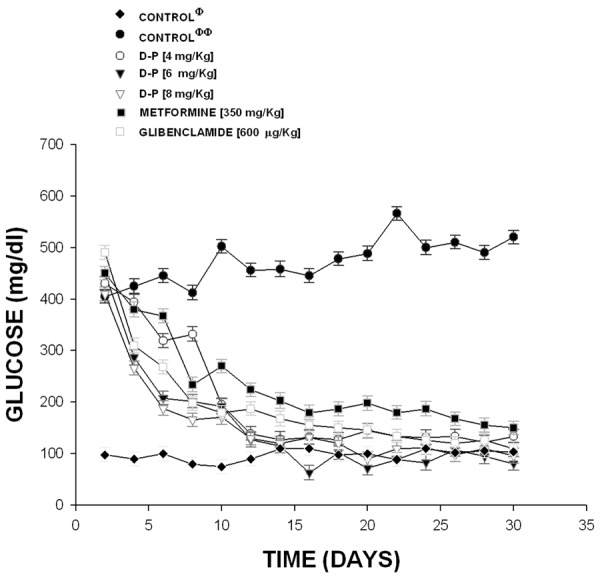
Activity induced by the pregnenolone-dihydrotestosterone conjugate (D-P) on glucose concentration in a diabetic rat model. The results showed that D-P significantly reduced (p=0.05) the blood glucose concentration at doses different in comparison with diabetic rats (without treatment). The effects are expressed as mean ± S.E. n=6 of each group. Φ=non-diabetic rats; ΦΦ=diabetic rats without treatment.
Other results indicate that the compound 2 (Figure 3) at doses of 4 mg/dl (452 to 502 mg/dl), 6 mg/dl (502 to 570 mg/d1) and 8.0 mg/dl (478 to 532 mg/dl) was not decreased the glucose levels in comparison with glibenclamide and metformin. It is important to mention that experimentscontrol with normal rats (non-diabetic) the level of glucose showedan average of 98 to 104. Finally, other experimental results (Figure 4) indicate that also the dihydrotestosterone derivative exert changes on glucose concentration in similar form that compound 3 at dose of 4 mg/Kg (502 to 138 mg/dl; p=0.05); at 6 mg/kg (456 to 116 mg/dl; p=0.05); at 8 mg/Kg (522 to 124 mg/dl; p=0.05).
Figure 3.
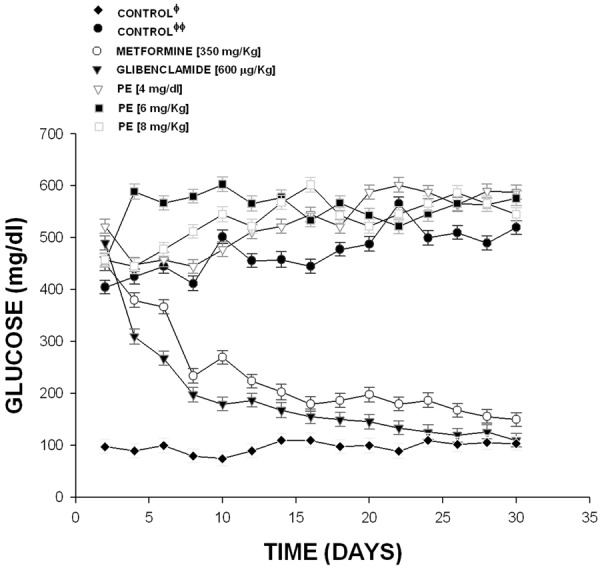
Effect exerted by pregnenolone derivative (PE) on glucose concentration in a diabetic rat model. The results showed that blood glucose concentration was not decreased by administration of PE at doses different in comparison with glibenclamide and metformin. The effects are expressed as mean ± S.E. n=6 of each group. Φ=non-diabetic rats; ΦΦ=diabetic rats without treatment.
Figure 4.
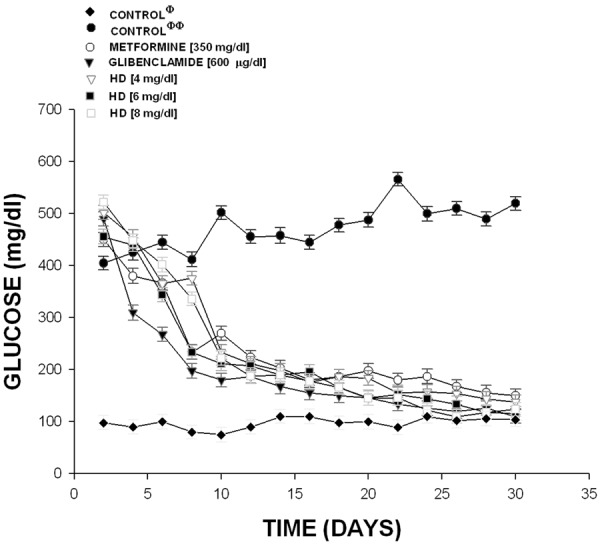
Activity induced by the hemisuccinate of dihydrotestosterone (HD) on glucose concentration in a diabetic rat model. The results showed that HD significantly decreased (p=0.05) the blood glucose levels at doses different in comparison with diabetic rats (without treatment). The effects are expressed as mean ± S.E. n=6 of each group. Φ=non-diabetic rats; ΦΦ=diabetic rats without treatment.
Radiochemical study
Thin Layer Chromatography method shows that steroidderivatives were bound to Tc-99m (≈90%) under the conditions previous described. The purities of Tc-99m-steroid conjugates determined by paper electrophoresis showed a value of Rf of 0.56 for Tc-99m-steroid derivative (compound 3) and a Rf of 0.45 for Tc-99m-hemisuccinate of dihydrotestosterone (compound 1).
Pharmacokinetics activity
The biodistribution of Tc-99m-steroidderivatives (Figure 5; Tables 4 and 5) showed thatbiodistribution of compounds 3 was significantly higherin the brain than in spleen, stomach, intestine liver andkidney in comparison with the compound 1.
Figure 5.
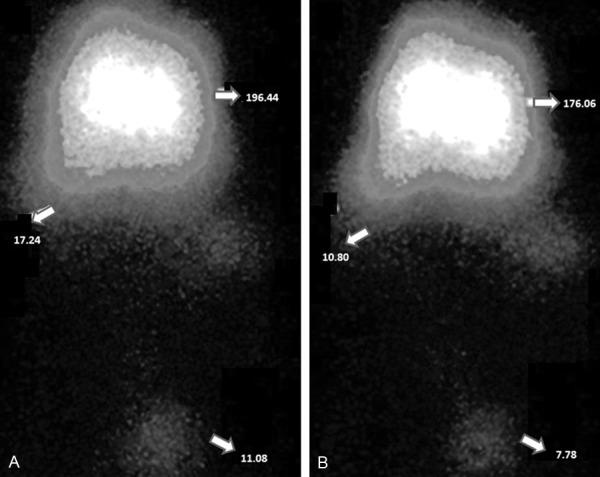
Scintigrams (μCi) were taken 15 min after the administration of the Tc-99m-steroid derivatives. The pregnenolone-dihydrotestosterone conjugate (A) show different values on brain (196.44 μCi) in comparison with others biological compartments such as spleen (17.24 μCi) and gonads (11.08 μCi). Other data showed a biodistribution of the hemisuccinate of dihydrotestosterone (B) inseveral issues such as brain (176.9 μCi) spleen (10.80 μCi), and gonads (7.78 μCi).
Table 4.
Biodistribution of Tc-99m-dihydrotestosterone succinate conjugate (μCi). Each value is mean ± S.E. n=6
| Time (minutes) | ||||
|---|---|---|---|---|
|
|
||||
| Organ | 15 | 30 | 45 | 60 |
| Brain | 176.02 | 170.12 | 168.44 | 156.08 |
| Spleen | 11.22 | 10.78 | 10.68 | 10.02 |
| Stomach | 10.80 | 9.78 | 10.00 | 8.78 |
| Intestine | 5.80 | 5.66 | 5.78 | 5.40 |
| Liver | 16.24 | 15.02 | 14.04 | 13.22 |
| Kidney | 3.88 | 3.90 | 4.02 | 3.68 |
| Gonads | 7.78 | 7.56 | 7.86 | 6.65 |
| Blood | 3.76 | 3.24 | 2.04 | 1.80 |
Table 5.
Biodistribution of Tc-99m-steroid conjugate (μCi). Each value is mean ± S.E. n=6
| Time (minutes) | ||||
|---|---|---|---|---|
|
|
||||
| Organ | 15 | 30 | 45 | 60 |
| Brain | 196.44 | 196.12 | 195.88 | 197.08 |
| Spleen | 10.22 | 10.02 | 9.44 | 9.02 |
| Stomach | 17.24 | 16.44 | 16.38 | 15.76 |
| Intestine | 7.02 | 6.54 | 5.04 | 5.00 |
| Liver | 18.24 | 18.04 | 17.86 | 17.88 |
| Kidney | 5.10 | 4.87 | 4.44 | 4.32 |
| Gonads | 11.08 | 10.65 | 10.02 | 12.44 |
| Blood | 3.32 | 2.88 | 2.22 | 2.04 |
Physicochemical parameters
Data of compounds 1 and 3 are showed in the Table 6. These data indicate a higher degree of liposolubility of 3 with respect to 1.
Table 6.
Physicochemical parameters [log P (log Kow), and π] of both compounds hemisuccinate of dihydrotestosterone (1) and steroid derivative (3)
| Compounds | LogKow Fragment | Contributions |
|---|---|---|
| 1 | -CH3 [aliphatic carbon] | 1.0946 |
| -CH2- [aliphatic carbon] | 5.4021 | |
| -CH [aliphatic carbon] | 1.8070 | |
| -C(=O)- [carbonyl, aliphatic attach] | -1.5586 | |
| -COOH [acid, aliphatic attach] | -0.6895 | |
| -C(=O)O [ester, aliphatic attach] | -0.9505 | |
| -tert Carbon [3 or more carbon attach] | 0.5352 | |
| Fused aliphatic ring unit correction | -2.0526 | |
| Equation Constant | 0.2290 | |
| Log Kow | 3.8167 | |
| π | 0.7508 | |
| 3 | -CH3 [aliphatic carbon] | 2.7365 |
| -CH2- [aliphatic carbon] | 11.2953 | |
| -CH [aliphatic carbon] | 3.6140 | |
| =CH- or =C < [olefin carbon] | 0.7672 | |
| -NH- [aliphatic attach] | -2.9924 | |
| -C(=O)- [carbonyl, aliphatic attach] | -3.1172 | |
| -C(=O)O [ester, aliphatic attach] | -1.9010 | |
| -C(=O)N [aliphatic attach] | -1.0472 | |
| -tert Carbon [3 or more carbon attach] | 1.0704 | |
| Fused aliphatic ring unit correction | -4.1052 | |
| Equation Constant | 0.2290 | |
| Log Kow | 6.5494 | |
| π | 2.7327 |
Discussion
Chemical synthesis
There are several experimental approaches to develop new drugs to treatment of diabetes; however, expensive reagents and special conditions are required [23-25]. Therefore in this study a new hypoglycemic drug was prepared by the reaction of a pregnenolonederivative andhemisuccinate of dihydrotestosterone to form the pregnenolone-dihydrotestosterone conjugate using boric acid as catalyst. The chemical structure of the pregnenolone-dihydrotestosterone conjugate was confirmed using IR and NMR spectroscopy (Tables 2 and 3). The IR spectra contained characteristic vibrations at 1744 for ester group; at 1720 for ketone group; at 1658 for both amide groups. The 1H NMR spectrum of the steroid derivative shows signals at 0.62 and 1.00 ppm for methyl groups bound to pregnenolone fragment; at 0.78 and 0.98 ppmfor methyl groups bound to dihydrotestosterone fragment; at 2.13 ppm for methyl group bound to carbonyl group; at 0.90, 1.05-1.15, 1.19-1.24, 1.32, 1.36, 1.51-1.56, 1.73-1.88, 2.06, 2.14-2.23, and 4.65 ppm for dihydrotestosterone fragment; at 1.04, 1.16, 1.20, 1.35, 1.39-1.46, 1.61-1.70, 1.89-1.96, 2.07-2.08, 2.31-2.38, 2.51, 4.53 and 5.40 ppm for pregnenolone fragment; at 2.42, 2.48, 2.54 and 2.56 ppm for methylene groups bound to both ester and amide groups; at 3.32 and 3.44 ppm for methylene groups bound to both amide groups; at 8.60 ppm for both amide groups. The 13C NMR spectrum of 3 contains peaks at 12.06 and 17.06 ppm for methyl groups bound to dihydrotestosterone fragment; at 13.10 and 19.58 ppm for methyl groups bound to pregnenolone fragment; at 23.58 for methyl group bound to carbonyl group; at 20.75, 25.50, 27.00, 27.60, 28.84, 35.16-36.92, 37.50-37.78, 42.66-43.40, 45.40-47.20, 50.65 and 82.44 ppm for protons involved in the dihydrotestosterone fragment; at 21.90, 27.76, 31.40-31.78, 37.02, 37.94-38.80, 43.90, 50.02, 56.78-73.95 and 122.70-139.60 ppm for methylene groups involved in the pregnenolone fragment; at 29.42-30.43 and 31.90 ppm for methylene groups bound to both ester and amide groups; at 37.37 and 42.40 ppm for methylene groups bound to both amide groups; at 169.81 and 171.66 ppm for both amide groups; at 171.28 and 171.40 ppm for both ester groups; at 209.20 and 212.36 for both ketone groups. Finally, the presence of the pregnenolone-dihydrotestosterone conjugate was further confirmed from mass spectrum which showed a molecular ion at m/z 830.52.
Biological activity
In this study, the hypoglycemic activity of the pregnenolone-dihydrotestosterone conjugate was evaluated in a diabetic rat model. It is important to mention, that diabetes was induced with alloxan. There are reports that alloxan causes massive reduction in insulin release, through the destruction of β-cells of the islets of Langerhans [26].
On the other hand, the results showed that pregnenolone-dihydrotestosterone conjugate had potency similar in comparison with glibenclamide; nevertheless, this effect was higher with respect to metformin. Analyzing these data and physicochemical properties of these steroid derivative, alternative experiments were performed using as pharmacological tools to hemisuccinate of dihydrotestosterone and the pregnenolonederivative to test if any of the fragments involved in the pregnenolone-dihydrotestosterone conjugate could be the responsible for its hypoglycemic activity. The results indicate that pregnenolone-dihydrotestosterone conjugate have similar effect in comparison with hemisuccinate of dihydrotestosterone and different with respect to pregnenolone derivative. These data indicate that only the hemisuccinate of dihydrotestosterone is the responsible of hypoglycemic activity of the pregnenolone-dihydrotestosterone conjugate.
Pharmacokinetics activity
Analyzing the results previously described, in thisstudy was evaluated the biodistribution of steroidderivative using radioimmunoassay methods (17-19). In thistechnique the steroid derivatives were easily labeledwith Tc-99m by the conventional stannous chloridemethod. The steroid conjugates involved in thisstudy are excellent chelating agents, with both carbonylgroups binding to Tc-99m.
On the other hand, to evaluate the pharmacokineticsof the compounds hemisuccinate of dihydrotestosterone and pregnenolone-dihydrotestosterone conjugate as a consequence ofincreases in time (15, 30, 45 and 60 min) the Tc-99m-steroid conjugates were used. The results indicatethat the biodistribution of bothsteroid derivativeswas significantly higher in brain in comparison with spleen, stomach, intestine liver and kidney; however, there are differences in the distribution of the compounds hemisuccinate of dihydrotestosterone and pregnenolone-dihydrotestosterone conjugate in each organ studied. Thisphenomenon could be conditioned by interaction betweenthe steroid derivatives and some endogenous substancesinvolved in the brain or by the degree of lipophilicity exerted by both steroid derivatives; this effectmay depend of some physicochemical parameters involved in its structure chemical such as happening with other type of compounds [27].
Physicochemical parameters
In order to delineate the structural chemical requirementsof the compounds hemisuccinate of dihydrotestosterone and pregnenolone-dihydrotestosterone conjugate in volved in the degree of lipophilicity, some parameters such as thedescriptors log P and π [28] were calculated. The descriptor log P estimates the logarithmicoctanol-water partition coefficient; therefore, log P representsthe lipophilic effects of a molecule which includesthe sum of the lipophilic contributions of the parent moleculeand its substituent [29]. The differencebetween the substituted and unsubstituted log P values isconditioned by the π value for a particular substituent. Several years ago, Hammett showed that π valuesmeasure the free energy change caused by particular substituentto relate to biological activity [30]. Therefore, in this study, the logP and π parameters werecalculated by previously methods reported (Mannhod andWaterbeemd, 2001). The results (Table 6) showed an increase inlogP and π values in the pregnenolone-dihydrotestosterone conjugate with respect to hemisuccinate of dihydrotestosterone. Thisphenomenon is conditioned mainly, by the contribution of allsubstituent atoms involved in the chemical structure of two compounds. All these data indicate that aliphatic carbons involved in thepregnenolone-dihydrotestosterone conjugate contributes to increase the degree oflipophilicity in comparison with the compound hemisuccinate of dihydrotestosterone which consequently brings higher biodistribution of the pregnenolone-dihydrotestosterone conjugate in all biological compartments in comparison with hemisuccinate of dihydrotestosterone.
Conclusions
The new steroid derivative exerts hypoglycemic activity and this phenomenon could depend of its physicochemical properties which could be related to the degree of lipophilicity of the steroid derivative.
Disclosure of conflict of interest
None.
References
- 1.Gaziano A. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112:3547–3553. doi: 10.1161/CIRCULATIONAHA.105.591792. [DOI] [PubMed] [Google Scholar]
- 2.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1997;97:596–601. doi: 10.1161/01.cir.97.6.596. [DOI] [PubMed] [Google Scholar]
- 3.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U. S. adults. The third national health and nutrition examination survey, 1988-1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 4.Zárate A, Tene C. Nuevos fármacos en el tratamiento de la diabetes mellitus tipo 2. Gac Med Mex. 1995;135:91–93. [PubMed] [Google Scholar]
- 5.Knowler W, Barrett-Connor E, Fowler S, Hamman R, Lachin J. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wongpaitoon V, Mills P, Russell R, Patrick R. Intrahepatic cholestasis and cutaneous bullae associated with glibenclamide therapy. Postgrad Med J. 1981;57:244–246. doi: 10.1136/pgmj.57.666.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Arch Intern Med. 2003;163:2594–2602. doi: 10.1001/archinte.163.21.2594. [DOI] [PubMed] [Google Scholar]
- 8.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;259:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 9.Saltiel A, Olefsky J. Thiazolidinediones in the Treatment of Insulin Resistance and Type II Diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Javorschi S, Hevener A, Kruszynska Y, Norman R, Sinha M, Olefsky J. The Effect of Thiazolidinediones on Plasma Adiponectin Levels in Normal, Obese, and Type 2 Diabetic Subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 11.Cottineau B, Toto P, Marot C, Pipaud A, Chenault J. Synthesis and Hypoglycemic Evaluation of Substituted Pyrazole-4-carboxylic Acids. Bioorg Med Chem Lett. 2002;12:2105–2118. doi: 10.1016/s0960-894x(02)00380-3. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa-Valverde L, Díaz-Cedillo F, Camacho-Luis A, López-Ramos M, García-Cervera E. Design and Synthesis of Carbamazepine-Alkyne Conjugate as Antidiabetic Agent: Study of Chemical Descriptors (logP and π) Asian J Chem. 2010;22:7057–7064. [Google Scholar]
- 13.Figueroa-Valverde L, Diaz-Cedillo F, Lopez-Ramos M, Garcia-Cervera E, Pool-Gomez E, Cardeña-Arredondo C, Ancona-Leon G. Glibencla-mide-pregnenolone derivative has greater hypoglycemic effects and biodistribution than glibenclamide-OH in alloxan-rats. Biomed Pap. 2012;156:122–127. doi: 10.5507/bp.2012.028. [DOI] [PubMed] [Google Scholar]
- 14.Figueroa-Valverde L, Díaz-Cedillo F, Tolosa L, Maldonado G, Ceballos-Reyes G. Synthesis of Pregnenolone-Pregnenolone Dimer via Ring A-Ring A connection. J Me Chem Soc. 2006;50:42–45. [Google Scholar]
- 15.Figueroa-Valverde L, Diaz-Cedillo F, Lopez-Ramos M, Garcia-Cervera E. A facile Synthesis of two quaternary aminosteroids derivatives and its correlation with descriptors LogP and π. Intern J Chem Tech Res. 2010;2:1553–1559. [Google Scholar]
- 16.Bayne K. Revised Guide for the Care and Use of Laboratory Animals available. American Physiological Society. Physiologist. 1996;39:208–211. [PubMed] [Google Scholar]
- 17.Kawashima K, Kuzuya T, Matsuda A. Radioimmunoassay of glibenclamide. Diabetes. 1979;28:221–226. doi: 10.2337/diab.28.3.221. [DOI] [PubMed] [Google Scholar]
- 18.Yoda R, Arube Y, Takata J, Nagata Y, Matsushima Y. Technetium-99m Complexes with Steroid-2-aminooxyethyliminodiaceticAcid Conjugates. Chem Pharm Bull. 1994;47:413–416. doi: 10.1248/cpb.47.413. [DOI] [PubMed] [Google Scholar]
- 19.Schneider S, Ueberberg S, Korobeynikov A, Schechinger W, Schwanstecher C, Schwanstecher M, Klein HH, Schirrmacher E. Synthesis and evaluation of a glibenclamide glucose-conjugate: a potential new lead compound for substituted glibenclamide derivatives as islet imaging agents. Regul Pept. 2007;139:122–127. doi: 10.1016/j.regpep.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Yumisley A, Rivero A, Zayas F, Mesa N, Hernández I, León M. Formulación de 99M TC-Macroagregadosde albúmina para estudios de perfusión pulmonar. Nucleus. 2009;45:19–25. [Google Scholar]
- 21.Mannhod R, Waterbeemd H. Substructure and whole approaches for calculating logP. J Comput Aided Mol Des. 2001;15:337–354. doi: 10.1023/a:1011107422318. [DOI] [PubMed] [Google Scholar]
- 22.Hocht C, Opezzo L, Gorzalczany S. Una Aproximación Cinética y Dinámica de Metildopa en Ratas con Coartación Aórtica Mediante Microdiálisis. Rev Argent Cardiol. 1999;67:769–773. [Google Scholar]
- 23.Meurer L, Tolman R, Chapin E, Saperstein R, Vicario P, Zrada M andMacCoss M. Synthesis and hypoglycemic activity of substituted 8-(1-piperazinyl)imidazo[1,2-a] pyrazines. J Med Chem. 1992;35:3845–3857. doi: 10.1021/jm00099a012. [DOI] [PubMed] [Google Scholar]
- 24.Woo L, Bok K, Jong A, Sung K, Jung L, Jae S, Soon A, Sang L, Seung Y. Molecular design, synthesis, and hypoglycemic and hypolipidemic activities of novel pyrimidine derivatives having thiazolidinedione. Eur J Med Chem. 2005;40:862–874. doi: 10.1016/j.ejmech.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Tunçbilek M, Bozdağ-Dündar O, Ayhan-Kılcıgil G, Ceylan M, Waheed A, Verspohl E, Ertan R. Synthesis and hypoglycemic activity of some substituted flavonylthiazolidinedione derivatives-fifth communication: flavonyl benzyl su bstituted 2,4-thiazolidinediones. Farmaco. 2003;58:79–83. doi: 10.1016/S0014-827X(02)01241-7. [DOI] [PubMed] [Google Scholar]
- 26.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 27.Figueroa-Valverde L, Díaz-Cedillo F, López-Ramos M, Garcia-Cervera E. Activity induced by two Steroid-Dihydropyrimidine derivatives on Glucose levels in a Diabetic Rat Model. Relationship between descriptors logP and p and its Antidiabetic activity. Int J Pharm Tech Res. 2010;2:2075–2080. [Google Scholar]
- 28.Leo A, Jow P, Silipo C. Calculation of hydrophobic constant (log P) from pi and f constants. J Med Chem. 1975;18:865–868. doi: 10.1021/jm00243a001. [DOI] [PubMed] [Google Scholar]
- 29.Leo A, Hoekman D. Calculating log P(oct) with no missing fragments; The problem of estimating new interaction parameters. Perspect Drug Discov Des. 2000;18:19–38. [Google Scholar]
- 30.Hansch C, Leo A, Taft R. A survey of Hammett substituent constants and resonance and field parameters. Chem Rev. 1991;91:165–195. [Google Scholar]


