Abstract
Background and objectives: An increasing number of studies have examined the ability of mesothelin to be a marker for the diagnosis of pancreatic cancer (PCa). The exact role of mesothelin needs to be elucidated. The aim of this study is to determine the overall accuracy of mesothelinin PCa through a meta-analysis of published studies. Materials and methods: Publications addressing the accuracy of mesothelin in the diagnosis of PCa were selected from Pubmed, Embase, Cochrane Library, Web of Science, and The Chinese Journals Full-text Database (CNKI). The following indexes of test accuracy were computed for eachstudy: sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). The diagnostic threshold identified for each study wasused to plot a summary receiver operating characteristic (SROC) curve. Statistical analysis was performed by Meta-Disc1.4 and STATA 12.0 software. Results: 12 studies met the inclusion criteria. The summary estimates for mesothelin in the diagnosis of PCa were: sensitivity 0.71 (95% CI, 0.67-0.75), specificity 0.88 (95% CI, 0.85-0.91), positive likelihood ratio (PLR) 8.53 (95% CI, 3.42-21.27), negative likelihood ratio (NLR) 0.36 (95% CI, 0.28-0.46)and diagnostic odds ratio 33.93 (95% CI, 10.71-107.5). The SROC curveindicated that the maximum joint sensitivity and specificity (Q-value) was 0.81; the area under the curve was 0.88. Conclusion: Our findings suggest that mesothelin may be a useful diagnostic adjunctive tool for confirming PCa. However, further large scale studies are needed to confirm these findings.
Keywords: Pancreatic cancer, mesothelin, diagnosis, accuracy, meta-analysis
Introduction
Pancreatic cancer (PCa) is one of the most difficult cancers to treat with increasing incidence and mortality worldwide [1]. Despite surgical resection, radiation, and chemotherapy, more than 94% of people with PCado not survive beyond 5 years [2]. Most PCa patients are diagnosed with metastatic disease at the time of presentation, with median survival duration less than 6 months [3]. Therefore, to make an early and accurate diagnosis will be very importance to the treatment and prognosis of PCa.
Diagnosis of PCa mainly relies upon pathology findings together with radiological information or clinical and cytological data [4-7]. However, a wide range of histopathologic features may present in PCa and mimic other kinds of cancers. Similarly, cytological analysis requires the distinction of malignant pancreatic epithelial cells from reactive pancreatic and bile duct cells as well as other gastrointestinal contaminants, which often makes the diagnosis difficult [8]. One potential way of improving diagnostic accuracy is to use immunohistochemical (IHC) biomarkers as an adjunct in difficult to diagnose cases [9]. Several diagnostic IHC biomarkers have been investigated both as single biomarkers and as part of biomarker panels to improve the diagnosis of PCa. Mesothelin, a 40-kD phosphatidyl-inositol linkedcell-surface glycoprotein, has been observed in an increasing number of human malignancies [10,11], but not in normal pancreatic ductal epithelium [12,13]. Therefore, mesothelin may have utility as a marker for discriminating between benign and malignant pancreatic epithelium.
Although an increasing number of studies have examined the ability of mesothelin to be a marker for thediagnosis of PCa [14-25], the exact role of mesothelin needs to be elucidated. As meta-analysis is an essential tool for accurately and reliably summarizing evidence, we performed this meta-analysis to assess the potential value of mesothelin in the diagnosis of PCa, which, to the best of our knowledge,has not been previously performed.
Material and methods
Search strategy and study selection
Electronic databases Pubmed, Embase, CochraneLibrary, Web of Science, and The Chinese Journals Full-text Database (CNKI) (updated to June 30, 2014) were searched for suitable studies. The search terms were “pancreatic cancer/pancreatic carcinoma/pancreatic adenocarcinoma/pancreatic ductal adenocarcinoma/pancreatic neoplasm”, “mesothelin”, “sensitivity”, “specificity”, and “diagnosis”. The reference lists of all articles reviewedwere also searched for eligible studies. A study was included if it met the following inclusion criteria: (1) e-clinical studies on evaluation of mesothelin in the diagnosis of PCa, (2) each study contains morethan ten specimens, and (3) studies must provide sufficient data tocalculate both sensitivity and specificity. Conference abstracts, reviewsand letters to editor were excluded because of the limited data.
Data extraction and quality assessment The final set of articles was assessed independentlyby two reviewers. The following data from each publication were collected: author, publication year, study of state, diagnosticstandard, patient number, specimen, test method, mesothelin expression signature, sensitivity and specificitydata and methodological quality. The methodological quality of each study was assessed by QUADAS (quality assessment for studies of diagnostic accuracy, an evidence-based quality assessment tool for use in systematic reviews of diagnostic accuracy studies, maximum score 14) [26].
Statistical analysis
The standard methods recommended for diagnostic accuracy were used in this meta-analysis [27]. Analyses were performed using two statistical software programs: Stata, version 12 (StataCorporation, College Station, TX, USA) and Meta-Disc 1.4 for Windows (XI Cochrane Colloquium, Barcelona, Spain). The following indexes of test accuracy were computed for eachstudy: sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). The diagnostic threshold identified for each study was used to plot a summary receiver operating characteristic (SROC) curve [28]. To detect cut-off threshold effects, the relationship between sensitivity and specificity was evaluated by the Spearman correlation coefficient. The chi-square-based Q test and the inconsistency index I2 were used to detect statistically significant heterogeneity across studies. When a significant Q test (p < 0.05 or I2 > 50%) indicated heterogeneity among studies, the random-effectmodel (DerSimonian-Laird method) was conducted for the meta-analysis to calculate the pooled sensitivity, specificity, and other related indexes of the studies; otherwise, the fixed-effect model (Mantel-Haenszel method) was chosen. Chi-square test was usedto detect statistically significant heterogeneity across studies. If there were enough studies, meta-regression was performed to investigate the source of heterogeneity within the included studies (inverse variance weighted) [29]. Since publication bias is of concern for meta-analyses of diagnostic studies, we tested for the potential presence of this bias using Deeks’ funnel plots [30]. All statistical tests were two-sided and p < 0.05 was considered to indicate a statistically significant result.
Results
Quality of reporting and study characteristics The literature selection process were presented in a flow chart in Figure 1. In accordance with the inclusion and exclusion criteria, 12 publications dealing with mesothelin for diagnosis of PCa were included in the present meta-analysis. The clinical characteristics of the sestudies, along with QUADAS score, were outlined in Table 1. Overall, 12 selected studies including 928 patients were available for analysis. All patients with PCa were diagnosed based on the histological evaluation of surgically resected tissue specimens or endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) biopsy and/or clinical data. Of the 12 articles included, 7 had QUADAS scores ≥ 10.
Figure 1.
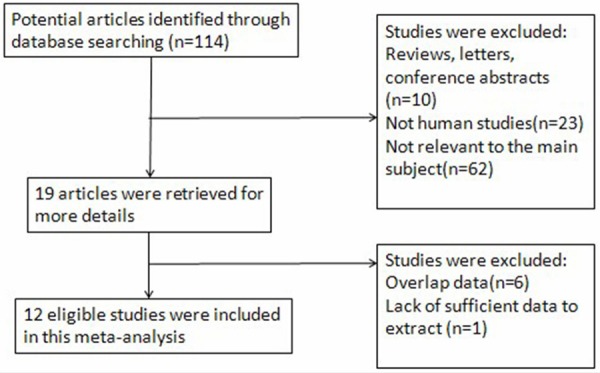
Flow chart of selection process for eligible articles.
Table 1.
Summary of the studies included in the meta-analysis
| First author | Year | Country | Specimen type | Cut-off | Sample size | TP | FP | FN | TN | QUADASscores |
|---|---|---|---|---|---|---|---|---|---|---|
| McCarthy DM | 2003 | America | FNA | Strong cytoplasmic and membranous staining | 30 | 13 | 1 | 6 | 10 | 10 |
| Zhu QY | 2005 | China | FNA | Membranous staining | 27 | 14 | 1 | 5 | 7 | 12 |
| Hornick JL | 2005 | America | surgical | Membranous staining | 60 | 16 | 0 | 9 | 35 | 9 |
| Hassan R | 2005 | America | surgical | ≥ 1% cells stained | 74 | 38 | 1 | 1 | 34 | 10 |
| Jhala N | 2006 | America | surgical | ≥ 5% cells with ≥ 2+ intensity cytoplasmic staining | 65 | 28 | 0 | 17 | 20 | 10 |
| Baruch AC | 2007 | America | FNA | Cytoplasmic and membranous staining | 36 | 18 | 0 | 10 | 8 | 10 |
| Chen ZR | 2008 | China | surgical | Cytoplasmic and membranous staining | 82 | 32 | 0 | 11 | 39 | 11 |
| Agarwal B | 2008 | America | FNA | Cytoplasmic staining | 56 | 20 | 9 | 1 | 26 | 7 |
| Glass JP | 2011 | America | FNA | ≥ 2+ intensityapical staining | 58 | 24 | 0 | 18 | 16 | 9 |
| Liu H | 2012 | America | surgical | ≥ 5% cells stained | 180 | 35 | 28 | 25 | 92 | 9 |
| Dim DC | 2014 | America | FNA | Cytoplasmic and membranous staining | 62 | 37 | 8 | 13 | 4 | 11 |
| Ali A | 2014 | UK | surgical | Cytoplasmic and membranous staining | 198 | 72 | 4 | 27 | 95 | 8 |
TP, true positive; FP, false positive; FN, false negative; TN, true negative;FNA, fine-needle aspiration.
Quantitative data analysis
The I2 of sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and DOR were 71.7% (p = 0.0001), 86% (p < 0.0001), 88% (p < 0.0001), 61.1% (p = 0.0029), and 78.4% (p < 0.0001), respectively. Since heterogeneity is obvious in the study, the random effects model was used for calculating pooled sensitivity, specificity, PLR, NLRand DOR. The pooled sensitivity and specificity of mesothelin test for the diagnosis of PCa calculated was 0.71 (95% CI, 0.67-0.75)and 0.88 (95% CI, 0.85-0.91), respectively. The forest plots of sensitivity and specificity of eachincluded study were shown in Figure 2. The summary positiveand negative likelihood ratios were 8.53 (95% CI, 3.42-21.27) and 0.36 (95% CI, 0.28-0.46). The pooled diagnostic oddsratio was 33.93 (95% CI, 10.71-107.5) (Figure 3). Figure 4 displays the SROC curve, which presentsa global summary of test performance and shows the tradeoff between sensitivity and specificity [31 chest].
Figure 2.
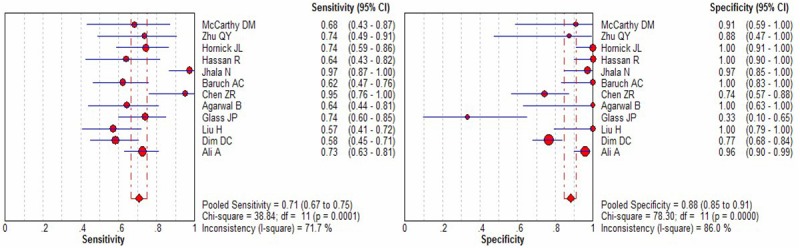
Forest plots of the sensitivity and specificity for mesothelin in the diagnosis of PCa for all studies. The point estimates of sensitivity and specificity for each study are shown as solid circles and the size of each solid circle indicates the sample size of each study. Error bars are 95% confidence intervals.
Figure 3.
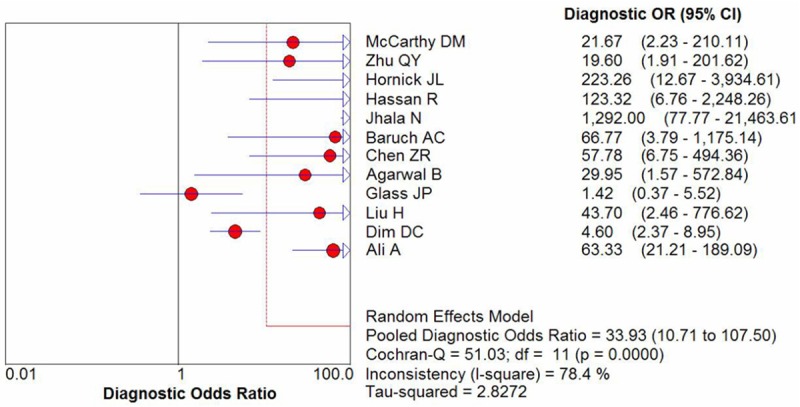
Summary receiver operating characteristic (SROC) curve for mesothelinin the diagnosis of PCa for all studies. Solid circles represent each study included in the meta-analysis. The size of each solid circle indicates the size of each study. The regression SROC curve summarizes the overall diagnostic accuracy.
Figure 4.
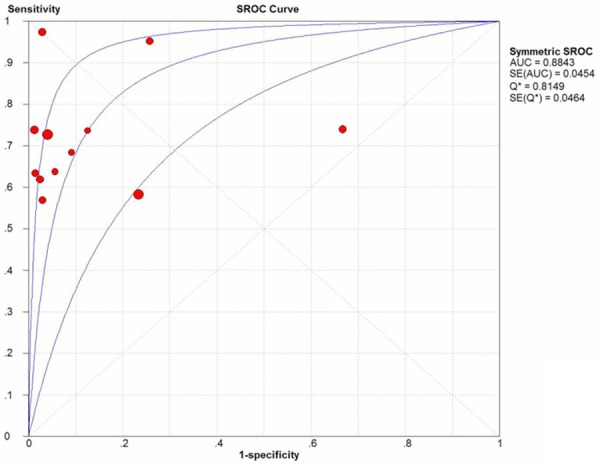
Forest plots of pooleddiagnostic odds ratio (DOR) for mesothelinin the diagnosis of PCa for all studies. Solid circles represent each study included in the meta-analysis. The size of each solid circle indicates the size of each study. Error bars are 95% confidence intervals.
As a global measure of test efficacy we used the Q-value, the intersection point of the SROC curve with a diagonal line from the left upper corner to the right lower corner of the ROC space, which corresponds to the highest common value of sensitivity and specificity for the test. This point does not indicate the only or even the best combination of sensitivity and specificity for a particular clinical setting but represents an overall measure of the discriminatory power of a test. In the present meta-analysis, the maximum joint sensitivity and specificity was 0.81 (the Q value), the AUC was 0.88, indicating the level of overall accuracy was good.
Meta-regression and publication Bias
As I2 test for the pooled sensitivity, specificity, NLR and DOR showed a significant heterogeneity between the studies, a meta-regression analysis was performed to explore the possible reasons for the heterogeneity. We used specimen type (surgical or fine-needle aspiration (FNA) specimens), sample size (≥ 100 or < 100) and QUADAS scores (≥ 10 or < 10) as covariates in our meta-regression. In the present study, none of the above covariates were found to be the significant source of heterogeneity (p = 0.4432, 0.3987 and 0.8775, respectively).
Publication bias was explored through Deeks’ funnel plots. The shape of the funnel plot of the pooled DOR of mesothelin for the diagnosis of PCa did not reveal any evidence of obvious asymmetry (Figure 5), while the Deeks’ test showed a statistically non-significant value (p = 0.96), indicating that there was no potential publication bias.
Figure 5.
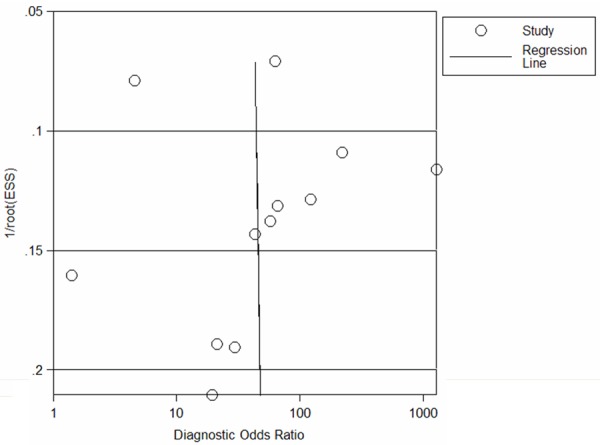
Funnel graph for the assessment of potential publication bias of the 12 included studies. The funnel graph plots the log of the diagnostic oddsratio (DOR) against the standard error of the log ofthe DOR (an indicator of sample size). Solid circles represent each study in the meta-analysis. The line indicates the regression line.
Discussion
The diagnosis of PCa is an important clinical challenge because of the late clinical presentation with advanced disease. In recent years, molecular techniques such as serial analysis of gene expression and RNA-based global gene expression profiling have identified several potential new markers of pancreatic cancer. Among these, mesothelin expression isreported to distinguish benign from malignant pancreatic tissue [12,13] and an increasing number of diagnostic tests have focused on the value of mesothelin in the differential diagnosis of benign and malignant pancreatic diseases, but the results remain controversial because of several factors, including the differences in study designs, sample size, statistical methods, etc [32]. As meta-analysis is an essential tool for accurately and reliably summarizing evidence, we performed this meta-analysis to comprehensively assess the diagnostic accuracy of mesothelin for PCa.
In our meta-analysis, the data has shown that the pooled sensitivity and specificity were 0.71 and 0.88, respectively, suggesting its potentia diagnosis value of PCa, though the relatively low sensitivity of mesothelin may be not sufficient to screen PCa. The SROC curve presents a global summary of test performance, and shows the trade-off between sensitivity and specificity. The DOR, the ratio of the odds of positivity in disease relative to the odds of positivity in the non-diseased, is a single indicator of diagnostic test performance [33] that combines the data from sensitivity and specificity into a single number. The value of a DOR ranges from 0 to infinity, with higher values indicating better discriminatory test performance (higher accuracy). A DOR of 1.0 indicates that a test cannot discriminate between patients with the disorder and those without it. In this meta-analysis, the maximum joint sensitivity and specificity (Q value) was 0.81 while the AUC was 0.88, and the pooled DOR was 33.93, suggesting a moderate diagnostic accuracy for diagnosing PCa. However, the SROC curve and the DOR are not easy to interpret and use in clinical practice, while the likelihood ratio (PLR and NLR) is more clinically meaningful for our measures of diagnostic accuracy. A PLR value of 8.53 suggests that patients with PCa have about 9-fold higher chance of being mesothelin-positive compared to non-PCa, and this was high enough for the clinical practice. On the other hand, the NLR was 0.36, which means that the probability of having PCa in mesothelin-negative patients is 36% in theory, which is not low enough to rule out PCa.
The results of the present meta-analysis suggest that mesothelin may, to a certain extent, play a role in the diagnosis of malignant effusions. However, no single biomarker is 100% perfect; therefore, different biomarkers should be investigated in various combinations, toselect an optimum panel for potential clinical application. Some biomarkers were proved to be useful in distinguishing PCa from other benign pancreatic diseases. For instance, Lok Tet al. have reported that S100 Pand MUC5AC were frequently expressed in pancreatic ductal adenocarcinomas, seen in 95% and 67% cases, respectively [34]. In addition, it has been reported that using a panel of KOC, S100P and mesothelin with at least 2 positive biomarkers achieved almost 100% sensitivity and specificity in detecting pancreatico-biliary adenocarcinomas [25]. Nevertheless, due to the varying degrees of diagnostic accuracy of identical markers reported between studies, it remains unclear which marker has a superior performance. Therefore, more immunomarkers should be comprehensively evaluated for their diagnostic accuracy and larger sample-size diagnostic tests are needed to find the optimum panel of antibodies for the diagnosis of malignant effusions [35].
This meta-analysis has limitations. First of all, we excluded conference abstracts and letters to the editor, which may have contributed to the observed publication bias. Secondly, the small sample-sized studies appeared to overestimate the true diagnostic accuracy of mesothelin for the diagnosis of PCa and might be vulnerable to selection bias. Third, the diagnosis of PCa was made by histological assessment (gold standard) insome studies, while other PCa patients were diagnosed on thebasis of clinical course. This issue of diagnostic accuracy may have caused non-random misclassification, leading to biased results. Also, because of a lack of required data reported in the original publications, it was not possible to analyze the effect of factors such as laboratory infrastructure, expertise with immunological technique, patient spectrum and setting on the accuracy of the mesothelin measurements. And for the same reason, we could not explore whether the study design, such as blinded, cross-sectional, consecutive/random and prospective design, affects the diagnostic accuracy, either. Therefore, further studies are still needed to evaluate the diagnostic accuracy of mesothelin in clinical applications.
Despite the above limitations, our meta-analysis used a statistical approach to combine the results of multiple studies. The data demonstrated that mesothelin may be a useful adjunct to conventional diagnostic tools for detecting PCa, while the results of immunostaining should be interpreted in parallel with the gold standard of morphology and clinical findings.
Acknowledgements
We thank all authors of primary studies included in our meta-analyses.
Disclosure of conflict of interest
None.
References
- 1.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Plate JM. Advances in therapeutic vaccines for pancreatic cancer. Discov Med. 2012;14:89–94. [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. Br Med J. 2012;344:e2476. doi: 10.1136/bmj.e2476. [DOI] [PubMed] [Google Scholar]
- 5.Miura F, Takada T, Amano H, Yoshida M, Furui S, Takeshita K. Diagnosis ofpancreatic cancer. HPB. 2006;8:337–342. doi: 10.1080/13651820500540949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabizzi E, Assef MS, Raimondo M. Diagnostic management of pancreatic cancer. Cancer. 2011;3:494–509. doi: 10.3390/cancers3010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A. Pancreatic headcancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30:488–500. doi: 10.1007/s00261-004-0279-z. [DOI] [PubMed] [Google Scholar]
- 8.Harewood GC, Wiersema LM, Halling AC, Keeney GL, Salamao DR, Wiersema MJ. Influence of EUS training and pathology interpretation on accuracyof EUS-guided fine needle aspiration of pancreatic masses. Gastrointest Endosc. 2002;55:669–673. doi: 10.1067/mge.2002.123419. [DOI] [PubMed] [Google Scholar]
- 9.Sopha SC, Gopal P, Merchant NB, Revetta FL, Gold DV, Washington K, Shi C. Diagnostic and therapeutic implications of a novel immunohistochemical panel detecting duodenal mucosa linvasion by pancreatic ductal adenocarcinoma. Int J Clin Exp Pathol. 2013;6:2476–86. [PMC free article] [PubMed] [Google Scholar]
- 10.Ordonez NG. Application of mesothelinimmunostaining in tumordiagnosis. Am J Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Frierson HF Jr, Moskaluk CA, Powell SM, Zhang H, Cerilli LA, Stoler MH, Cathro H, Hampton GM. Large-scale molecularand tissue microarray analysis of mesothelin expression incommon human carcinomas. Hum Pathol. 2003;34:605–609. doi: 10.1016/s0046-8177(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 12.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellström KE, Hellström I. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable insera from patients with ovarian carcinoma. Proc Natl Acad Sci U S A. 1999;96:11531–11536. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Mesothelin is overexpressedin the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serialanalysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 14.McCarthy DM, Maitra A, Argani P, Rader AE, Faigel DO, Van Heek NT, Hruban RH, Wilentz RE. Novel markers of pancreatic adenocarcinoma in fine-needle aspiration: mesothelin and prostate stem cell antigen labeling increases accuracy in cytologically borderline cases. Appl Immunohistochem Mol Morphol. 2003;11:238–43. doi: 10.1097/00129039-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Zhu QY, Li ZS, Pan X, Sun ZX. Expression and diagnostic value of mesothelin in specimen of pancreas fine-needle aspiration. Acta Acad Med Sin. 2007;27:615–618. [PubMed] [Google Scholar]
- 16.Chen ZR, Bao HM, Xu TF, Zhang CB. Expressionand clinical significance of mesothelin in pancreatic carcinoma. Labeled Immunoassays Clin Med. 2008;15:359–361. [Google Scholar]
- 17.Hornick JL, Lauwers GY, Odze RD. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol. 2005;29:381–9. doi: 10.1097/01.pas.0000149710.01559.fe. [DOI] [PubMed] [Google Scholar]
- 18.Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–45. [PubMed] [Google Scholar]
- 19.Jhala N, Jhala D, Vickers SM, Eltoum I, Batra SK, Manne U, Eloubeidi M, Jones JJ, Grizzle WE. Biomarkers in diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–9. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal B, Ludwig OJ, Collins BT, Cortese C. Immunostaining as an adjunct to cytology for diagnosis of pancreatic adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1425–31. doi: 10.1016/j.cgh.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Baruch AC, Wang H, Staerkel GA, Evans DB, Hwang RF, Krishnamurthy S. Immunocytochemical study of the expression of mesothelin in fine-needle aspiration biopsy specimens of pancreatic adenocarcinoma. Diagn Cytopathol. 2007;35:143–7. doi: 10.1002/dc.20594. [DOI] [PubMed] [Google Scholar]
- 22.Dim DC, Jiang F, Qiu Q, Li T, Darwin P, Rodgers WH, Peng HQ. The usefulness of S100P, mesothelin, fascin, prostate stem cell antigen, and 14-3-3 sigma in diagnosing pancreatic adenocarcinoma in cytological specimens obtained by endoscopic ultrasound guided fine-needle aspiration. Diagn Cytopathol. 2014;42:193–9. doi: 10.1002/dc.21684. [DOI] [PubMed] [Google Scholar]
- 23.Glass JP, Parasher G, Arias-Pulido H, Donohue R, Prossnitz ER, Cerilli LA. Mesothelin and GPR30 staining among a spectrum of pancreatic epithelial neoplasms. Int J Surg Pathol. 2011;19:588–96. doi: 10.1177/1066896911409575. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Shi J, Anandan V, Wang HL, Diehl D, Blansfield J, Gerhard G, Lin F. Reevaluation and identification of the best immunohistochemical panel (pVHL, Maspin, S100P, IMP-3) for ductal adenocarcinoma of the pancreas. Arch Pathol Lab Med. 2012;136:601–9. doi: 10.5858/arpa.2011-0326-OA. [DOI] [PubMed] [Google Scholar]
- 25.Ali A, Brown V, Denley S, Jamieson NB, Morton JP, Nixon C, Graham JS, Sansom OJ, Carter CR, McKay CJ, Duthie FR, Oien KA. Expression of KOC, S100P, mesothelin and MUC1 in pancreatico-biliary adenocarcinomas: development and utility of apotential diagnostic immunohistochemistry panel. BMC Clin Pathol. 2014;14:35. doi: 10.1186/1472-6890-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones CM, Ashrafian H, Skapinakis P, Arora S, Darzi A, Dimopoulos K, Athanasiou T. Diagnostic accuracy meta-analysis: a review of the basic principles of interpretation and application. Int J Cardiol. 2010;140:138–144. doi: 10.1016/j.ijcard.2009.05.063. [DOI] [PubMed] [Google Scholar]
- 28.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12:1293–1316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 29.Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI. Understanding heterogeneity in meta-analysis: the role of metaregression. Int J Clin Pract. 2009;63:1426–34. doi: 10.1111/j.1742-1241.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- 30.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–93. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Jiang J, Shi HZ, Liang QL, Qin SM, Qin XJ. Diagnostic value of interferon-gamma in tuberculous pleurisy: a metaanalysis. Chest. 2007;131:1133–41. doi: 10.1378/chest.06-2273. [DOI] [PubMed] [Google Scholar]
- 32.Niu Y, Huang T, Lian F, Li F. Contrast-enhanced ultrasonography for the diagnosis of small hepatocellular carcinoma: a meta-analysis and meta-regression analysis. Tumour Biol. 2013;34:3667–74. doi: 10.1007/s13277-013-0948-z. [DOI] [PubMed] [Google Scholar]
- 33.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 34.Lok T, Chen L, Lin F, Wang HL. Immunohistochemical distinction between intrahepatic cholangiocarcinoma andpancreaticductal adenocarcinoma. Hum Pathol. 2014;45:394–400. doi: 10.1016/j.humpath.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Li D, Wang B, Hu Q, Shen Y, Xu D, Wang T, Wen F. Diagnosticaccuracy of MOC-31 formalignanteffusions: ameta-analysis. Tumour Biol. 2014;35:6003–9. doi: 10.1007/s13277-014-1795-2. [DOI] [PubMed] [Google Scholar]


