Abstract
Background: Baicalin is one of flavonoid extracts from Scutellaria baicalensis, which has several functions including anti-inflammation, anti-bacteria, antitumor and et al. However, the mechanisms of anti-inflammatory of baicalin in ulcerative colitis is not clear. Methods: Mice colitis models were established by dextran sodium sulfate, Mice administrated with baicalin (100 mg/kg) and mesalazine (100 mg/kg) twice daily by intragastric injection for 7 days after colitis induced were defined as treated group. Then the mice were sacrificed and the colon samples were collected. Toll-like receptor-2, 4, 9 were detected by immunohistochemistry. Signaling proteins such as TLR4, MyD88, and NF-κB p65 were analyzed by western blotting. Cytokine’s mRNA include TNF-α, IL-6 IL-10 and IL-13 were measured by reverse transcription polymerase chain reaction. Modified disease activity index were used to analyse the severity of the disease by assessed of diarrhea, stool (occult) blood and body weight loss of the mice. Results: Compared with control and model groups, modified disease activity index in baicalin and mesalazine treated, mice decreased gradually. Immunohistochemistry analysis showed the expression of TLR4, but not TLR2 and TLR9, in the mucosa of mice colon were decreased. Western blot analysis showed that in colitis model, the expression of NF-κB p65 and TLR4 decreased (P < 0.05), while the expression of MyD88 increased significantly compared to control group, and MyD88 expression can not be repressed by baicalin (P < 0.05). Baicalin and mesalazine treatment suppressed the expression of TNF-α, IL-6 and IL-13 mRNA (P < 0.05), yet up-regulated the expression of IL-10 mRNA (P < 0.05), compared to the DDS and control groups. Conclusions: Baicalin administration by intragastric injection ameliorates the severity of colon inflammation. The possible mechanism of anti-inflammatory response by baicalin may involve in the blocking of the TLR4/NF-κB-p65/IL-6 signaling pathway.
Keywords: Baicalin, ulcerative colitis, NF-κB, TLR, MyD88
Introduction
Ulcerative colitis (UC), as well as Crohn’s Disease, is one of inflammatory bowel disease (IBD) which pathogenesis is not clear. One of the potential pathogenesis may be associated with excessive immune responses against intestinal bacteria and lead to the damage of intestinal epithelial barrier via abnormal activity of some pro-inflammatory signals. Which mainly including P38/MAPK, JNK/MAPK, PI3K/Akt, and NF-κB signaling pathways [1]. Recently, an inflammation and cancer related signal transduction pathway, MyD88/NF-κB, raised the attention of the researchers, Which is triggered by pattern recognition receptors (PRRs), Toll-like receptors (TLRs) via interacting with the antigen from intestinal flora. Studies have identified the immune response that induced by TLRs mediated MyD88/NF-κB signal are exist in many conditions such as experimental cerebral ischemia [2], inflammation and carcinoma [3,4], allergy [1,5] and so on. UC is one of autoimmune tendency condition with a high risk of developing colorectal cancer [6] or other hematologic tumor [7]. Recent studies have showed that, TLRs/MyD88/NF-κB signal-transducing pathway plays an important part in UC [1,8,9].
Traditional drug for UC such as 5-aminosalicylates (5-ASA) and corticosteroids are wildly used, and some immunomodulators are used in refractory or steroid dependent UC patients. In addition, biological agents have a good effect in moderate to severe UC. Although many drugs have been used for UC treatment, the adverse effects of first line drugs will limit the use.
Baicalin (5,6-dihydroxy-4-oxygen-2-phenyl-4H-1-benzopyran-7-beta-D- glucopyranose acid, BCLN), one of flavonoid extracts from Scutellaria baicalensis, a well-known herbal medicine wildly used in China, have several biological activitys including anti-inflammatory [10,11], antimicrobial [12], antitumor [13,14]. Although, the anti-inflammatory mechanism of baicalin in the intestinal tract is not so clear, the decoction of Scutellaria baicalensis, with a large number of flavonoids, is wildly used in China and East Asia [15]. Recent years, the efficacy of flavonoid extractions is wildly studied [16,17], and it was found that, the extraction of Scutellaria inhibit the activation of NF-kB and then block inflammatory response [16,18,19], but which are seldom studied in IBD. Base on these theories mentioned above, we suppose that baicalin may inhibit the activation of NF-kB and ultimately be benefic for the treatment of UC.
In order to explore the anti-inflammatory mechanism of baicalin in UC, we built a mice colitis model by administrating dextran sulfate sodium (DSS), and explored the expression of PRR molecular (TLR2, 4, 9) in the intestinal mucosa, the expression of signal transduction proteins (TLR4, MyD88, NF-κB p65) in mice colonic epithelial cells, and the expression of mRNA of cytokines (TNF-α, IL-6, IL-10 and IL-13 mRNA) in the colon tissue. We expected to clarify whether TLRs/MyD88/NF-κB signaling pathway were influenced by baicalin in UC model and provide experimental basis for the use of baicalin as a new drug candidate for UC treatment.
Materials and methods
Animals
Thirty two female C57BL/6 (B6) mice, 6-7 weeks old, weighting 17-21 g, were purchased from Medical Experimental Animal Center of Guangdong province (SCXK2008-0002). This study was approved by the Ethics Committee of Guangdong Medical College.
Colitis induction
All mice were randomly divided into four groups, A: Control group, B: model group (DSS induced colitis without treatment, DSS group), C: baicalin treated group (DSS+BCLN group), and, D: mesalazine treated group (DSS+MSLZ group). For group B, C and D, mice were fed with 2.5% (w/v) DSS (36 to 50 kDa, Lot number: 160110, MP Biomedicals, USA) solution in purified water for 7 days as Wirtz’s described [20].
Drug administration
One percent (w/v) baicalin (Nanjing Zelang Medical Technology Co., Ltd, China) at a dose of 100 mg/kg, or 1% (w/v) mesalazine (Ethypharm Industries Co., Ltd, France) at a dose of 100 mg/kg was administrated to mice in group C and D twice daily for another 7 days by intragastric injection. Mice in group B were fed normally from day 8 to day 14, and mice in group A were fed normally for 14 days.
Modified disease activity index (mDAI)
Body weight and hematochezia or fecal occult blood of mice in all groups was examined daily. Weight loss and hematochezia of mice were scored based on modified disease activity index (mDAI), as described previously [21]. The mDAI follow the scoring system as below: body weight: 0: < 5% weight loss; 1: 5 to 10% weight loss; 2: 10 to 15% weight loss; 3: 15 to 20% weight loss; and 4: > 20% weight loss. A fecal occult blood test (Nanjing Jiancheng Bioengineering Institute, China, C027) were used to screen for occult blood in the stool and scored as follows: 0: no blood; 2: positive; and 4: gross blood. Grade 1 and 3 do not exist in this scale.
Samples preparation
Mice were sacrificed on day 15, colon samples were collected from mice instantly. Part of colon tissue were fixed in 10% formalin, treated with conventional dehydration, embedded in paraffin, sliced into sections, which were used for hematoxylin and eosin (H&E), immunohistochemical staining. Others parts were frozen at -80°C for RNA and protein extraction.
Immunohistochemistry (IHC) analysis
Four μm-thick tissue sections were dewaxed, epitope retrieval was performed using citrate buffer (pH=6), and then the activity of endogenous peroxidase were blocked by incubated with H2O2 solution for 10 minutes at room temperature. After epitope retrieval, tissue sections were incubated with rabbit purified anti-mouse TLR2 (BioLegend), rabbit anti-mouse-TLR4 (Abcam) and rabbit anti-mouse-TLR9 antibody (Abcam) respectively for 30 minutes at room temperature, washed and incubated with secondary peroxidase anti-rabbit IgG at 1:500 dilution for another 30 minutes at room temperature. After that, DAB reagents were used for chromogenic reaction. Counterstaining was performed with hematoxylin, dehydrated, cleaned in xylene, and mounted on coated slides. The results of IHC were analyzed by Image-Pro Plus (IPP 6.0 Media Cybernetics, US) software.
Western blot (WB) analysis
Colon tissues were lysed with lysis buffer (20 mM Tris-HCl, pH 7.4, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 1 mM PMSF, 10 nM phosphatase inhibitor microcystin). Protein were separated on 10% SDS-PAGE and then transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with 2% BSA in TBST (0.05% Tween 20 in Tris-buffered saline) for 1 hour at room temperature, the membranes were separately incubated with anti-mouse CD282 (TLR2) (BioLegend), anti-MyD88 (Biovision), anti-NFκBp65(ARP) and anti-β-actin (Santa Cruz, US) antibodies at a 1:1000 dilution in TBST for 1 hour at room temperature. Then membranes were incubated with HRP-conjugated secondary antibodies (Santa Cruz, US) at a 1:10,000 dilution for 1 hr at room temperature before visualizing by using ECL detection reagents.
Reverse transcription polymerase chain reaction (RT-PCR) analysis
The RNA in colon tissue was extracted by using Trizol reagent (Life Technologies, Copenhagen, Denmark) and chloroform following the manufacturer’s instructions in the RT-PCR kit. To analyze the expression of TNF-α, IL-6, IL-10 and IL-13 mRNA, the oligonucleotide primers (Sangon, China) designed for TNF-α were 5’-AGCACAGAAAGCATGATCCG-3’ (forward) and 5’-CTGATGAGAGGGA GGCCATT-3’ (reverse), for IL-13 were 5’-TCTTGCTTGCCTTGG TGGTCTCGC-3’ (forward) and 5’-GATGGCATTGCAATTGGAGATGTTG-3 (reverse), for IL-6 were 5’-GAGGATACCACTCCCAACAGACC-3’ (forward) and 5’-AAGTGCATCATCGTTGTTCATACA-3’ (reverse), for IL-10 were 5’-ATGCTGCCTGCTCTTACT GACTG-3’ (forward) and 5’-CCCAAGTAACCCTTAAAGTCCTGC-3’ (reverse). The oligonucleotide primers for β-actin used as a house-keeping gene were 5’-ATCACTATTGGCAACGAGCG-3’ (forward) and 5’-TCAGCAATGCCTGGGT ACAT-3’ (reverse). Image-Pro Plus 6.0 (IPP, Media Cybernetics, US) was used to analyze relative concentration values of mRNA expression.
Statistical analysis
All the data were expressed as mean ± standard deviation. SPSS 19.0 software was used for one-way ANOVA and variance analyses of repeated measurement data analysis. A P-value of less than 0.05 was considered significant.
Result
Protective effects of baicalin in colitis induced by DSS
mDAI scores increased in colitis model group (group B, C, D) significantly (Figure 1A, 1B; Tables 1, 2) and baicalin and mesalazine treatment markedly suppressed the mDAI scores (Figure 1C). In addition, baicalin and mesala zine dramatically improved the inflammatory lesions in colon tissue by the histologic analysis (Figure 2B-D).
Figure 1.
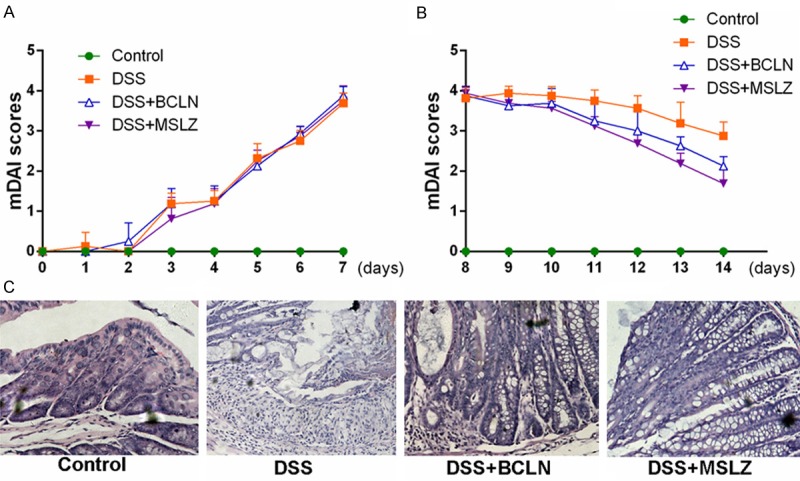
Protective effects of baicalin and mesalazine in mice with DSS induced colitis. A. Modified disease activity index after DSS administration during colitis induction experiment. B. Modified disease activity index of colitis during drugs intervention. C. Histological presence of colon mucosa of all 4 groups (H&E-staining).
Table 1.
mDAI score of mice in each group during the period of modeling
| Groups | mDAI score | Sum | F | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| day 1 | day 2 | day 3 | day 4 | day 5 | day 6 | day 7 | |||||
| Control (n=8) | x̅ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | — | 0.000 |
| s | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||
| DSS (n=8) | x̅ | 0.125 | 0.000 | 1.188 | 1.250 | 2.313 | 2.750 | 3.688 | 1.616 | 204.465 | 0.000 |
| s | 0.354 | 0.000 | 0.259 | 0.267 | 0.372 | 0.267 | 0.259 | 0.254 | |||
| DSS+BCLN (n=8) | x̅ | 0.000 | 0.250 | 1.188 | 1.250 | 2.125 | 2.938 | 3.875 | 1.661 | 171.428 | 0.000 |
| s | 0.000 | 0.463 | 0.372 | 0.378 | 0.231 | 0.177 | 0.231 | 0.265 | |||
| DSS+MSLZ (n=8) | x̅ | 0.000 | 0.000 | 0.813 | 1.188 | 2.250 | 2.875 | 3.750 | 1.554 | 187.204 | 0.000 |
| s | 0.000 | 0.000 | 0.530 | 0.372 | 0.267 | 0.231 | 0.378 | 0.254 | |||
| Sum | x̅ | 0.031 | 0.063 | 0.797 | 0.922 | 1.672 | 2.141 | 2.828 | 1.208 | 556.751* | 0.000* |
| s | 0.177 | 0.246 | 0.594 | 0.611 | 1.013 | 1.271 | 1.678 | 0.799 | |||
| F | 1.000 | 2.333 | 20.615 | 34.350 | 151.667 | 418.333 | 432.616 | 439.921* | (F=62.994, P=0.000)# | ||
| P | 0.407 | 0.096 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000* | |||
F statistic and P value of main effect;
F statistic and P value of crossover effect.
Table 2.
mDAI score of mice in each group during the period of treatment
| Groups | mDAI score | Sum | F | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| day 8 | day 9 | day 10 | day 11 | day 12 | day 13 | day 14 | |||||
| Control (n=8) | x̅ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | — | 0.000 |
| s | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||
| DSS (n=8) | x̅ | 3.813 | 3.938 | 3.875 | 3.750 | 3.563 | 3.188 | 2.875 | 3.571 | 17.398 | 0.000 |
| s | 0.259 | 0.177 | 0.231 | 0.267 | 0.320 | 0.530 | 0.354 | 0.306 | |||
| DSS+BCLN (n=8) | x̅ | 3.875 | 3.625 | 3.688 | 3.250 | 3.000 | 2.625 | 2.125 | 3.170 | 25.516 | 0.000 |
| s | 0.231 | 0.354 | 0.372 | 0.463 | 0.535 | 0.231 | 0.231 | 0.345 | |||
| DSS+MSLZ (n=8) | x̅ | 3.938 | 3.438 | 3.188 | 2.688 | 2.063 | 1.625 | 1.250 | 2.598 | 102.365 | 0.000 |
| s | 0.177 | 0.563 | 0.530 | 0.259 | 0.496 | 0.443 | 0.535 | 0.429 | |||
| Sum | x̅ | 2.906 | 2.750 | 2.688 | 2.422 | 2.156 | 1.859 | 1.563 | 2.335 | 108.323* | 0.000* |
| s | 1.715 | 1.656 | 1.630 | 1.498 | 1.428 | 1.278 | 1.134 | 1.477 | |||
| F | 791.961 | 506.613 | 495.227 | 275.456 | 176.235 | 154.933 | 149.695 | 1004.906* | (F=16.823, P=0.000)# | ||
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000* | |||
F statistic and P value of main effect;
F statistic and P value of crossover effect.
Figure 2.
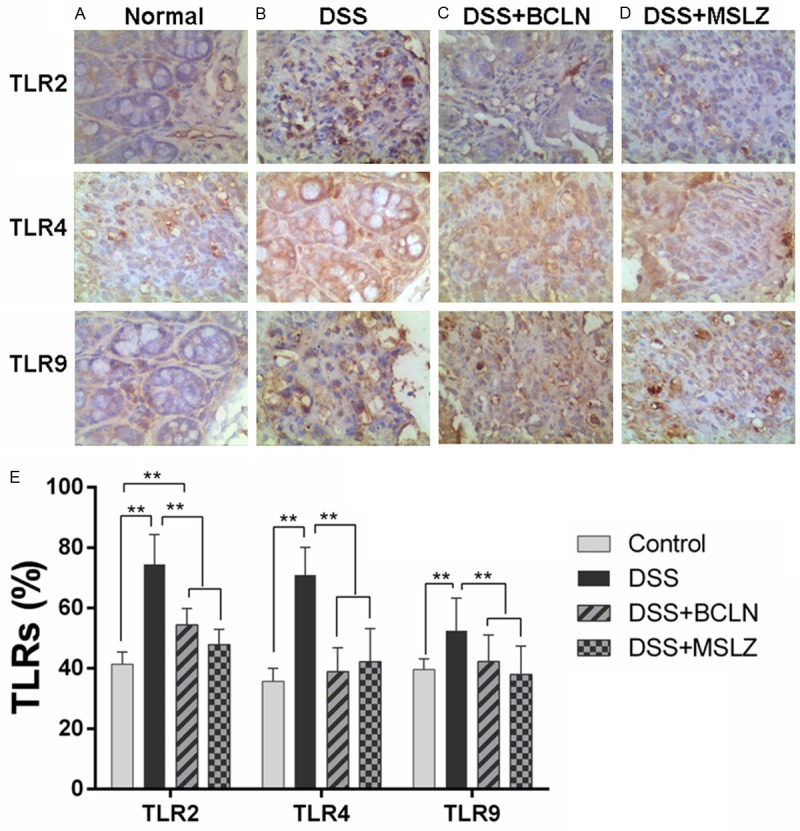
TLR2, 4, 9 expression on colon mucosa. A-D. The immunohistochemical analysis of TLR2, 4, 9 expressed on colon tissue; E. The calculation of TLR2, 4, 9 positive cells was performed by IPP software. Values are shown as mean ± SEM (*P < 0.05, **P < 0.01).
Baicalin treatment suppressed TLR4 expression in colon mucosa
Results of IHC in colon tissues (Figure 2A) showed the expression of TLR2, 4, 9 was increased in mice with colitis (Figure 2B-D) and The majority of the TLR2 and TLR9 positive cells were lamina propria mononuclear cells and the expression of TLR2, TLR9 were located on cytomembrance (Figure 2A). The expression of TLR4 decreased dramatically after baicalin and mesalazine treatment. The expression of TLR9 decreased by mesalazine treatment. However, the expression of TLR2 remained unchange after both drugs treatment.
Baicalin and mesalazine treatment decreased NF-κB p65 expression in colon tissue
WB analysis revealed that NF-κB p65 and MyD88 expression in colon tissue decreased by baicalin or mesalazine treatment. Whereas the decreased expression of MyD88 in baicalin and mesalazine treated group did not reach statistical levels (P > 0.05, Figure 3).
Figure 3.
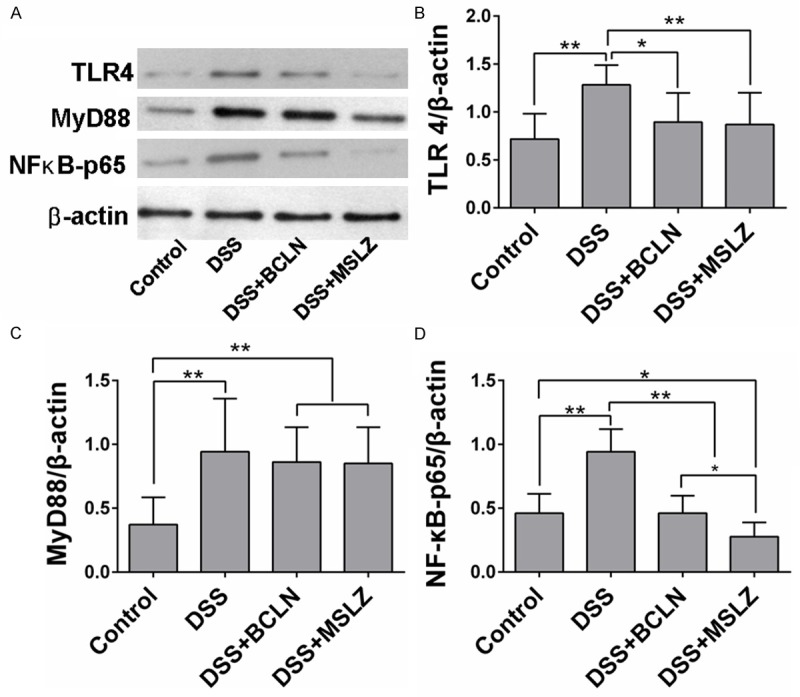
TLR4, MyD88, NF-κB p65 Expression in colon tissue. A. Western blot analysis of TLR4, MyD88, NF-κB p65 and β-actin. B-D. Relative concentration of TLR4, MyD88, NF-κB p65 were analyzed by IPP software. Values are shown as mean ± SEM (*P < 0.05, **P < 0.01).
Baicalin and mesalazine treatment increased IL-10 mRNA but decreased TNF-α, IL-6 and IL-13 mRNA expressions
The mRNA of cytokines in mice colon tissues were detected by RT-PCR analysis. DSS substantially increased the TNF-α, IL-6, IL-10 and IL-13 mRNA expressed in colon tissues. On the contrary, baicalin and mesalazine treatment suppressed TNF-α, IL-6 and IL-13 mRNA expressions while elevated IL-10 mRNA expression (P < 0.05, Figure 4).
Figure 4.
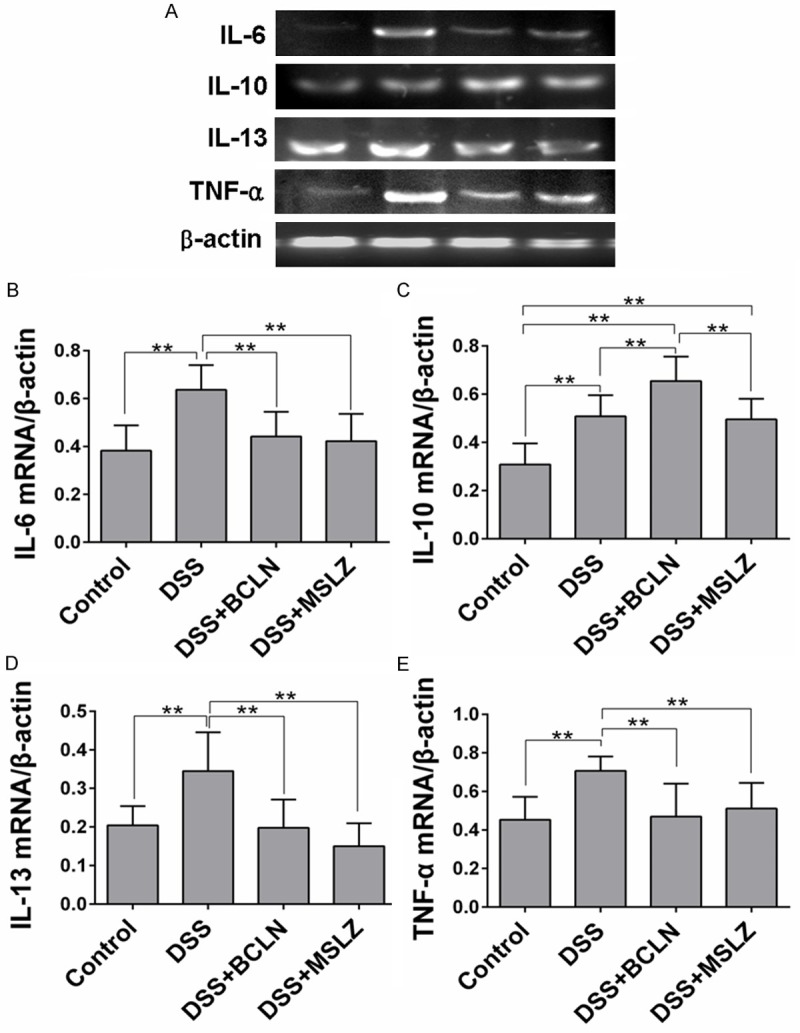
TNF-α, IL-6, IL-10 and IL-13 mRNA expression in colon tissue. A. TNF-α, IL-6 IL-10, IL-13 and mRNA expression in colon tissue were detected by RT-PCR analysis. B-E. Relative concentration of IL-6, IL-10, IL-13 and TNF-α mRNA were analyzed by IPP software. Values are shown as mean ± SEM (*P < 0.05, **P < 0.01).
Discussion
NF-κB is a critical signaling molecule in inflammatory process, which facilitates the expression and secretion of pro-inflammatory cytokines, and then lead to a series of inflammatory responses and mucosal damage. It have been identified that, inhibiting the activation of NF-κB by blocking the MyD88 signal (an upstream signal molecular of NF-κB signal pathway) will reduce the release of proinflammatory cytokines, alleviate the inflammatory response and achieve a therapeutic effect [4]. Previous studies have demonstrated the important role of TLRs/MyD88/NF-κB signaling pathway in different conditions [2,22-24].
Our current study show that, mice induced by DSS, developed a series of inflammatory responses in the intestinal mucosa, the expression of PRRs such as TLR2, TLR4 and TLR9 were significantly increased, the expression of signal-transducing protein assayed by WB, TLR4 and NF-κB p65 were significantly increased, and the clinical parameters such as weight loss, hematochezia or fecal occult blood were increased accordingly. In addition, the mDAI were significantly increased. The result of histological assessment improved significantly.
After baicalin and mesalazine treatment, the symptoms of weight loss and fecal occult blood of mice were significantly ameliorated. The histological change of the intestinal mucosa were close to normal, expression of NF-κB p65, TLR4 and expression of TNF-α, IL-6 and IL-13 mRNA were significantly elevated, accompany with an increasing levels of IL-10 mRNA. These results indicated that, TLR4/NF-κB signal was activated in the colon mucosa of colitis induced by DSS. Baicalin inhibited the signal of NF-κB and then suppress the production of proinflammatory cytokines such as TNF-α, IL-6 and IL-13.
In order to compare the protective effects of baicalin with traditional drug used for UC, we selected a first line drug, mesalazine, as a control drug, which was well known for anti-inflammatory effect with a mechanism of blocking NF-κB signaling in UC patients [25]. In our study, baicalin showed a similar effect of anti-inflammatory presenting by immunological parameters in DSS+BCLN group which is closed to DSS+MSLZ group.
Considering the imbalance of pro-inflammatory and anti-inflammatory cytokines in extracellular under inflammatory condition, RT-PCR was performed to analyze the mRNA of intracellular cytokines in colon tissues. Our data suggested baicalin were able to modulate the mRNA expression of pro-inflammatory (TNF-α, IL-6 and IL-13) and anti-inflammatory (IL-10) cytokines in colon tissues, while mesalazine can only modulate the mRNA expression of pro-inflammatory (TNF-α, IL-6 and IL-13). It is well known that NF-κB or MyD88 is a crucial factor involved in the regulation of inflammation [26], therefore, we focused on the relationship between baicalin and the activity of NF-κB or MyD88. Whether baicalin induces anti-inflammatory cytokines via MyD88 is not yet known.
Interestingly, one of the signal-transduction proteins, MyD88, increased in DSS+BCLN and DSS+MSLZ group in our study. This change is not consistent with NF-κB p65 and TLR4 (Figure 4B-D). To our knowledge, signals through TLRs 1/2/6, 5, 7-9 are mediated exclusively by MyD88 and led to activation of NF-KB or AP-1 [27]. Our data might suggest that, baicalin might exert regulatory function via MyD88 signal, including increasing the expression of IL-10 mRNA, which needs further study.
TLRs mainly expressed on antigen-presenting cells which are important in the modulation of host immune responses. To date, 10 and 12 functional TLRs have been identified in humans and mice, respectively [28]. TLRs recognize distinct pathogen-associated molecular patterns derived from bacteria, viruses, fungi, and parasites [29]. TLR2 recognizes lipopeptides from bacteria, while TLR4 recognizes lipopolysaccharide (LPS), and TLR9 recognizes ssRNA and CpG DNA. Research indicates that TLR2 may direct tolerogenic responses, induce an increase in anti-inflammatory cytokines, such as IL-10 production [30].
The role of mediating protective effects of baicalin via TLRs signals in DSS-induced colitis was also investigated in this study. We found that, baicalin down regulated the expression of TLR4, but not TLR2 and TLR9. The level of IL-10 mRNA were elevated, which indicated baicalin might up regulated IL-10 through intervening TLR2 or TLR9 signals or other pathway. It’s reported that, IL-10 was induced via TLR/MyD88/NF-κB signaling pathway in some regulatory lymphocytes such as regulatory B cells. Whether baicalin increased IL-10 mRNA via TLR4/MyD88 pathway needs further investigate.
Our study has demonstrated the efficacy of baicalin in UC via relieving the clinical symptoms, reducing the mDAI and histological scores, decreasing the expression of TLR4 and NF-kB p65 and also the expression of TNF-α, IL-6 and IL-13 mRNA which are mediated by NF-κB signal, but increasing the expression of IL-10 mRNA. All these results indicate the inflammatory response may be alleviated by baicalin via blocking TLR4/NF-κB signal transduction pathway. Therefore, TLR4/NF-κB signaling pathway is expected to become a new target for the treatment of UC. Baicalin might contribute to the development of new pharmaceutical products for UC treatment.
Acknowledgements
This study was supported by Medical Research Foundation of Guangdong Province (B2014294), Scientific Funds of Guangdong Medical College (M2013054) and Special Funds for discipline construction from Ministry of Education of Guangdong province (JB1211).
References
- 1.Wei J, Feng J. Signaling pathways associated with inflammatory bowel disease. Recent Pat Inflamm Allergy Drug Discov. 2010;4:105–17. doi: 10.2174/187221310791163071. [DOI] [PubMed] [Google Scholar]
- 2.Ma Y, He M, Qiang L. Exercise Therapy Downregulates the Overexpression of TLR4, TLR2, MyD88 and NF-kappaB after Cerebral Ischemia in Rats. Int J Mol Sci. 2013;14:3718–33. doi: 10.3390/ijms14023718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guven Maiorov E, Keskin O, Gursoy A, Nussinov R. The structural network of inflammation and cancer: Merits and challenges. Semin Cancer Biol. 2013;23:243–51. doi: 10.1016/j.semcancer.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Liang B, Chen R, Wang T, Cao L, Liu Y, Yin F, Zhu M, Fan X, Liang Y, Zhang L, Guo Y, Zhao J. Myeloid differentiation factor 88 promotes growth and metastasis of human hepatocellular carcinoma. Clin Cancer Res. 2013;19:2905–16. doi: 10.1158/1078-0432.CCR-12-1245. [DOI] [PubMed] [Google Scholar]
- 5.Mastrandrea F, Semeraro FP, Coradduzza G, Manelli M, Scarcia G, Pezzuto F, Serio G. CD34+ hemopoietic precursor and stem cells traffic in peripheral blood of celiac patients is significantly increased but not directly related to epithelial damage severity. Eur Ann Allergy Clin Immunol. 2008;40:90–103. [PubMed] [Google Scholar]
- 6.Beaugerie L, Svrcek M, Seksik P, Bouvier AM, Simon T, Allez M, Brixi H, Gornet JM, Altwegg R, Beau P, Duclos B, Bourreille A, Faivre J, Peyrin-Biroulet L, Fléjou JF, Carrat F. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166–75. e8. doi: 10.1053/j.gastro.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 7.Claessen MM, Siersema PD, Vleggaar FP. IBD-related carcinoma and lymphoma. Best Pract Res Clin Gastroenterol. 2011;25(Suppl 1):S27–S38. doi: 10.1016/S1521-6918(11)70007-5. [DOI] [PubMed] [Google Scholar]
- 8.Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong L, Li J, Liu Y, Yue W, Luo X. Toll-like receptor 2 monoclonal antibody or/and Toll-like receptor 4 monoclonal antibody increase counts of Lactobacilli and Bifidobacteria in dextran sulfate sodium-induced colitis in mice. J Gastroenterol Hepatol. 2012;27:110–9. doi: 10.1111/j.1440-1746.2011.06839.x. [DOI] [PubMed] [Google Scholar]
- 10.Dou W, Mukherjee S, Li H, Venkatesh M, Wang H, Kortagere S, Peleg A, Chilimuri SS, Wang ZT, Feng Y, Fearon ER, Mani S. Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PLoS One. 2012;7:e36075. doi: 10.1371/journal.pone.0036075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002;68:268–71. doi: 10.1055/s-2002-23143. [DOI] [PubMed] [Google Scholar]
- 12.Brackman G, Cos P, Maes L, Nelis HJ, Coenye T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother. 2011;55:2655–61. doi: 10.1128/AAC.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Y, Shuai L, Chen S, Huang L, Qin S, Yang Z. Flavonoids and antioxidative enzymes in temperature-challenged roots of Scutellaria baicalensis Georgi. Z Naturforsch C. 2012;67:77–85. doi: 10.1515/znc-2012-1-210. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Polednik C, Gruensfelder P, Roller J, Hagen R. The effects of PC-Spes on chemosensitive and chemoresistant head and neck cancer cells and primary mucosal keratinocytes. Oncol Rep. 2009;21:1297–305. doi: 10.3892/or_00000354. [DOI] [PubMed] [Google Scholar]
- 15.Lam W, Bussom S, Guan F, Jiang Z, Zhang W, Gullen EA, Liu SH, Cheng YC. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med. 2010;2:45ra59. doi: 10.1126/scitranslmed.3001270. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Hou J, Fu J, Li D, Zhang C, Liu J. Baicalin protects rat brain microvascular endothelial cells injured by oxygen-glucose deprivation via anti-inflammation. Brain Res Bull. 2013;97:8–15. doi: 10.1016/j.brainresbull.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Lixuan Z, Jingcheng D, Wenqin Y, Jianhua H, Baojun L, Xiaotao F. Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models. Pulm Pharmacol Ther. 2010;23:411–9. doi: 10.1016/j.pupt.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Xie C, Kang J, Li Z, Schauss AG, Badger TM, Nagarajan S, Wu T, Wu X. The acai flavonoid velutin is a potent anti-inflammatory agent: blockade of LPS-mediated TNF-alpha and IL-6 production through inhibiting NF-kappaB activation and MAPK pathway. J Nutr Biochem. 2012;23:1184–91. doi: 10.1016/j.jnutbio.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Funakoshi-Tago M, Nakamura K, Tago K, Mashino T, Kasahara T. Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int Immunopharmacol. 2011;11:1150–9. doi: 10.1016/j.intimp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–6. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 21.Sina C, Arlt A, Gavrilova O, Midtling E, Kruse ML, Muerkoster SS, Kumar R, Folsch UR, Schreiber S, Rosenstiel P, Schafer H. Ablation of gly96/immediate early gene-X1 (gly96/iex-1) aggravates DSS-induced colitis in mice: role for gly96/iex-1 in the regulation of NF-kappaB. Inflamm Bowel Dis. 2010;16:320–31. doi: 10.1002/ibd.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu QF, Yang Z, Wang SR, Wang X, Hao SL. Bi-directional interaction of NF-κB in nervous system damage, learning and memory. Neurology, Psychiatry and Brain Research. 2012;18:186–90. [Google Scholar]
- 23.Mccall MB, Netea MG, Hermsen CC, Jansen T, Jacobs L, Golenbock D, Van Der Ven AJ, Sauerwein RW. Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J Immunol. 2007;179:162–71. doi: 10.4049/jimmunol.179.1.162. [DOI] [PubMed] [Google Scholar]
- 24.Sun Q, Dai Y, Zhang X, Hu YC, Zhang D, Li W, Zhang XS, Zhu JH, Zhou ML, Hang CH. Expression and cell distribution of myeloid differentiation primary response protein 88 in the cerebral cortex following experimental subarachnoid hemorrhage in rats: A pilot study. Brain Res. 2013;1520:134–44. doi: 10.1016/j.brainres.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Bantel H, Berg C, Vieth M, Stolte M, Kruis W, Schulze-Osthoff K. Mesalazine inhibits activation of transcription factor NF-kappaB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol. 2000;95:3452–7. doi: 10.1111/j.1572-0241.2000.03360.x. [DOI] [PubMed] [Google Scholar]
- 26.Napetschnig J, Wu H. Molecular basis of NF-kappaB signaling. Ann Rev Biophys. 2013;42:443–68. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frederiksen RF, Jakobsen H, Nissen IF, Rohde A, Sebamalairashan D. Rationale, efficacy and safety of probiotics in the treatment of Inflammatory Bowel Disease. 2007 [Google Scholar]
- 28.Kamdar K, Nguyen V, Depaolo RW. Toll-like receptor signaling and regulation of intestinal immunity. Virulence. 2013;4:207–12. doi: 10.4161/viru.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahla RS, Reddy MC, Prasad DV, Kumar H. Sweeten PAMPs: Role of Sugar Complexed PAMPs in Innate Immunity and Vaccine Biology. Front Immunol. 2013;4:248. doi: 10.3389/fimmu.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borrello S, Nicolo C, Delogu G, Pandolfi F, Ria F. TLR2: a crossroads between infections and autoimmunity? Int J Immunopathol Pharmacol. 2011;24:549–56. doi: 10.1177/039463201102400301. [DOI] [PubMed] [Google Scholar]


