Abstract
Hyperbaric oxygen therapy (HBOT) protects brain tissue from inflammatory injury by suppressing mitochondrial apoptotic pathways. However, its neuroprotective mechanism via anti-apoptosis in spinal cord injury (SCI) is still unclear. In our study, Male Sprague-Dawley rats were randomly divided into three groups: sham-operated (SH), SCI model, and SCI + HBOT. Rats in each group were randomly divided into four sub-groups in a time-dependent manner (1 day, 3 days, 7 days and 14 days after surgery). Expression of adaptor molecule apoptosis-associated speck-like protein (ASC) and caspase-3 was evaluated at the indicated time after injury. Our data showed that HBOT downregulated expression of ASC in SCI rats at the mRNA and protein levels. HBOT mitigated caspase-3 release in injured spinal cord tissue. We conclude that HBOT prevents inflammation apoptosis after SCI, likely through suppression of ASC and caspase-3.
Keywords: Hyperbaric oxygen therapy, spinal cord injury, apoptosis-associated speck-like protein, caspase-3
Introduction
Spinal cord injury (SCI) is a devastating disease that results in permanent neurological deficits, bringing a heavy burden to patients and their families [1-3]. The pathophysiological process of SCI includes primary injury that damages cord integrity and secondary injury which causes vascular abnormalities, excitotoxicity, oxidative stress, and cell death [4]. Cell death includes necrosis and apoptosis. Increasing evidence demonstrates the presence of apoptosis at the stage of secondary injury. Apoptosis occurs mainly in neurons and oligodendrocytes, and apoptosis of oligodendrocytes contributes to post-injury demyelination [5,6].
Many treatment strategies have been focused on the second phase of SCI, but few of these are effective. Hyperbaric oxygen therapy (HBOT) involves administering 100% oxygen which higher than atmospheric pressure at sea level for a prescribed amount of time. Some researchers have confirmed that HBOT has a protective effect by decreasing apoptosis of spinal cord motor neurons after SCI [7,8]. However, the molecular mechanism of the antiapoptotic effects after SCI is largely unknown. Adaptor molecule apoptosis-associated speck-like protein (ASC) is a proapoptotic protein originally found in apoptotic cells with a “speck” that is important in regulating development of inflammation and apoptosis through activation of different caspases [9]. Caspase-3 is so called “executioner” which initiates process of apoptosis. Much attention has been given to searching for inhibitors of caspase-3 but a few effective agents have been reported [10,11].
Here, we investigated the effects of HBOT on the expression of ASC and caspase-3 after SCI. We aimed to determine the molecular mechanism of ASC-induced apoptosis and the antiapoptotic effect of HBOT. This study implies that HBOT plays an important role in the antiapoptotic and myelin-preserving effects in SCI.
Materials and methods
Animals
Healthy male Sprague-Dawley rats (aged 2-3 months, weighing 250-300 g) were kept two per cage for at least 5 days after their arrival at our laboratory. The rats had access to food and water ad libitum and were housed within a room with a 12:12 h light/dark cycle. This study was performed in accordance with the ethical guidelines laid down by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Capital Medical University (Beijing, China).
SCI model
SCI was induced aseptically under anesthesia using intraperitoneal injection of 10% chloral hydrate at a dose of 350 mg/kg. Using the method described by Basso et al. [12], the rats were positioned prone on the operating table, the T10 spinous process area was sterilized, laminectomy was conducted to expose the spinal cord. SCI model was performe by the Multicenter Animal Spinal Cord Injury Study (MASCIS) impactor. Moderate SCI was induced by dropping a 10 g rod from a distance of 25 mm. The characteristics of a successful model include: the wagging tail reflex, retraction of the lower limbs, and flaccid paralysis of both lower extremities. Animals were allowed to recover from anesthesia and surgical procedures in an intensive care facility. Postoperatively, the bladder was compressed by manual abdominal pressure twice daily until the bladder reflex was restored. Penicillin G sodium was administered for 3 days.
Experimental groups
Seventy-two rats were randomly divided into three groups (each group n = 24): sham-operated (SH), SCI, and SCI and HBOT (SCI + HBO). Rats in each group were randomly divided into four sub-groups in a time-dependent manner (1 day, 3 days, 7 days, 14 days after surgery, each group n = 6). The rats in the SH group were subjected only to laminectomy, without SCI or HBOT.
HBOT
For groups of SCI + HBO, rats were placed in a custom-made pressure chamber of transpar ent acrylic plastic (701 Space Research Institute, Beijing, China) immediately after surgery and received 1 h HBOT at 2.0 ATA twice daily (8 h intervals) for the first 3 days and then daily for the following days. The air compression process was performed at a rate ascending of 1 kg/cm2/min to 2.0ATA/100% oxygen and maintained for 1 h. The chamber was ventilated with 100% O2 at a rate of 8 L/min. During HBO exposure, oxygen concentration was continuously monitored and maintained at ≥ 95%. To minimize the effects of diurnal variation, all HBOT was started at 08:00 and 16:00 h. The rats in the SH and SCI groups were exposed to normobaric air at 1.0 ATA for 1 h.
Evaluation of motor function and sample collection
Recovery of motor function was evaluated by Basso-Bettie-Bresnahan (BBB) scores using an open-field locomotor test at day 1, day 3, day 7 and day 14 after surgery [12]. In an open-field chamber (120 cm × 120 cm), the behavior of rats was observed for 5 min by three individuals who were blinded to the groups. The scale was designed to reflect progressive motor rating scores. The BBB score was counted based on movement of joints of the hindlimb, weight-bearing capability, coordinated and proper gait, and tail position. After evaluation of motor function, animals were deeply anesthetized by chloral hydrate. The spinal segments of the injured center site were removed. Each sample was kept in liquid nitrogen for future polymerase chain reaction (PCR) and western blotting experiments.
Real-time PCR
Total RNA was extracted from frozen spinal cord tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and RNA kit (Sangon, Shanghai, China). RNA was then reverse transcribed to synthesize first-strand cDNA. Quantitative PCR was performed using a Line-Gene sequence detector (ABI, Carlsbad, CA, USA). ASC and actin primers are listed in Table 1. PCR was performed in the Real-Time Detection System by SYBR Green I Dye Detection (Sangon). The PCR was 94°C for 30 s, followed by 45 cycles of 94°C for 20 s and 60°C for 25 s. Data were analyzed by the software attached to the detector (Sangon). RT-PCR products were verified by electrophoresis on 1% agarose gels and melting curve. The amplified production of ASC and actin were 103 bp and 128 bp, respectively. Relative quantification of mRNA expression was calculated with the 2-ΔΔCT method.
Table 1.
Sequences of primers of ASC
| Primer | Sequences |
|---|---|
| ASC Forward primer | 5’-GCACAGCCAGAACAGAACAT-3’ |
| ASC Reverse primer | 5’-AGCACATTGCCATACAGAGC-3’ |
| GAPDH Forward primer | 5’-CAACTCCCTCAAGATTGTCAGCAA-3’ |
| GAPDH Reverse primer | 5’-GGCATGGACTGTGGTCATGA-3’ |
Protein preparation
Spinal cord tissues were frozen in liquid nitrogen and stored at -80°C before analysis. The tissues were homogenized in an SDS sample buffer containing a mixture of proteinase and phosphatase inhibitors (Sigma, St. Louis, MO, USA). Homogenates were centrifuged at 12,000 rpm for 10 min at 4°C and the supernatants were collected. The protein concentrations were measured using a protein assay kit (Pierce, Rockford, IL, USA).
Western blotting
The proteins (20 μg/sample) were loaded on 12% SDS-PAGE. After electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes blocked with 5% nonfat dry milk for 1 h at room temperature in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) and incubated overnight at 4°C with anti-ASC antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing in TBS-T, the membranes were incubated with horseradish-peroxidase-labeled secondary antibody (Santa Cruz Biotechnology) for 2-3 h at room temperature. The membranes were developed with the enhanced chemiluminescence agents (ECL Plus; Pierce) before exposure to X-rays. For quantification, films of western blots were scanned using a Minolta scanner and analyzed by Adobe Photoshop software. The labeling density was quantities using LabWorks software (UVP, Upland, CA, USA).
Enzyme-linked immunosorbent assay
The activity of caspase-3 in the injured spinal cord tissue was detected using a colorimetric caspase-3 assay kit (Sigma) in accordance with the manufacturer’s instructions. In brief, synthetic caspase-3 substrate, acetyl-Asp-Glu-Val-Asp-p-nitroanilide was added to the reaction mixture. Meanwhile, in order to rule out nonspecific hydrolysis of substrate, a control reaction mixture was prepared. Both the mixtures were incubated at 37°C for 1-2 h and the absorbance was read at 405 nm. The activity of caspase-3 was expressed by the value of OD405.
Immunohistochemical staining
Histological sections of tissues (3-4 μm thick) were obtained and fixed in 10% formalin, and paraffin embedded. The sections were deparaffinized in xylene and rehydrated in ethanol, and endogenous peroxidase was blocked by immersion in methanol containing 0.3% hydrogen peroxidase for 20 min. Before incubation, the sections were permeabilized and blocked with normal goat serum. The sections were incubated overnight at 4°C with the primary antibodies (Histostain-Plus Kit; Sunbio, Beijing, China). On the following day, the sections were incubated with secondary antibodies and horseradish enzyme markers for 10-15 min, followed by staining with diaminobenzidine. The slides were examined using a Nikon i50 microscope. The proportion of positively stained cells was calculated as the number of positive cells divided by the total cell number.
TUNEL staining
Injured tissue of spinal cord was processed for motor neuron apoptosis by TUNEL assay kit (Cell Death Detection Kit; Roche), according to the manufacturer’s instructions. The main steps were as follows: frozen sections were dried at room temperature, fixed in 4% paraformaldehyde for 20 min, incubated with 0.3% H2O2/methanol for 30 min at 15-25°C, rinsed with PBS three times, and incubated with 0.1 M Triton HCl for 30 min at room temperature, rinsed with PBS three times, incubated with proteinase K for 2 h, rinsed with PBS three times, incubated with TUNEL reaction mixture for 1 h at 37°C, and observed under a fluorescence microscope.
Statistical analysis
Statistical analysis was performed using SPSS version 15.0 (SPSS, Chicago, IL, USA). All quantitative data were expressed as mean ± SD. One-way analysis of variance procedures were used to test the differences in BBB scores, expression of ASC and caspase-3, positive rate of immunohistochemical and TUNEL staining. Student’s t test was used for part of the BBB score because of the heterogeneity of variance. A p value < 0.05 was considered statistically significant.
Results
HBOT suppressed SCI-induced ASC expression
Western blotting and RT-PCR showed that the expression of ASC in the SCI groups was significantly increased compared with that in the SH groups at any time points (Figures 1 and 2, p < 0.05 or p < 0.01). ASC expression reached a peak at day 1 after surgery at both the mRNA and protein levels. Expression of ASC at the protein and mRNA levels was significantly decreased in the SCI + HBO group compared with the SCI group at day 3 and day 7 after surgery (Figures 1 and 2, p < 0.05).
Figure 1.
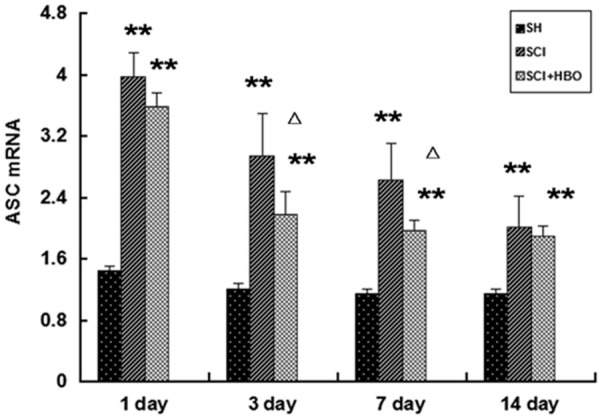
RT-PCR analysis of mRNA level of ASC in the spinal cords of all rats at different times after surgery. Data are presented as mean ± SD. **p < 0.01 for SCI and SCI + HBO groups versus SH and SH + HBO groups; #p < 0.05 for SCI and SCI + HBO groups versus SH and SH + HBO groups; ♦p < 0.01 and ΔP < 0.05 for SCI + HBO group versus SCI group.
Figure 2.
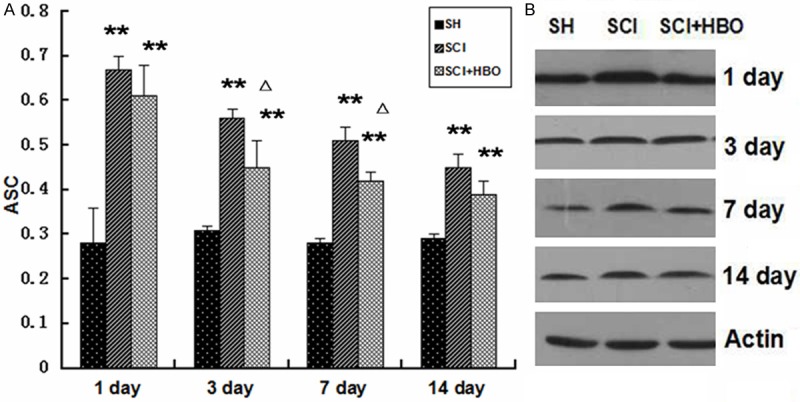
Western blotting of ASC in the spinal cords of all rats. Data are presented as mean ± SD. **p < 0.01 for SCI and SCI + HBO groups versus SH and SH+HBO groups; #p < 0.05 for SCI and SCI + HBO groups versus SH and SH+HBO groups; ♦p < 0.01 and ΔP < 0.05 for SCI + HBO group versus SCI group.
Immunohistochemical staining was applied to examine the distribution of ASC expression. ASC-positive cells were rarely detected in SCI tissue in the SH group. By contrast, the number of ASC-positive cells in the SCI groups was significantly increased in the gray and white matter. The percentage of positive staining in the SH and SCI on day 1 was 15.2% and 72.3%, respectively. However, HBOT significantly attenuated the SCI-induced ASC expression (p < 0.05). The percentage of positive staining in the HBO + SCI 3 day and HBO + SCI 7 day groups was 48.7% and28.9%, respectively (Figure 3; Table 2).
Figure 3.
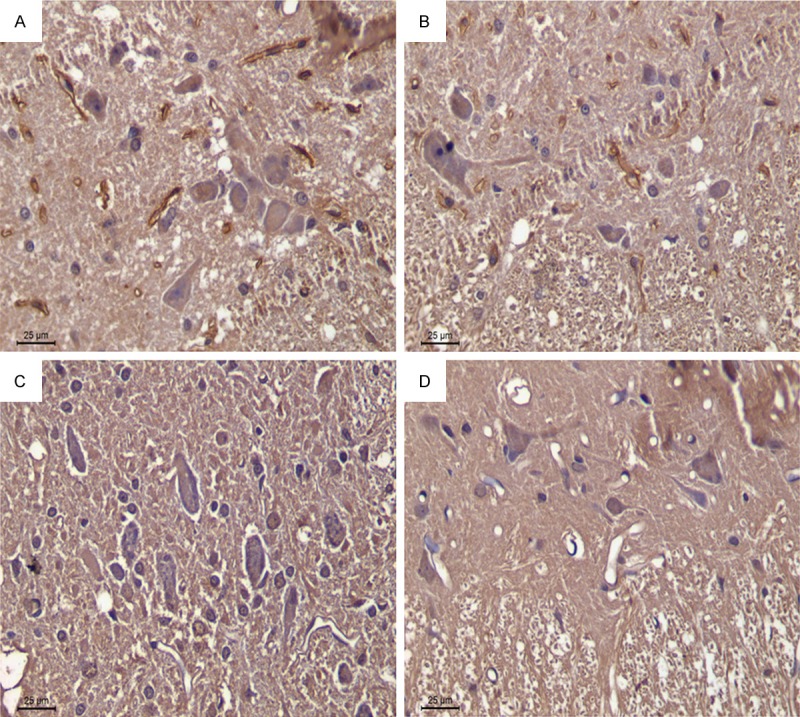
Immunohistochemical staining of ASC (right) and quantitative analysis of ASC in the spinal cords of all rats (left). Data are presented as mean ± SD. **p < 0.01 for SCI and SCI + HBO groups versus SH and SH + HBO groups; #p < 0.05 for SCI and SCI + HBO groups versus SH and SH + HBO groups; ♦p < 0.01 and ΔP < 0.05 for SCI+HBO group versus SCI group. A: SCI3d group; B: SCI + HBO3d group; C: SCI7d group; D: SCI + HBO7d gr.
Table 2.
Number of ASC-positive cells
| Group | 1 d | 3 d | 7 d | 14 d |
|---|---|---|---|---|
| SH | 15.2 ± 4.1 | 13.1 ± 3.6 | 13.8 ± 2.9 | 14.6 ± 4.0 |
| SCI | 72.3 ± 9.6** | 60.2 ± 7.7** | 41.6 ± 5.8** | 26.4 ± 3.7# |
| SCI + HBO | 65.8 ± 7.2** | 48.7 ± 5.1**,∆ | 28.9 ± 3.4**,∆ | 23.1 ± 2.8# |
Data are presented as mean ± SD.
p < 0.01 for SCI and SCI + HBO groups versus SH groups;
p < 0.05 for SCI and SCI + HBO groups versus SH and SH + HBO groups;
P < 0.05 for SCI + HBO group versus SCI group.
HBOT downregulated ASC-induced activation of caspase-3
Caspase-3 in SCI tissue was significantly increased compared with that in the SH groups at any time points, and reached peak at day 1 after surgery. Compared to the SCI group, the caspase-3 level in the SCI + HBO group was significantly decreased at days 3 and 7 after surgery (p < 0.05, p < 0.01). These changes were synchronized with ASC (Table 3).
Table 3.
Caspase 3 activatity in groups
| Group | SH | SCI | SCI + HBO |
|---|---|---|---|
| 1 day | 3.98 ± 1.25 | 13.44 ± 2.37** | 11 .35 ± 5.35** |
| 3 days | 4.15 ± 1.22 | 12.27 ± 3.85** | 9.44 ± 4.55#,∆ |
| 7 days | 4.88 ± 1.32 | 11.24 ± 2.20** | 8.26 ± 3.77#,*,∆ |
| 14 days | 5.19 ± 2.27 | 9.16 ± 1.65# | 8.22 ± 4.58# |
Data are presented as mean ± SD.
p < 0.01 for SCI and SCI + HBO groups versus SH groups;
p < 0.05 for SCI and SCI + HBO groups versus SH groups;
p < 0.05 for SCI + HBO group versus SCI group.
HBOT inhibited apoptosis of neurons and oligodendroglial cells
Apoptotic nuclei were found throughout the gray and white matter in SCI rats. The number of apoptotic cells in the SCI groups was greatly increased compared with the SH groups, and reached the peak of 62.2% (p < 0.01) at 3 days after surgery. In the SCI 7-day group, apoptotic cells were mainly oligodendroglial cells at the periphery of the injury site, with a positive rate of 42.8% (p < 0.01). In the SCI + HBO 3-day and 7-day groups, the positive rates were 53.7% and 31.9%, which were significant decreases compared with the SCI 3-day and 7-day groups (P < 0.05) (Figure 4; Table 4).
Figure 4.
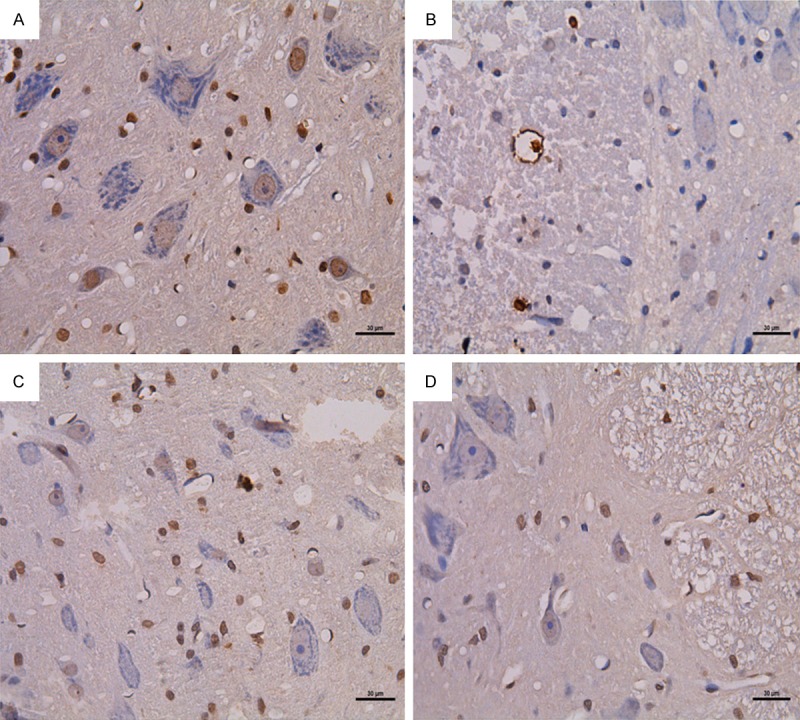
TUNEL staining of caspase-1 (right) and quantitative analysis of caspase-1 in the spinal cords of all rats (left). Data are presented as mean ± SD. **p < 0.01 for SCI and SCI + HBO groups versus SH and SH + HBO groups; #p < 0.05 for SCI and SCI + HBO groups versus SH and SH + HBO groups; ♦p < 0.01 and ΔP < 0.05 for SCI + HBO group versus SCI group. A: SCI3d group; B: SCI + HBO3d group; C: SCI7d group; D: SCI + HBO7d group.
Table 4.
Number of TUNEL-positive cells
| Group | 1 d | 3 d | 7 d | 14 d |
|---|---|---|---|---|
| SH | 11.2 ± 4.1 | 13.1 ± 3.6 | 12.8 ± 2.7 | 11.6 ± 3.5 |
| SCI | 52.3 ± 9.6** | 62.2 ± 7.7** | 42.8 ± 4.7** | 26.4 ± 2.7# |
| SCI+HBO | 51.8 ± 7.2** | 53.7 ± 5.1**,∆ | 31.9 ± 3.4**,∆ | 24.1 ± 2.6# |
Data are presented as mean ± SD.
p < 0.01 for SCI and SCI + HBO groups versus SH groups;
p < 0.05 for SCI and SCI + HBO groups versus SH groups;
p < 0.05 for SCI + HBO group versus SCI group.
HBOT increased BBB score of rats after SCI
The BBB scores in the SCI and SCI + HBO groups were significantly lower than in the SH groups (p < 0.01). Although a significant recovery was observed in the SCI and SCI + HBO groups over time, on day 7 and day 14 groups, HBOT significantly increased BBB score when compared to that in the SCI group (p < 0.05) (Table 5).
Table 5.
BBB scores of all groups
| Groups | SH groups | SCI groups | SCI + HBO groups |
|---|---|---|---|
| 1 day | 19.33 ± 7.29 | 0.27 ± 0.31** | 0.30 ± 0.26** |
| 3 days | 20.25 ± 7.34 | 1.03 ± 0.66** | 1.25 ± 0.75** |
| 7 days | 20.61 ± 7.58 | 2.56 ± 1.24** | 5.27 ± 2.14**,∆ |
| 14 days | 20.47 ± 6.81 | 7.39 ± 3.08** | 11.35 ± 4.22**,∆ |
Data are presented as mean ± SD.
p < 0.01 for SCI and SCI + HBO groups versus SH groups;
p < 0.05 for SCI + HBO group versus SCI group.
Discussion
In the course of SCI, the mechanical disruption of the primary injury leads to a cascade of inflammatory response called the secondary injury [13]. Strong biochemical evidence demonstrates that the main form of cell death caused by this secondary injury is apoptosis, especially in oligodendroglial cells [14,15]. Oligodendroglial cells are present around the periphery of the SCI tissue, which leads to demyelination of white matter tracts and axonal degeneration [16,17]. This contributes to further neurological damage, such motor deficit and chronic pain. Apoptosis usually occurs in the days to weeks after SCI, which could be prevented by treatment. Targeting of apoptosis could preserve the neurons and oligodendroglial cells around the injury site [18,19].
ASC is a proapoptotic protein which is a component of a “speck” in apoptotic cells and contains an N-terminal pyrin domain and a C-terminal caspase-recruitment domain (CARD) [20]. Ohtsuka et al. [21] demonstrated that activated ASC can interact directly with Bax and translocate it to the mitochondria in response to p53, which reduces mitochondrial membrane potential and activation of caspase-3. A previous study showed that ASC was increased in neutrophils and mediated their apoptosis at sites of severe inflammation in appendicitis [22]. In the present study, western blotting and RT-PCR showed that expression of ASC was upregulated in SCI groups compared to SH groups, and peaked at 1 day after surgery. The positive rate of ASC in immunohistochemical staining was 58.7% in the SCI 1-day group, which was higher than that in the corresponding SH group. Our findings suggested that functional ASC was activated at the stage of secondary injury after SCI. We investigated the pathway of ASC activation after SCI. Masumoto et al. [23] showed that co-expression of NALP3 (NACHT domain-leucine-rich-repeat-, and pyrindomain-containing protein 3) with ASC induces apoptosis in 293T cells. According to previous research [24,25], we propose that reactive oxygen species (ROS) generated in the process of second injury activate NALP3 protein, which is a cytoplasmic immunosensor. The exposed NATCH domain of NALP3 promotes the binding and activation of ASC. Activated ASC binding to NALP3 and caspase-8 forms a ternary signaling complex that mediates apoptosis [26,27].
Most of the protective effects of HBOT are usually attributed to suppression of injury-induced inflammation, but few studies have focused on the antiapoptotic mechanism of HBOT after SCI. Tian et al. [28] found that HBOT reduced cell toxicity and oxidative stress, decreasing malondialdehyde levels, Bax expression, and cytochrome c release. HBOT significantly reduces the rate of apoptosis in Alzheimer’s disease. In our present study, expression of ASC at the protein and mRNA levels was significantly decreased in the SCI + HBO group compared with the SCI group at 3 days and 7 days after surgery. Downregulation was confirmed by immunohistochemical staining, and the number of ASC-positive cells was significantly decreased in the SCI + HBO 3-day and 5-day groups, compared to the corresponding SCI groups. We confirmed that HBOT increased tissue oxygen tension, resistance to hypoxia–ischemia after SCI, and decreased formation of ROS. HBOT could inhibit formation of ASC complex and interaction of Bax, blocking the ASC-mediated apoptosis signaling pathway.
Caspase-3 is a member of the caspase (cysteine aspartic proteinases) family of enzymes, which is activated both in the intrinsic and extrinsic apoptotic pathways [29], causing chromatin condensation and DNA fragmentation. Apoptotic events are usually marked by activated caspase-3. Caspase-3 activation has been demonstrated during the process of secondary injury after SCI [30]. We examined the expression of caspase-3 in SCI tissue by ELISA. The level of caspase-3 was increased in the SCI compared to SH groups, and peaked at 1 day in the SCI 1-day group. HBOT had no effect on this change, and downregulated expression of caspase-3 compared to that in SCI groups. The synchronized increase of caspase-3 accompanied with ASC indicated that caspase 3 activation contributes to ASC-mediated apoptosis after SCI. HBOT decreases the expression of caspase-3, which could be a consequence of ASC inhibition induced by ROS.
We detected apoptotic cells in the spinal cord tissues with TUNEL staining. Lu et al. [31] studied the effect of preconditioning with HBO on neural cell apoptosis after SCI in rats, and showed that the number of apoptotic cells decreased following HBO preconditioning. Our results were in accordance with those of the previous study. The increase in ASC and caspase-3 was accompanied by a massive increase in apoptotic cells in the center of the injury site in the SCI 1-day group. The rate of apoptosis peaked in the SCI 3-day group. In the SCI 7-day group, apoptosis was mainly seen in oligodendroglial cells at the periphery of the injury site. Accordingly, there was a significant reduction in the apoptosis rate in the SCI + HBO 3-day and 7-day groups compared with the SCI 3-day and 7-day groups.
Inhibition of oligodendroglial cells improved axonal recovery of sublethally injured cells and improved neurological recovery. To evaluate the recovery of motor function, BBB scores were measured. The BBB scores were significantly lower in the SCI and SCI + HBO groups compared with the SH group. Meanwhile, BBB score was significantly increased in the SCI + HBO groups compared with the SCI groups on 7 days and 14 days after surgery.
In this study, we demonstrated that HBOT significantly prevented ASC-induced neuronal apoptosis via suppressing caspase-3 during secondary damage, preserving neural function after SCI.
Acknowledgements
This work was supported by program of International S&T cooperation, grant number 2012DFA31240.
Disclosure of conflict of interest
None.
References
- 1.Porter R. The Cambridge Illustrated History of Medicine. New York: Cambridge University Press; 1996. [Google Scholar]
- 2.Dobkin BH, Curt A, Guest J. Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair. 2006;20:5–13. doi: 10.1177/1545968305284675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price C, Makintubee S, Herndon W, Istre GR. Epidemiology of traumatic spinal cord injury and acute hospitalization and rehabilitation charges for spinal cord injuries in Oklahoma, 1988-1990. Am J Epidemiol. 1994;139:37–47. doi: 10.1093/oxfordjournals.aje.a116933. [DOI] [PubMed] [Google Scholar]
- 4.Tator CH, Koyanagi I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J Neurosurg. 1997;86:483–92. doi: 10.3171/jns.1997.86.3.0483. [DOI] [PubMed] [Google Scholar]
- 5.Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–25. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- 6.Crowe MJ, Bresnahan JC, Shuman S, Masters JN, Crowe MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–6. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 7.Wang YF, Qin GH, Yang MC. Effect of Hyperbaric Oxygen on Bcl-2 Expression after Spinal Cord Injury in Rats. Journal of China Medical University. 2013;42:293–300. [Google Scholar]
- 8.Li HP, Ba F, Bai D, Gao J. Apoptosis of anterior horn motor neurons after hyperbaric oxygen preconditioning in spinal cord injury rats. Chinese Journal of Tissue Engineering Research. 2012;16:295–8. [Google Scholar]
- 9.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–22. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 10.Häcker HG, Sisay MT, Gütschow M. Allosteric modulation of caspases. Pharmacol Ther. 2011;132:180–95. doi: 10.1016/j.pharmthera.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Ottmann C, Hauske P, Kaiser M. Activation instead of inhibition: targeting proenzymes for small-molecule intervention. Chem Bio Chem. 2010;11:637–9. doi: 10.1002/cbic.201000024. [DOI] [PubMed] [Google Scholar]
- 12.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight drop device versus transaction. Exp Neurol. 1996;139:244–56. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 13.Cramer SC, Lastra L, Lacourse MG, Cohen MJ. Brain motor system function after chronic, complete spinal cord injury. Brain. 2005;128:2941–50. doi: 10.1093/brain/awh648. [DOI] [PubMed] [Google Scholar]
- 14.Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–25. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- 15.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–6. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 16.Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998;89:911–20. doi: 10.3171/jns.1998.89.6.0911. [DOI] [PubMed] [Google Scholar]
- 17.Li GL, Brodin G, Farooque M, Funa K, Holtz A, Wang WL, Olsson Y. Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J Neuropathol Exp Neurol. 1996;55:280–9. doi: 10.1097/00005072-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Jiang S, Bendjelloul F, Ballerini P, D’Alimonte I, Nargi E, Jiang C, Huang X, Rathbone MP. Guanosine reduces apoptosis and inflammation associated with restoration of function in rats with acute spinal cord injury. Purinergic Signal. 2007;3:411–21. doi: 10.1007/s11302-007-9079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald JW, Belegu V. Demyelination and remyelination after spinal cord Injury. J Neurotrauma. 2006;23:345–59. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- 20.Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, Hidaka E, Katsuyama T, Higuchi T, Sagara J. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274:33835–8. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 21.Ohtsuka T, Ryu H, Minamishima YA, Macip S, Sagara J, Nakayama KI, Aaronson SA, Lee SW. ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol. 2004;6:121–8. doi: 10.1038/ncb1087. [DOI] [PubMed] [Google Scholar]
- 22.Shiohara M, Taniguchi S, Masumoto J, Yasui K, Koike K, Komiyama A, Sagara J. ASC, which is composed of a PYD and a CARD, is up-regulated by inflammation and apoptosis in human neutrophils. Biochem Biophys Res Commun. 2002;293:1314–8. doi: 10.1016/S0006-291X(02)00384-4. [DOI] [PubMed] [Google Scholar]
- 23.Masumoto J, Dowds TA, Schaner P, Chen FF, Ogura Y, Li M, Zhu L, Katsuyama T, Sagara J, Taniguchi S, Gumucio DL, Núñez G, Inohara N. ASC is an activating adaptor for NF-κB and caspase-8-dependent apoptosis. Biochem Biophys Res Commun. 2003;303:69–73. doi: 10.1016/s0006-291x(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 24.Inohara N, Nuñez G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene. 2001;20:6473–81. doi: 10.1038/sj.onc.1204787. [DOI] [PubMed] [Google Scholar]
- 25.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O’Reilly L, Mason K, Gross O, Ma S, Guarda G, Anderton H, Castillo R, Häcker G, Silke J, Tschopp J. Inhibitor of apoptosis proteins limit RIP3 kinas-dependent interleukin-1 activation. Immunity. 2012;36:215–27. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Richards N, Schaner P, Diaz A, Stuckey J, Shelden E, Wadhwa A, Gumucio DL. Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J Biol Chem. 2001;276:39320–9. doi: 10.1074/jbc.M104730200. [DOI] [PubMed] [Google Scholar]
- 27.Stehlik C, Fiorentino L, Dorfleutner A, Bruey JM, Ariza EM, Sagara J, Reed JC. The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor kB activation pathways. J Exp Med. 2002;196:1605–15. doi: 10.1084/jem.20021552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian X, Zhang L, Wang J, Dai J, Shen S, Yang L, Huang P. The protective effect of hyperbaric oxygen and Ginkgo biloba extract on Aβ25-35-induced oxidative stress and neuronal apoptosis in rats. Behav Brain Res. 2013;242:1–8. doi: 10.1016/j.bbr.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–32. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 30.Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–68. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu PG, Feng H, Yuan SJ, Zhang RW, Li M, Hu R, Liu ZS, Yin J. Effect of preconditioning with hyperbaric oxygen on neural cell apoptosis after spinal cord injury in rats. J Neurosurg Sci. 2013;57:253–8. [PubMed] [Google Scholar]


