Abstract
Cholecystolithiasis is a common disease, and gallbladder dysmotility is considered as a pivotal pathogenesis. Interstitial cells of Cajal (ICCs) serve as pacemakers and mediators of neuromuscular transmission for gastrointestinal motility. Reduction of ICCs has been reported in gallstone diseases. However, there are no reasonable mechanisms for the cholecystolithiasis-associated loss of ICCs in humans. Stem cell factor (SCF) and its ligand c-kit are essential for normal development and survival of ICCs. To date, little is known about the SCF/c-kit signaling pathway in gallstone diseases. The purpose of this study was to investigate the role of the SCF/c-kit signaling pathway in the loss of ICCs in cholecystolithiasis. Data from 18 patients with gallstones and 14 individuals without gallstones were compared. The gallbladder contractility was assessed by measuring the gallbladder ejection fraction (GEF) ultrasonographically. Tissues samples were obtained during surgery, changes of ICC quantities were analyzed by immunohistochemistry, and the mRNA and protein expression of SCF and c-kit were detected by Real-Time PCR and Western-blot analysis. Compared with the controls, the GEF was significantly reduced in the gallstone group, and decreased number of ICCs was present obviously in the gallstone group. Furthermore, the mRNA and protein expression of SCF and c-kit were significantly attenuated in the gallstone group. These data indicate that gallbladder motility may be affected by reduction of ICCs in gallstone disease. Additionally, the decreased of SCF/c-kit signaling pathway play an important role in the loss of ICCs.
Keywords: Gallstone disease, interstitial cells of Cajal, stem cell factor, c-kit, gallbladder motility
Introduction
Cholecystolithiasis is one of the most common gastrointestinal diseases in developed countries, affecting 10-15% of the adult population [1]. Surgical procedures are the main treatment methods for gallstones [2]. Several factors are involved in the pathogenesis of this disease, which include biliary cholesterol hypersecretion, supersaturation and crystallization, bile stasis and mucus hypersecretion and gel formation within the gallbladder [3]. However, gallbladder hypomotility may be a key factor in the pathogenesis of cholelithiasis [4]. Because precipitation of excess cholesterol in bile as solid crystals is a prerequisite for gallstone formation [5], impaired gallbladder motility allows these microcrystal to be retained and eventually grow over months or years into macroscopic gallstones [6].
Interstitial cells of Cajal (ICCs) were first described by the Spanish neuropathologist, Ramón Santiago y Cajal in 1889 [7] and are known to generate and propagate the slow waves in the alimentary tract, act as mediators of electrical coupling between nerve fiber terminals and smooth muscles, and play a pivotal role in the regulation of gastrointestinal (GI) motility [8-10]. An important characteristic feature of ICCs is expression of transmembrane tyrosine kinase receptor protein (c-kit), and c-kit protein (CD117) can be identified by immunohistochemical and molecular methods throughout the GI tract of humans as well as other species [11]. Absence, reduction in number or altered integrity of the ICCs network may have a dramatic effect on GI system motility. Several disorders, including chronic idiopathic intestinal pseudoobstruction, achalasia, afferent loop syndrome, and chronic constipation have been associated with loss of ICCs in dysfunctional regions of the GI tract [9,12-15]. Recently, cholecystolithiasis was added to the list of diseases. Pasternak A et al. [16] were the first to report that the density of ICCs in the muscularis propria was significantly lower in patients with gallstones than in controls subjects and suggested that the gallbladder motility may be affected by the decreased number of ICCs in patients with cholelithiasis. Thus far, no reasonable pathological and physiological mechanisms have been identified for the cholecystolithiasis-associated loss of ICCs in humans.
It is well known that the development, phenotype maintenance, and survival of ICCs and their networks require cellular signaling through the c-kit ligand, termed stem cell factor (SCF), via the SCF/c-kit signaling pathway [17]. SCF is the natural ligand for c-kit, and the SCF/c-kit system plays a crucial role in the functional activity of ICCs in the GI tract [18]. Disruption of c-kit signaling results in a severe anomaly of gut motility with depletion of ICCs and aberrant electromechanical coupling movement [19]. To date, little is known about the SCF/c-kit signaling pathway and its role in the changes of ICCs in patients with gallstone disease. Therefore, can we hypothesize that disorders of the SCF/c-kit signaling pathway may participate in the pathogenesis of reduction of ICCs in cholecystolithiasis? The purpose of this study was to investigate the role of SCF/c-kit signaling pathway in the loss of ICCs in gallstone disease.
Materials and methods
Subjects
This study was approved by the Ethics Committee of Human Study of Zhong Da Hospital Southeast University and the Affiliated Cancer Hospital of Xiangya School Of Medicine Central South University conforming to the Declaration of Helsinki guidelines. Thirty-two patients who had been surgically treated in 2013 were enrolled in this study. Patients with symptomatic gallstone disease (n=18, mean age 51.2±13.4 years, 10 females, 8 males) were qualified to participate in the study group. None of these patients had associated acute cholecystitis or choledocholithiasis. The control group (n=14, mean age 52.6±15.1 years, 8 females, 6 males) had early resectable pancreatic head tumors without any signs of cholelithiasis or jaundice, and the gallbladders were not affected by tumors, and the serum bilirubin levels were within the normal range.
Gallbladder contractile function evaluation
The gallbladder contractile function in these patients was assessed by measuring the gallbladder ejection fraction (GEF) ultrasonographically [20]. Briefly, all patients were investigated the gallbladder volume after 12 hours of fasting and then 15th-, 30th-, 45th-, 60th-, 75th-, 90th-minute after consuming a fatty meal. Using the ellipsoid method, the long axis and transverse figures of the gallbladder were obtained by ultrasonography using the ellipse formula: Gallbladder volume (ml)=0.52 × length × width × height [21]. The GEF at each postprandial time point (15th-, 30th-, 45th-, 60th-, 75th-, and 90th-minute) was calculated as follows: GEF (%)=(Fasting gallbladder volume–postprandial gallbladder volume)/(fasting gallbladder volume) × 100.
Gallbladder tissue specimens’ preparation
Patients in control group undergone a Whipple or a Traverso-Longmire procedure, and patients in study group underwent laparoscopic cholecystectomy. The fresh cholecystectomy tissues were washed three times with cold phosphate-buffered saline (PBS) and placed on a flatplate. The mucosa and submucosa were carefully removed. Then, some of gallbladder specimens were cut into small pieces and preserved in liquid nitrogen for standby.
Immunohistochemical examination
Gallbladder specimens fixed in 10% buffered formalin was embedded in paraffin. Sections from paraffin embedded gallbladder tissues were cut at 4 μm thickness and mounted on positively charged slides. Immunohistochemistry was performed on paraffin sections using a microwave-based antigen retrieval technique. Briefly, the sections were incubated at room temperature for 1 h with primary rabbit anti-human CD117 monoclonal antibody (Maixin Biotechnology Development Co. Ltd., Fuzhou, China), and followed by appropriate secondary antibodies incubation. Subsequently, the sections were stained with diaminobenzidine and counterstained with hematoxylin. Negative controls were prepared by omitting primary antibodies in order to check the specificity of the immunostaining. Images of CD117-positive cells were taken in 5 randomly chosen fields (× 200 magnification) per tissue section. The positive cell density was assessed with the Image-Pro plus 6.0 software (Media Cybernetics, Bethesda, MD, United States).
Real-Time PCR analysis
Total RNA from frozen gallbladder tissues was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA, USA), and DNase set was performed with an RNase-free DNase set (Qiagen). Real-Time PCR was performed to determine the expression of human SCF and c-kit in a SYBR Green PCR Master mix (Applied Biosystems) using the Bio-Rad iCycler iQ Multicolor RT-PCR Detection systems (version 3.0 software) with the following protocol: initial cycle: 95°C for 5 min; 40 cycles at 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s, and finally 10 min of synthesis at 72°C. The primer sequences are summarized in Table 1. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a control for target genes for reaction efficiency. The ΔΔCt method was used to determine the relative amounts of product using GAPHD as an endogenous control.
Table 1.
Primer sequences for Real Time PCR analysis
| Primer | Accession No. | Forward Primer (5’-3’) | Reverse Primer (5’-3’) |
|---|---|---|---|
| GAPDH | NM_001256799 | TGGTATCGTGGAAGGACTCA (726-745) | CCAGTAGAGGCAGGGATGAT (838-857) |
| SCF | NM_000899 | AACCCAGGTGCTTTGAGAAG (2749-2768) | CAATGCCACACACTGAGACA (2858-2877) |
| C-kit | NM_000222 | ATTCCCAGAGCCCACAATAG (1383-1402) | ACCACTAGCTTTCCAAACGG (1489-1508) |
Western-blot analysis
Liquid nitrogen-frozen gallbladder tissues were homogenized in ice-cold RIPA lysis buffer (Beyotime, Shanghai, China), disrupted by sonication three times for 10 s and centrifuged at 12000 r/min for 30 min at 4°C, and the supernatants were used as the total proteins. The total protein concentration was measured using the BCATM protein assay kit (POERCE, Rockford, IL, USA). Equal amounts of proteins (50 μg) were loaded onto each lane of 10% SDS polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Non-specific binding was reduced by incubating the membrane in 5% skimmed milk for 1 hour at room temperature. The membranes were then incubated overnight at 4°C with the primary antibodies c-kit (1:5000, abcam, UK) and SCF (1:5000, abcam, UK), washed with PBS three times for 15 min, and followed incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. Finally, the immunoreactive bands were analyzed using an ECL advanced system (GE Healthcare, Chalfont St. Giles, UK). β-actin was used as the internal control.
Statistical analysis
The statistical analyses were performed using the SPSS 13.0 statistical software package (SPSS Inc., USA). The data were expressed as mean ± standard deviation (SD). Differences between groups were analyzed with Student’s t-test. P<0.05 was considered to be statistically significant.
Results
Gallbladder contractile function evaluation
The gallbladder contractile function analysis showed that the 15th-, 30th-, 45th-, 60th-, 75th-, and 90th-minute postprandial GEFs of patients with gallstones were lower that of the controls, and the difference was statistically significant (Table 2; Figure 1).
Table 2.
Postprandial Gallbladder Ejection Fraction in the study and controlgroups
| Gallbladder Ejection Fraction, % | Gallstone group (n=18) | Control group (n=14) | P Value |
|---|---|---|---|
| 15th minute | 12±2.2 | 32±2.1 | <0.05 |
| 30th minute | 18±2.4 | 41±2.3 | <0.05 |
| 45th minute | 24±2.0 | 53±1.8 | <0.05 |
| 60th minute | 28±2.3 | 58±2.2 | <0.05 |
| 70th minute | 32±2.1 | 62±2.6 | <0.05 |
| 90th minute | 35±1.9 | 65±2.4 | <0.05 |
Figure 1.
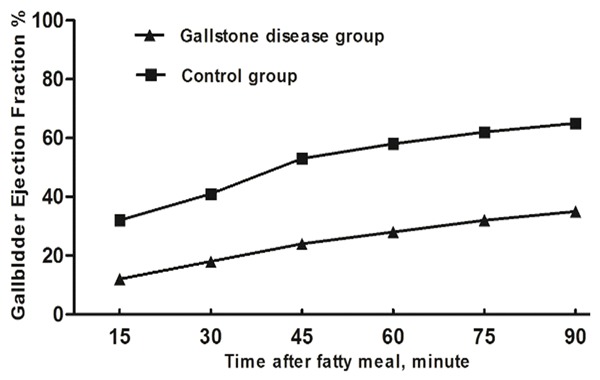
The variation in gallbladder ejection fractions (%) between gallstone group and control group. The 15th-, 30th-, 45th-, 60th-, 75th-, and 90th-minute postprandial GEFs of patients with gallstones were lower that of the controls (P<0.05).
Immunohistochemical examination
CD117 positive cells could be divided into two cell populations (Figure 2). One was mast cells situated within the lamina propria, another was ICCs with large oval nuclei, sparse cytoplasm and branching processes. These cells predominantly located in the muscularis propria. The density of ICCs in gallstone group was significantly lower than the control group (24.24± 11.29 vs. 56.29±12.43, P<0.05).
Figure 2.
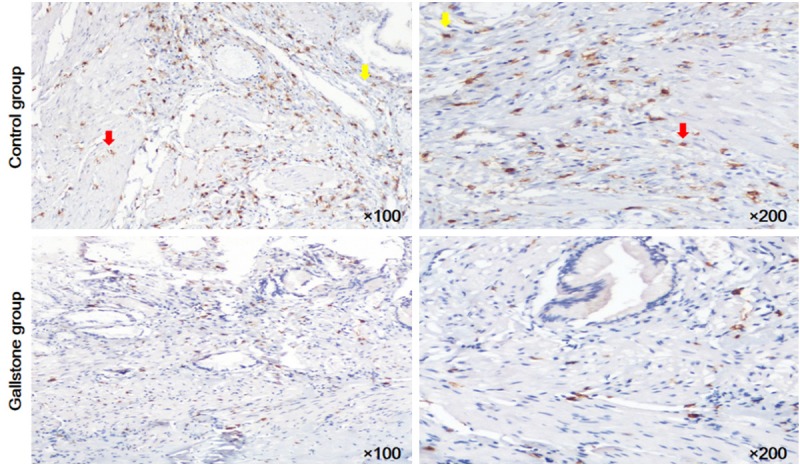
Immunohistochemistry for CD117. Mast cells (yellow arrows) within the muscularis propria. CD117-positive ICCs (red arrows) in the the muscularis propria. The density of CD117 positive ICCs in gallstone group was significantly lower than the control group (P<0.05).
Real-Time PCR analysis
The RT-PCR analysis, normalized with respect to GAPHD mRNA, revealed that the levels of SCF and c-kit mRNA were significantly lower in the gallstone disease group than the control group (Figure 3). The relative amount of SCF was 0.66±0.30 in the control group and 0.12±0.03 in the gallstone disease group. The relative amount of c-kit was 0.79±0.20 in the control group and 0.21±0.03 in the gallstone disease group.
Figure 3.
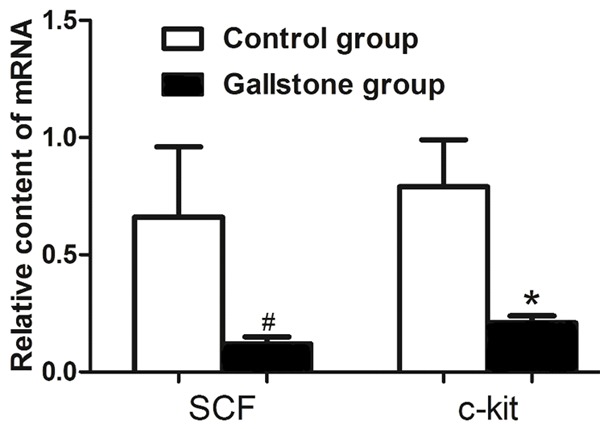
Expression of SCF and c-kit mRNA. Decreased expressions of SCF and c-kit mRNA were demonstrated when compared with the control group. Each bar represents the mean ± SD. #P<0.05; *P<0.05.
Western-blot analysis
The results from Western-blot strengthened the immunohistochemistry and RT-PCR observations. The protein expression levels of c-kit and SCF were normalized to the internal control β-actin. The presence of 145 kDa c-kit protein and 31 kDa SCF protein were revealed by Western-blot analysis (Figure 4A). The protein expression levels of c-kit and SCF were significantly lower in the study group than in the control group (Figure 4B). The ratio of SCF and β-actin was 0.93±0.78 and 0.52±0.11 in the gallstone disease group. The ratio of c-kit and β-actin was 0.96±0.16 in the control group and 0.42±0.26 in the gallstone disease group.
Figure 4.
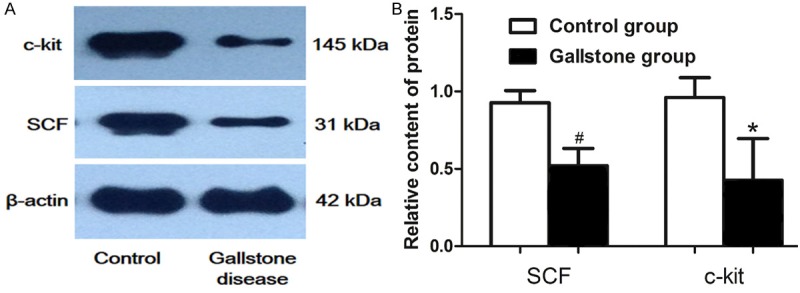
Expression of SCF and c-kit protein. A. The protein expression of c-kit and SCF were downregulated in the gallstone disease group compared with the control group. β-actin was used as the internal control. B. quantification of SCF and c-kit protein expression. Each bar represents the mean ± SD. #P<0.05; *P<0.05.
Discussion
Cholecystolithiasis is a prevalent and costly disease. The following factors have been shown cause gallstone formation: cholesterol supersaturation, hydrophobic bile salts, mucus hypersecretion with gel formation, pronucleating proteins and disrupted gallbladder motility. The gallbladder dysmotility seems to be a “triggering” event in the pathogenesis of the procedure, which provides the space and time necessary for the precipitation of cholesterol microcrystals from bile supersaturated with cholesterol and their subsequent growth to macroscopic stones [22]. Ultrasonography is an accurate and useful method for studying gallbladder motility and thus indirectly gallbladder emptying and refilling in humans [23]. Portincasa P et al. [24] described a subset of cholesterol gallstone patients (“bad contractors”) have severely decreased or even absent postprandial gallbladder emptying, whereas patients whose gallbladders empty (“good contractors”) mostly have increased fasting and residual gallbladder volumes compared with controls. Our results also illustrated the impaired gallbladder motility result in the lower postprandial GEF of patients with gallstones.
Gallbladder motility involves several regulating mechanisms, including enteric nervous circuit activity, smooth muscle contraction function, and the recently described interstitial cells of Cajal (ICCs). ICCs are recognized as pacemaker cells and mediators of neuromuscular transmission in the GI tract. It is now clear from previous studies that ICCs serve as the following: (i) pacemaker cells, generating the spontaneous electrical rhythms of the gut known as slow waves; (ii) a propagation pathway for slow waves so that large areas of the musculature can be entrained to a dominant pacemaker frequency; (iii) mediators of excitatory cholinergic and inhibitory nitrergic neural inputs from the enteric nervous system, and (iv) stretch receptors that modulate membrane potential and electrical slow wave frequency [25]. An important characteristic feature of ICCs in the GI tract is expression of transmembrane tyrosine kinase receptor protein (c-kit) and can be identified by immunohistochemical and molecular methods. However, mast cells are the only other cells expressing c-kit in the GI tract. ICCs were predominantly located in the muscularis propria and mast cells in the lamina propria [26]. The distinctive size, shape, and relative distributions of these cells make a minor interference in identification of ICCs [27]. To reduce the interference of mast cells, we carefully removed the mucosa and submucosa of the gallbladder before the Western-blot analysis and RT-PCR testing.
Hinescu et al. [28] initially verified that ICCs were presented in the human gallbladder, and this point was definitively reinforced in 2012 [26]. Ahmadi et al. [29] suggested for the first time that ICCs are present in human extrahepatic bile ducts. In the gallbladder, ICCs intimately contact smooth muscle cells with gap junction, and they are responsible for the generation of smooth muscle rhythmic activity [30]. Damaging ICCs by light and methylene blue, which were specific only to ICCs, but not the associated smooth muscle cells, the contractile ability of guinea pig gallbladder muscle strips was sharply weakened [31]. These teams demonstrated that the roles of ICCs are essential for propagation of spontaneous rhythmicity and involved in biliary tract motility and its related disorders. Recently, the deficiency of ICCs has been regarded as a novel factor associated with gallbladder dysmotility in gallstone disease [16]. Our result also shows the significant decreased number of CD117 positive cells in the muscularis propria of gallbladder wall in patients with gallstones, which is exactly consistent with the previous research. Some hypotheses, including an increased bile lithogenicity index, chronic inflammatory processes, apoptosis mechanism, may be involved in the reduction of ICC density in gallbladders of patients with gallstones [3,16]; however, there is no data to support these suspicions directly.
One of the important breakthroughs in this field has been the discovery that the SCF/c-kit signal pathway is essential for normal development, maturation and survival of ICCs and is required for maintenance phenotype and function of ICC networks. This finding was first suggested after observing that a severe anomaly of GI tract movement with depletion of ICCs resulted from the blockade of KIT postnatally by an antagonistic anti-KIT antibody [32]. Numerous studies have subsequently revealed an association between loss of ICCs and disorder of the SCF/c-kit signal pathway in human clinical diseases [12,13,33-35]. Could we hypothesize that blocking the SCF/c-kit signal pathway results in the loss of ICCs in gallstone disease? To determine the overall expression of SCF and c-kit in cholecystolithiasis and normal gallbladder tissues, Western-blot and Real-Time PCR analyses were performed. Our results illustrate that the mRNA and protein expression of SCF and c-kit were substantially decreased in patients with gallstone diseases. The downregulation of SCF and its receptor c-kit suggested that the disorder of this ligand/receptor system might be involved in the pathogenesis of ICC depletion in gallstone disease. Data from animal models also support our results. Fan et al. [36] revealed the decreased expression of c-kit and scf in terminal ileum are present in gallstone model of guinea pigs fed on high cholesterol diet, which suggested the c-kit/scf pathway inhibition might be involved in the decline of intestinal transit function during cholesterol gallstone formation. T Yamamoto et al. [37] presented disturbed GI motility diabetic db/db mice with reduced areas of ICCs and expression of SCF, which showed that the expression levels of SCF by quantitative Real-Time PCR were reduced to half and two-thirds in the small intestine and colon, respectively. Another study reported a decrease in the content of SCF in the stomach of non-obese diabetic mice, which showed the number of ICCs to be drastically decreased and the expression level of SCF to be reduced to one-third of that in normal mice [38]. W Tong et al. [17] indicated that the inhibition of the SCF/c-kit signaling pathway by the c-kit neutralized antibody ACK2 in mice after birth could cause the loss of ICCs and the aberrant electrical activity; however, application of SCF containing medium in cultured tissue significantly improved the recovery of c-kit mRNA and protein expression and the electrical activity in the jejunal muscle strips of mice. These previous findings and our research confirm the hypothesis that disorder SCF/c-kit signaling pathway may underlie the decrease in ICC number and its network function in gallstone disease.
SCF is produced by enteric neurons and smooth muscle cells in the GI tract [39]. Although previous work has showed that inhibition of the c-kit signaling pathway with neutralized antibody can induce transdifferentiation of ICCs to a smooth muscle phenotype [40], and c-kit signaling may stabilize the phenotype of ICCs, and the opportunity for SCF/c-kit signaling would favor the development of ICCs, whereas a lack of signaling would favor development of a smooth muscle phenotype [41]. In our study, reduction in SCF/c-kit mRNA and proteins levels also suggested that blockage of the c-kit/SCF signaling pathway may be involved in the depletion of ICCs in gallstone diseases. However, the reasons of decreased SCF content and the exact fate of ICCs in gallstone diseases are still unclear, and further investigations are awaited to clarify these questions.
In summary, our study reconfirmed that the SCF/c-kit signal pathway is critically associated with the control of the development of ICCs, survival, maintenance of phenotype and function, and suggested that the decreased SCF/c-kit signaling pathway may be involved in the depletion of ICCs in gallstone diseases.
Disclosure of conflict of interest
None.
References
- 1.Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39:157–169. doi: 10.1016/j.gtc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Tan YY, Zhao G, Wang D, Wang JM, Tang JR, Ji ZL. A new strategy of minimally invasive surgery for cholecystolithiasis: calculi removal and gallbladder preservation. Dig Surg. 2013;30:466–471. doi: 10.1159/000357823. [DOI] [PubMed] [Google Scholar]
- 3.Matyja A, Gil K, Pasternak A, Sztefko K, Gajda M, Tomaszewski KA, Matyja M, Walocha JA, Kulig J, Thor P. Telocytes: new insight into the pathogenesis of gallstone disease. J Cell Mol Med. 2013;17:734–742. doi: 10.1111/jcmm.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–239. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann AF, Amelsberg A, vanSonnenberg E. Pathogenesis and treatment of gallstones. N Engl J Med. 1993;328:1854–1855. [PubMed] [Google Scholar]
- 6.Portincasa P, Di Ciaula A, Vendemiale G, Palmieri V, Moschetta A, Vanberge-Henegouwen GP, Palasciano G. Gallbladder motility and cholesterol crystallization in bile from patients with pigment and cholesterol gallstones. Eur J Clin Invest. 2000;30:317–324. doi: 10.1046/j.1365-2362.2000.00639.x. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Lopez P, Garcia-Marin V, Martinez-Murillo R, Freire M. Updating old ideas and recent advances regarding the Interstitial Cells of Cajal. Brain Res Rev. 2009;61:154–169. doi: 10.1016/j.brainresrev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Huizinga JD, Chen JH. Interstitial cells of Cajal: update on basic and clinical science. Curr Gastroenterol Rep. 2014;16:363. doi: 10.1007/s11894-013-0363-z. [DOI] [PubMed] [Google Scholar]
- 9.Sanders KM. Interstitial cells of Cajal at the clinical and scientific interface. J Physiol. 2006;576:683–687. doi: 10.1113/jphysiol.2006.116814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torihashi S, Ward SM, Sanders KM. Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997;112:144–155. doi: 10.1016/s0016-5085(97)70229-4. [DOI] [PubMed] [Google Scholar]
- 11.Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, Cordon-Cardo C. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994;42:1417–1425. doi: 10.1177/42.11.7523489. [DOI] [PubMed] [Google Scholar]
- 12.Jain D, Moussa K, Tandon M, Culpepper-Morgan J, Proctor DD. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003;98:618–624. doi: 10.1111/j.1572-0241.2003.07295.x. [DOI] [PubMed] [Google Scholar]
- 13.Tong WD, Liu BH, Zhang LY, Xiong RP, Liu P, Zhang SB. Expression of c-kit messenger ribonucleic acid and c-kit protein in sigmoid colon of patients with slow transit constipation. Int J Colorectal Dis. 2005;20:363–367. doi: 10.1007/s00384-004-0679-0. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZH, Zhang YC, Jiang WF, Yang C, Zou GM, Kong Y, Cai W. Characterization of interstitial Cajal progenitors cells and their changes in Hirschsprung’s disease. PLoS One. 2014;9:e86100. doi: 10.1371/journal.pone.0086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiyohara T, Shinomura Y, Isozaki K, Nakahara M, Tsutsui S, Nishibayashi H, Miyazaki Y, Miyagawa J, Matsuzawa Y. A decreased number of c- kit-expressing cells in a patient with afferent loop syndrome. J Gastroenterol. 2003;38:390–394. doi: 10.1007/s005350300069. [DOI] [PubMed] [Google Scholar]
- 16.Pasternak A, Gil K, Matyja A, Gajda M, Sztefko K, Walocha JA, Kulig J, Thor P. Loss of gallbladder interstitial Cajal-like cells in patients with cholelithiasis. Neurogastroenterol Motil. 2013;25:17–24. doi: 10.1111/nmo.12037. [DOI] [PubMed] [Google Scholar]
- 17.Tong W, Jia H, Zhang L, Li C, Ridolfi TJ, Liu B. Exogenous stem cell factor improves interstitial cells of Cajal restoration after blockade of c-kit signaling pathway. Scand J Gastroenterol. 2010;45:844–851. doi: 10.3109/00365521003782371. [DOI] [PubMed] [Google Scholar]
- 18.Ro S, Park C, Jin J, Zheng H, Blair PJ, Redelman D, Ward SM, Yan W, Sanders KM. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterology. 2010;138:1068–1078. doi: 10.1053/j.gastro.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Du L, Xiao YT, Cai W. Disruption of interstitial cells of Cajal networks after massive small bowel resection. World J Gastroenterol. 2013;19:3415–3422. doi: 10.3748/wjg.v19.i22.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sari R, Balci MK, Coban E, Karayalcin U. Sonographic evaluation of gallbladder volume and ejection fraction in obese women without gallstones. J Clin Ultrasound. 2003;31:352–357. doi: 10.1002/jcu.10191. [DOI] [PubMed] [Google Scholar]
- 21.Dodds WJ, Groh WJ, Darweesh RM, Lawson TL, Kishk SM, Kern MK. Sonographic measurement of gallbladder volume. AJR Am J Roentgenol. 1985;145:1009–1011. doi: 10.2214/ajr.145.5.1009. [DOI] [PubMed] [Google Scholar]
- 22.Reshetnyak VI. Concept of the pathogenesis and treatment of cholelithiasis. World J Hepatol. 2012;4:18–34. doi: 10.4254/wjh.v4.i2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pauletzki J, Cicala M, Holl J, Sauerbruch T, Schafmayer A, Paumgartner G. Correlation between gall bladder fasting volume and postprandial emptying in patients with gall stones and healthy controls. Gut. 1993;34:1443–1447. doi: 10.1136/gut.34.10.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portincasa P, Di Ciaula A, Baldassarre G, Palmieri V, Gentile A, Cimmino A, Palasciano G. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function and gallbladder wall inflammation. J Hepatol. 1994;21:430–440. doi: 10.1016/s0168-8278(05)80324-1. [DOI] [PubMed] [Google Scholar]
- 25.Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasternak A, Gajda M, Gil K, Matyja A, Tomaszewski KA, Walocha JA, Kulig J, Thor P. Evidence of interstitial Cajal-like cells in human gallbladder. Folia Histochem Cytobiol. 2012;50:581–585. doi: 10.5603/19673. [DOI] [PubMed] [Google Scholar]
- 27.Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, Allescher HD, Vanderwinden JM, Hofmann F, Schemann M, Rad R, Storr MA, Schmid RM, Schneider G, Saur D. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat Commun. 2013;4:1630. doi: 10.1038/ncomms2626. [DOI] [PubMed] [Google Scholar]
- 28.Hinescu ME, Ardeleanu C, Gherghiceanu M, Popescu LM. Interstitial Cajal-like cells in human gallbladder. J Mol Histol. 2007;38:275–284. doi: 10.1007/s10735-007-9099-0. [DOI] [PubMed] [Google Scholar]
- 29.Ahmadi O, Nicholson Mde L, Gould ML, Mitchell A, Stringer MD. Interstitial cells of Cajal are present in human extrahepatic bile ducts. J Gastroenterol Hepatol. 2010;25:277–285. doi: 10.1111/j.1440-1746.2009.05980.x. [DOI] [PubMed] [Google Scholar]
- 30.Lavoie B, Balemba OB, Nelson MT, Ward SM, Mawe GM. Morphological and physiological evidence for interstitial cell of Cajal-like cells in the guinea pig gallbladder. J Physiol. 2007;579:487–501. doi: 10.1113/jphysiol.2006.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu D, Yu BP, Luo HS, Chen LD. Control of gallbladder contractions by cholecystokinin through cholecystokinin-A receptors on gallbladder interstitial cells of Cajal. World J Gastroenterol. 2008;14:2882–2887. doi: 10.3748/wjg.14.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 33.Hu B, Colletti LM. Stem cell factor and c-kit are involved in hepatic recovery after acetaminophen-induced liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:45–53. doi: 10.1152/ajpgi.00024.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stankov K, Popovic S, Mikov M. C-KIT signaling in cancer treatment. Curr Pharm Des. 2014;20:2849–2880. doi: 10.2174/13816128113199990593. [DOI] [PubMed] [Google Scholar]
- 35.Mukhopadhyay A, Do DV, Ong CT, Khoo YT, Masilamani J, Chan SY, Vincent AS, Wong PK, Lim CP, Cao X, Lim IJ, Phan TT. The role of stem cell factor and c-KIT in keloid pathogenesis: do tyrosine kinase inhibitors have a potential therapeutic role? Br J Dermatol. 2011;164:372–386. doi: 10.1111/j.1365-2133.2010.10035.x. [DOI] [PubMed] [Google Scholar]
- 36.Fan Y, Wu SD, Fu BB, Weng C, Wang XP. Decreased number of interstitial cells of Cajal play an important role in the declined intestinal transit during cholesterol gallstone formation in guinea pigs fed on high cholesterol diet. Int J Clin Exp Med. 2014;7:1262–1268. [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T, Watabe K, Nakahara M, Ogiyama H, Kiyohara T, Tsutsui S, Tamura S, Shinomura Y, Hayashi N. Disturbed gastrointestinal motility and decreased interstitial cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol. 2008;23:660–667. doi: 10.1111/j.1440-1746.2008.05326.x. [DOI] [PubMed] [Google Scholar]
- 38.Horvath VJ, Vittal H, Lorincz A, Chen H, Almeida-Porada G, Redelman D, Ordog T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 39.Wu JJ, Rothman TP, Gershon MD. Development of the interstitial cell of Cajal: origin, kit dependence and neuronal and nonneuronal sources of kit ligand. J Neurosci Res. 2000;59:384–401. doi: 10.1002/(SICI)1097-4547(20000201)59:3<384::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 41.Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:G602–611. doi: 10.1152/ajpgi.2001.281.3.G602. [DOI] [PubMed] [Google Scholar]


