Abstract
Epidemiologic studies that investigate whether vitamin C and E intake protects against bladder cancer have yielded inconsistent results. We conducted a systematic review and meta-analysis of published cohort and case-control studies to summarize the epidemiologic evidence investigating vitamin C and E intake and bladder cancer. Studies were identified through a search of PubMed and Embase databases and of references from relevant publications. Meta-analyses were conducted to estimate summary risk estimates (REs) and 95% confidence intervals (CIs) for vitamin C and E intake using fixed- or random-effects model depending on the heterogeneity of the studies. Subgroup analyses were performed according to study design, sex, geographical regions and source of vitamins intake. The summary REs of bladder cancer for all published studies was 0.90 (95% CI, 0.79-1.00) and 0.82 (95% CI, 0.72-0.90) for vitamin C and E intake, respectively, with no evidence of between-study heterogeneity for vitamin E, but some heterogeneity for vitamin C intake. Although some of the summary effects were non-significant, subgroup analyses showed that these inverse relationships were not modified by study design, sex, geographical regions and source of vitamins intake for vitamin E intake. Our results indicated that high intake of vitamin E could reduce bladder cancer risk. However, the inverse association between vitamin C and bladder cancer seemed to be limited. Further studies using larger samples and a rigorous methodology are warranted.
Keywords: Antioxidants, urinary bladder neoplasms, meta-analysis, vitamin C, vitamin E
Introduction
Bladder cancer ranks as the seventh common cancer among men with an estimated 386,300 new cases and 150,200 deaths worldly in 2008 worldwide [1]. It has the highest lifetime treatment costs per patient of all cancers due to its high recurrence rate, long-term survival rate, and costs associated with disease surveillance and treatment [2,3]. Thus, identifying risk factors of bladder cancer could potentially be life-saving, and reduce the health economic burden. Environmental factors, particularly dietary factors, have been postulated to play important roles in the etiology of bladder cancer [4]. Some epidemiologic studies have suggested that vegetable and fruit consumption may protect against bladder cancer [5-8]. There are many candidate agents in fruits and vegetables that influence bladder cancer risk, including vitamin C, vitamin E, carotenoids and folate. A number of epidemiological studies have investigated the relationships between vitamins C and E, both antioxidants, and the risk of bladder cancer, but the results are conflicting. In the present study, we assessed the influence of the intake of vitamin C and E on bladder cancer risk by meta-analysis of published epidemiological studies.
Materials and methods
Search strategy
This systematic review was planned, conducted, and reported in adherence to the standards of quality for reporting meta-analyses [9]. We conducted a literature search using PubMed and Embase databases to April 2014 with the following keywords: (“vitamin C” or “vitamin E” or “ascorbic acid” or tocopherol) and (“bladder cancer” or “urothelial cancer” or “urinary tract cancer” or “urinary bladder neoplasms” [MeSH Terms]). Additional articles were obtained from the reference lists of the selected articles and reviews.
Selection criteria
Studies were only included if they met the following criteria: (1) had a case-control or prospective study design; (2) reported results on vitamin C or vitamin E intake; (3) the outcome was bladder cancer or urothelial cancer incidence; (4) Sufficient information was provided to estimate the relative risk (RR) or odds ratio (OR) and 95% confidence intervals (95% CI) adjusted for at least age, sex, and smoking. Papers were restricted to human studies published in English. For articles with same population resources or overlapping datasets, the largest or most recent one was included.
Data extraction
The following information were collected: the first author, year of publication, country in which the study was performed, study design, years of follow-up or the study period, study participants sex and age range, number of subjects and cases, anatomical site of the neoplasm, research contents, study quality, and adjusted covariates. If a study provided several risk estimates (REs), the most completely adjusted estimate was extracted. If results were reported for both dietary and total vitamins intake (foods and supplements combined), we used the results for total vitamins in the main analysis. Data extraction was conducted independently by two authors (Y.Y.W and X.L.W) using a predefined data collection form. The discrepancies were resolved by consensus.
Study quality assessment
The study quality was assessed using the 9-star Newcastle-Ottawa Scale, a validated technique for assessing the quality of non-randomized studies in meta-analyses. (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). A quality score was calculated based on three major components: selection of the groups of study, comparability, and assessment of the outcome or exposure. The maximum score could be 9 points, representing the highest methodological quality. We assigned scores of < 7 and ≥ 7 for low- and high-quality studies, respectively.
Statistical analysis
We estimated a summary RE with 95% CI based on fixed- or random-effects model depending on the heterogeneity of the analysis. Statistical heterogeneity among studies was evaluated by using the Q [10] and I2 statistics [11]. In the sensitivity analysis, one study at a time was removed and the rest analyzed to evaluate whether the results could have been affected significantly by a single study. We also conducted subgroup analyses according to some characteristics of the studies-sex (male, female), geographical area (European countries, United States, Japan), and source of vitamin intake (foods, supplements, foods and supplements combined). We assessed publication bias using the tests of Egger [12] and Begg [13]. The influence of potential publication bias on risk estimates was further evaluated by implementing the Duval and Tweedie nonparametric “trim-and-fill” method [14]. All statistical analyses were performed with Stata software, version 11 (Stata Corp, College Station, Texas). p < 0.05 was considered statistically significant.
Results
Figure 1 gives the flowchart for selection of articles. We identified a total of 570 articles (412 from Embase and 158 from Medline) by searching the databases. 549 articles were excluded by examining their titles and abstracts and 21 were given a more detailed assessment [5,15-34]. Additional 11 articles were retrieved from reference lists or other sources [35-45]. Of these 32 articles [5,15-45], 12 were excluded because 7 studies did not provide risk estimates or 95% CI of bladder cancer associated with vitamin C or E intake [27,29,33-36,39], 3 measured serum or plasma vitamins levels [20,22,24], 1 evaluated the mortality of bladder cancer [41], and 2 studies shared the same population [26,43]. Finally, we identified 20 studies that were eligible for inclusion in the meta-analysis [5,15-19,21,23,25,26,28,30-32,37,38,40,42,44,45]. The 20 included studies represented 9 cohort [15-17,19,25,26,28,30,42] and 11 case-control studies [5,18,21,23,31,32,37,38,40,44,45]. The study quality scores, assessed by the Newcastle-Ottawa Quality Assessment Scale, ranged from 5 to 8 (with a mean of 7.35). Details of the included studies are given in Table 1.
Figure 1.
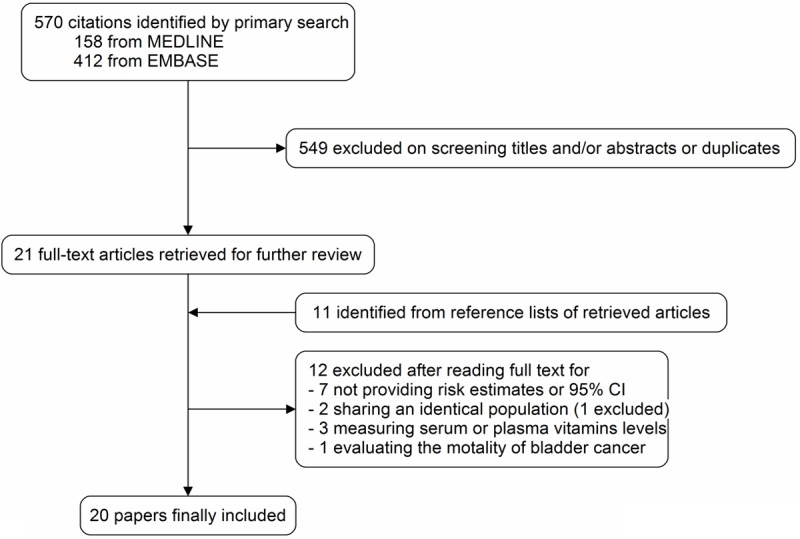
Flowchart of study identifying and including trials.
Table 1.
Study characteristics of published cohort and case-control studies on vitamin C and E and bladder cancer
| Authors and publication year | Study design | Country | Study period | Sex | Age | Cases/subjects | Anatomical site | Parameters examined | Study quality* | Variables of adjustment |
|---|---|---|---|---|---|---|---|---|---|---|
| Risch et al. 1988 | PCC | Canada | 1977-1982 | M/W | 35-79 | 826/1618 | Bladder | Vitamin C: diet | 7 | Age, sex, area of residence, smoking, and history of diabetes |
| Steineck et al. 1990 | PCC | Sweden | 1985-1987 | M/W | 40-74 | 418/929 | Urothelium | Vitamin C: diet and supplement | 7 | Age, sex, and smoking |
| Nomura et al. 1991 | PCC | USA | 1977-1986 | M/W | 30-93 | 261/783 | Urothelium | Vitamin C: diet, supplement and total | 8 | Age, sex, ethics, and smoking |
| Riboli et al. 1991 | PCC | Spain | 1985-1986 | M | < 80 | 432/1224 | Bladder | Vitamin C: diet Vitamin E: diet | 8 | Age, sex, smoking, and total calories |
| Shibata et al. 1992 | Cohort | USA | 1981-1989 | M | 65-84 | 71/70159 | Bladder | Vitamin C: diet and supplement Vitamin E: supplement | 5 | Age and smoking |
| Bruemmer et al. 996 | PCC | USA | 1981-1984 | M/W | 45-65 | 262/667 | Bladder | Vitamin C: diet, supplement and total Vitamin E: diet, supplement and total | 8 | Age, sex, county, smoking, and calories |
| Michand et al. 2000 | Cohort | USA | 1986-1998 | M | 40-75 | 320/51529 | Bladder | Vitamin C: supplement and total Vitamin E: supplement and total | 7 | Age, energy, pack-years of smoking history, current smoking status, geographic region of the United States, cruciferous vegetable intake, and total fluid intake |
| Wakai et al. 2000 | HCC | Japan | 1996-1999 | M/W | 20-99 | 297/692 | Bladder | Vitamin C: diet and total Vitamin E: diet and total | 6 | Age, sex, and smoking and occupational history as a cook |
| Zeegers et al. 2001 | Cohort | Netherlands | 1986-1992 | M/W | 55-69 | 569/3692 | Urinary tract | Vitamin C: total Vitamin E: total | 8 | Age, sex, cigarette smoking amount and cigarette smoking duration |
| Michand et al. 2002 | Cohort | Finland | 1985-1998 | M | 50-69 | 344/27111 | Bladder | Vitamin C: diet Vitamin E: diet | 7 | Age, duration of smoking, smoking dose, total energy, and trial intervention |
| Castelao et al. 2004 | PCC | USA | 1987-1996 | M/W | 25-64 | 1592/3184 | Bladder | Vitamin C: diet | 8 | Age, sex, education, number of cigarettes smoked per day, number of years of smoking, smoking status, lifetime use of nonsteroidal anti-inflammatory drugs, and number of years employed as a hairdresser/barber |
| Holick et al. 2005 | Cohort | USA | 1980-2000 | W | 30-55 | 237/88796 | Bladder | Vitamin C: total Vitamin E: total | 7 | Age, pack-years of cigarette smoking, current smoking, and total caloric intake |
| Kellen et al. 2006 | PCC | Belgium | 1999-2004 | M/W | Not mentioned | 178/540 | Bladder | Vitamin C: diet Vitamin E: diet | 7 | Sex, age, smoking status, number of cigarettes smoked per day, number of years smoking and occupational exposure to PAH or aromatic amines, total fruit and vegetable consumption, intake of vitamin E and C and TAS |
| Garcı´a-Closas et al. 2007 | HCC | Spain | 1998-2001 | M/W | Not mentioned | 912/1789 | Bladder | Vitamin C: diet Vitamin E: diet | 6 | Age, gender, region, smoking status and duration of smoking |
| Roswall et al. 2009 | Cohort | Denmark | 1993-2006 | M/W | 50-64 | 322/55557 | Urothelium | Vitamin C: diet, supplement and total Vitamin E: diet, supplement and total | 8 | Age, intake of vitamin C, vitamin E, and beta-carotene, smoking status, smoking duration, smoking intensity, passive smoking, and work exposure |
| Brinkman et al. 2010 | PCC | USA | 1997-2001 | M/W | 25-74 | 322/561 | Bladder | Vitamin C: total Vitamin E: total | 8 | Age, sex, smoking status, and total energy intake |
| Hotaling et al. 2011 | Cohort | USA | 2000-2007 | M/W | 50-76 | 330/77050 | Bladder | Vitamin C: supplement Vitamin E: supplement | 8 | Age, sex, race, education, family history of bladder cancer, smoking status/recency of smoking, pack-years of smoking, servings per day of fruits, and servings per day of vegetables |
| Wu et al. 2012 | PCC | USA | 2001-2004 | M/W | 30-79 | 1418/2589 | Bladder | Vitamin C: diet Vitamin E: diet | 8 | Age, gender, region, race, Hispanic status, smoking status, usual BMI, and total energy |
| Ros et al. 2012 | Cohort (nested) | Eurpoe | 1990-2005 | M/W | 25-70 | 856/1712 | Urothelium | Vitamin C: diet | 8 | Age at blood collection, study center, sex, date of blood collection, time of blood collection, and fasting status and further adjusted for smoking status, duration, and intensity |
| Park et al. 2013 | Cohort | USA | 1993-2007 | M/W | 45-75 | 581/185885 | Bladder | Vitamin C: diet Vitamin E: diet | 8 | Age, ethnicity, total energy intake, family history, employment in a high-risk industry, smoking, number of years since quitting, interactions of ethnicity with status |
FFQ, food-frequency questionnaire; HCC, hospital-based case-control study; PCC, population-based case-control study;
evaluated by 9-star Newcastle-Ottawa Scale.
As shown in Figure 2A, we observed a significantly borderline reduced risk of bladder cancer in a random-effects model for subjects with high vitamin C intake (RE = 0.90; 95% CI, 0.79-1.00). There was statistically significant heterogeneity among these studies (p = 0.02, I2 = 43.7%), but no evidence of significant publication bias either with test of Egger (p = 0.064) or Begg’s funnel plot (Figure 3A, p = 0.085). In analysis stratified by study design, the summary RE with 95% CI was 0.85 (95% CI, 0.69-1.00) and 0.94 (95% CI, 0.81-1.08) in case-control and cohort studies, respectively. There was statistically significant heterogeneity among the case-control studies (p = 0.012, I2 = 56.0%), but no heterogeneity in the cohort studies (p = 0.294, I2 = 16.6%). In the sensitivity analysis, after excluding the study by Bruemmer et al [5], which reported the lowest OR, no heterogeneity was detected across studies (p = 0.162 for case-control studies, p = 0.199 for total studies), while the summary RE became non-significant for both total studies (RE = 0.92; 95% CI, 0.83-1.01) and case-control studies (RE = 0.93; 95% CI, 0.77-1.03). We next pooled the REs by sex, geographical region, and source of vitamin C intake (Table 2). The REs showed vitamin C intake was generally associated with a decreased risk of bladder cancer, although most of the results were non-significant. When subgroup analysis was conducted by geographical regions, a statistical significant protective effect of vitamin C intake on bladder cancer was observed in US (RE = 0.85; 95% CI, 0.76-0.95), but not in Europe (RE = 0.93; 95% CI, 0.81-1.06) and Japan (RE = 0.81; 95% CI, 0.49-1.35). Also, a significant decreased association was observed among women (RE = 0.61; 95% CI, 0.25-0.97), but not in men (RE = 0.86; 95% CI, 0.71-1.01). In subgroup analysis by source of vitamin C intake, we observed a significantly decreased risk of bladder cancer in subjects with supplementary intake, but not with dietary or dietary plus supplementary intake.
Figure 2.
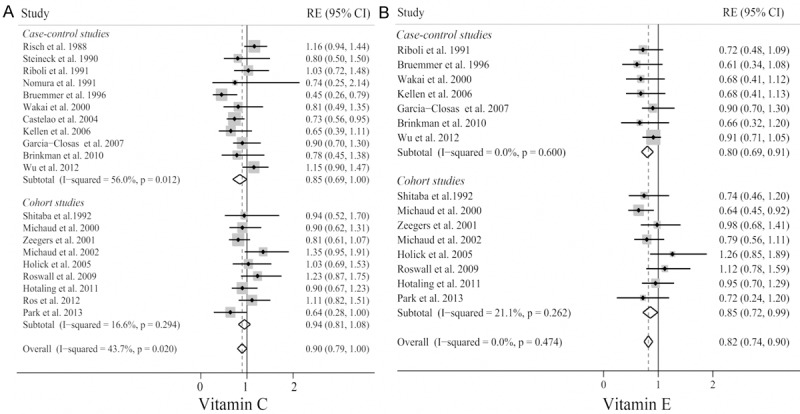
Forest plot of the highest compared with the lowest categories of intake of vitamin C (A) and E (B) bladder cancer risk.
Figure 3.
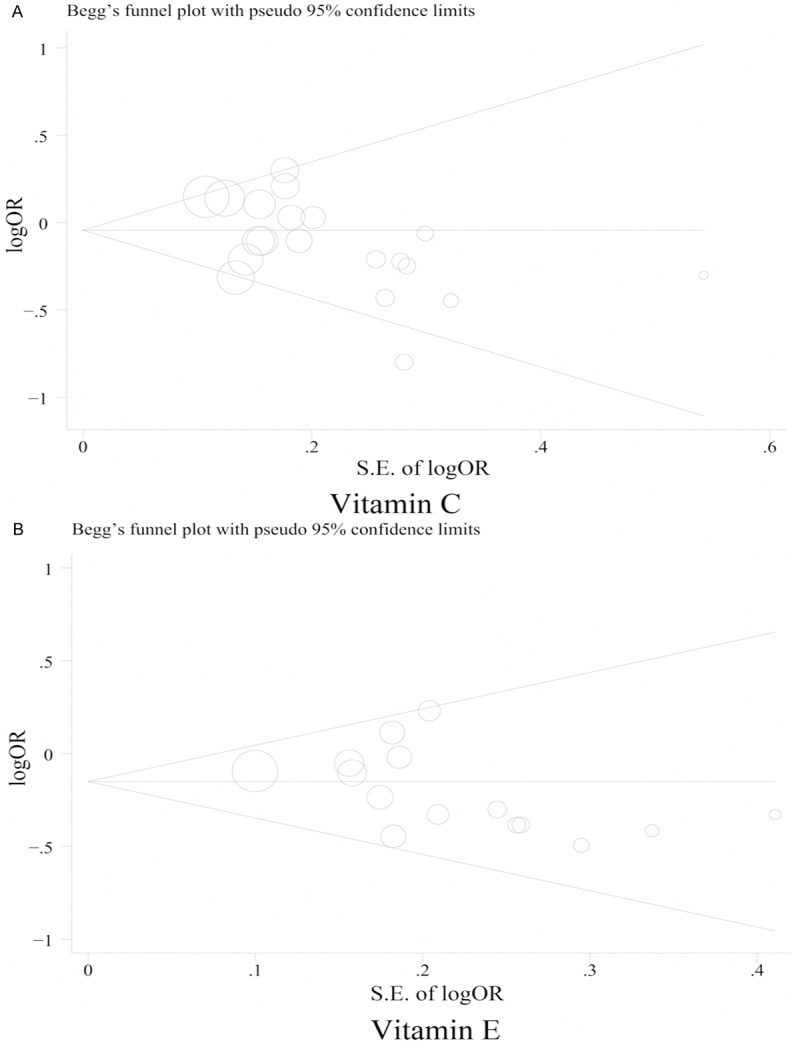
Funnel plot of vitamin C (A) and E (B) intake and bladder cancer risk.
Table 2.
Summary of pooled risk ratios of bladder cancer by study design, sex, geographical region, and source of intake
| Subgroup | Number of studies | Pooled RR (95% CI) | Q-test for heterogeneity p value (I2 score) | Egger’s test p value | Begg’s test p value |
|---|---|---|---|---|---|
| Vitamin C | 20 | 0.90 (0.79, 1.00) | 0.02 (43.7%) | 0.085 | 0.064 |
| Cohort studies | 9 | 0.94 (0.81, 1.08) | 0.294 (16.6%) | 0.67 | 1 |
| Case-control studies | 11 | 0.85 (0.69, 1.00) | 0.012 (56.0%) | 0.041 | 0.087 |
| European studies | 8 | 0.93 (0.81, 1.06) | 0.206 (27.8%) | 0.541 | 0.711 |
| US studies | 11 | 0.85 (0.76, 0.95) | 0.011 (56.2%) | 0. 213 | 0.07 |
| Japanese study | 1 | 0.81 (0.49, 1.35) | |||
| Men | 6 | 0.86 (0.71, 1.01) | 0.565 (0) | 0.847 | 1 |
| Women | 3 | 0.61 (0.25, 0.97) | 0.026 (49.6%) | 0.67 | 1 |
| Diet | 11 | 0.89 (0.75, 1.02) | < 0.001 (83.2%) | 0.117 | 0.086 |
| Supplement | 7 | 0.74 (0.54, 0.93) | 0.041 (54.3%) | 0.423 | 0.368 |
| Diet plus supplement | 8 | 0.82 (0.65, 1.00) | 0.015 (41.0%) | 0.641 | 0.536 |
| Vitamin E | 15 | 0.82 (0.74, 0.90) | 0.474 (0) | 0.534 | 0.138 |
| Cohort studies | 7 | 0.85 (0.72, 0.99) | 0.262 (21.1%) | 0.667 | 1 |
| Case-control studies | 7 | 0.80 (0.69, 0.91) | 0.6 (0) | 0.108 | 0.133 |
| European studies | 6 | 0.84 (0.71, 0.98) | 0.545 (0) | 0.272 | 0.260 |
| US studies | 8 | 0.82 (0.71, 0.92) | 0.259 (21.5%) | 0.280 | 0.536 |
| Japanese study | 1 | 0.68 (0.41, 1.12) | |||
| Men | 5 | 0.74 (0.60, 0.88) | 0.36 (8.1%) | 0.309 | 0.806 |
| Women | 2 | 0.83 (0.07, 1.06) | 0.008 (85.9%) | ||
| Diet | 7 | 0.78 (0.65, 0.90) | 0.583 (0) | 0.0321 | 0.0161 |
| Supplement | 5 | 0.79 (0.60, 0.97) | 0.251 (25.6%) | 0.166 | 0.221 |
| Diet plus supplement | 7 | 0.81 (0.63, 0.99) | 0.129 (39.4%) | 0.272 | 0.764 |
Abbreviations: CI, confidence interval; US, United States.
Trim and fill method was used to recalculate the summary RE and the result did not changed, suggesting the stability of the analysis.
A forest plot summarizing study-specific and summary associations between vitamin E and bladder cancer is illustrated in Figure 2B. The summary RE for vitamin E and bladder cancer for all published studies combined was 0.82 (95% CI, 0.74-0.90), with no evidence of between-study heterogeneity (p = 0.474) or publication bias (p Egger’s = 0.534, p Begg’s = 0.138, Figure 3B). The summary effect was slightly weaker, although still significant, for cohort findings (RE = 0.85; 95% CI, 0.72-0.99; p = 0.262) compared with case-control findings (RE = 0.80; 95% CI, 0.69-0.91; p = 0.6). Furthermore, the summary effect did not significantly change, as shown in Table 2, when we conducted separate meta-analyses by sex, geographical regions, and source of vitamin E intake. All analyses showed significant inverse associations except for women and Japanese subgroups.
Discussion
Previous meta-analysis conducted by Myung et al. [46] have demonstrate that vitamin C and E intake have a protective effect against cervical neoplasm. Consistently, Xu et al’s [47] meta-analysis suggested that there was an inverse association between vitamin C intake and risk of colorectal adenoma, the precursor of colorectal cancer. The present meta-analysis is the most up-to-date comprehensive review of vitamin C and E on bladder cancer. It included 20 observational studies and reported data of 7,693 bladder cancer cases. Our results suggested that vitamin C and E intake were statistically significantly associated with reduced risk of bladder cancer. To our knowledge, this is the first meta-analysis assessing the association between vitamin C and E intake and risk of bladder cancer.
However, the protective association between high vitamin C intake and bladder cancer risk was limited by borderline risk estimate and the cohort studies without significance, although the meta-analysis from case-control studies suggested an inverse association. Furthermore, we detected a significant heterogeneity among total studies and case-control studies but not in cohort studies. Therefore, a random-effects model was chosen over a fixed-effects model to determine the pooled risk estimates. Sensitivity analysis indicated that the study by Bruemmer et al. [5] contributed most to the variability among all studies, while other studies demonstrated a statistical homogeneity (p = 0.199). However, omission of this study altered the significance of observed protective effect. The inverse association was more evident between vitamin E intake and bladder cancer risk. No heterogeneity were detected across studies. Also, our subgroup analyses show that vitamin E intake is consistently associated with reduced bladder cancer risk, irrespective of study design, sex, study location, and source of vitamin E intake, which further confirmed the reliability of the results. The lack of significant findings for Japanese and women studies are likely due to statistical power limitations given the small number of studies included for analysis.
Several potential mechanisms could explain the association between vitamin C and E intake and the risk of bladder cancer. Vitamin C is considered to be one of the most prevalent antioxidative components in fruits and vegetables. It has generally been acknowledged that vitamin C prevents DNA damage by neutralizing free radicals and oxidants, thereby blocking the initiation of carcinogenesis [48]. Vitamin C was also shown to reduce inflammation, independently of its antioxidant activity, and slow the progression of gastric cancer [49]. However, Lee et al. [50] reported that a moderate daily dose of supplementary vitamin C induces the formation of genotoxins from lipid hydroperoxides, thereby resulting in DNA damage and initiation of carcinogenesis. Vitamin E has intracellular antioxidant properties [51]. α-tocopherol, the predominant and most active form of vitamin E in humans, can also enhance immune response, modulate gene expression, and inhibit cell proliferation, and cell adhesion [52,53]. α-tocopherol supplementation has been found to be significantly associated with lower incidence of prostate cancer, and higher serum α-tocopherol level was associated with a reduced risk of prostate cancer [54,55].
The main strength of the present meta-analysis lies in inclusion of all the epidemiological studies currently available, reporting data of 7,693 bladder cancer cases. However, several limitations of our review must be acknowledged. First, because our analyses were based on observational studies, we were not able to solve problems with confounding factors that could be inherent in the included studies, which may bias the results. For instance, although the analyses were all adjusted by smoking, confounding from smoking may not be completely removed. Castelao et al. [23] observed that the protective effect of vitamin C on bladder cancer was confined largely to ever smokers and were stronger in current than ex-smokers, but no such effect on never smokers. Liang et al. [20] also found that the inverse association between plasma a-tocopherol level and bladder cancer risk was more evident in ever smokers and heavy smokers, suggesting that high antioxidant content of vitamin C or E may reduce the oxidative damage caused by cigarette smoking, and smokers may benefit more from vitamin C or E intake than nonsmokers. However, the limited number of studies on separate analyses for smoking prevented us from performing meta-analyses. Second, although we conducted a comprehensive systematic literature search in selected databases, the imbalance among the location of the included articles still existed. All studies were conducted in developed countries, and whether the inverse relationship applies to low-income areas with a nutritional deficiency in vitamin C and E intake is unknown. Furthermore, the present meta-analyses included only one studies conducted in Asia [40], where people consume a lot of vegetables and fruits rich in vitamins. Third, the summary estimates from several subgroup meta-analyses are based on a small number of studies, which might limit the statistical effect of the results, and should be interpreted with caution. Finally, in this meta-analysis of published studies, publication bias could be of concern because we only included studies written in English, and small studies with null results tend not to be published, though we found no evidence of publication bias.
In conclusion, our findings from this meta-analysis suggest high intake of vitamin E was associated with a decreased risk of bladder cancer, and the inverse relation between vitamin C and bladder cancer risk seemed to be limited because of heterogeneity between studies. Although the reduced risk is moderate in size, our results have important clinical and public health significance given that vitamin C and E are two most commonly used vitamins around the world. More large prospective studies are needed to confirm these associations in the future.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 3.Sievert KD, Amend B, Nagele U, Schilling D, Bedke J, Horstmann M, Hennenlotter J, Kruck S, Stenzl A. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009;27:295–300. doi: 10.1007/s00345-009-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeegers MP, Kellen E, Buntinx F, van den Brandt PA. The association between smoking, beverage consumption, diet and bladder cancer: a systematic literature review. World J Urol. 2004;21:392–401. doi: 10.1007/s00345-003-0382-8. [DOI] [PubMed] [Google Scholar]
- 5.Bruemmer B, White E, Vaughan TL, Cheney CL. Nutrient intake in relation to bladder cancer among middle-aged men and women. Am J Epidemiol. 1996;144:485–495. doi: 10.1093/oxfordjournals.aje.a008955. [DOI] [PubMed] [Google Scholar]
- 6.D’Avanzo B, La Vecchia C, Negri E, Decarli A, Benichou J. Attributable risks for bladder cancer in northern Italy. Ann Epidemiol. 1995;5:427–431. doi: 10.1016/1047-2797(95)00057-7. [DOI] [PubMed] [Google Scholar]
- 7.Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. 1999;91:605–613. doi: 10.1093/jnci/91.7.605. [DOI] [PubMed] [Google Scholar]
- 8.Chyou PH, Nomura AM, Stemmermann GN. A prospective study of diet, smoking, and lower urinary tract cancer. Ann Epidemiol. 1993;3:211–216. doi: 10.1016/1047-2797(93)90021-u. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 14.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 15.Park SY, Ollberding NJ, Woolcott CG, Wilkens LR, Henderson BE, Kolonel LN. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J Nutr. 2013;143:1283–1292. doi: 10.3945/jn.113.174920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ros MM, Bueno-de-Mesquita HB, Kampman E, Aben KK, Buchner FL, Jansen EH, van Gils CH, Egevad L, Overvad K, Tjonneland A, Roswall N, Boutron-Ruault MC, Kvaskoff M, Perquier F, Kaaks R, Chang-Claude J, Weikert S, Boeing H, Trichopoulou A, Lagiou P, Dilis V, Palli D, Pala V, Sacerdote C, Tumino R, Panico S, Peeters PH, Gram IT, Skeie G, Huerta JM, Barricarte A, Quiros JR, Sanchez MJ, Buckland G, Larranaga N, Ehrnstrom R, Wallstrom P, Ljungberg B, Hallmans G, Key TJ, Allen NE, Khaw KT, Wareham N, Brennan P, Riboli E, Kiemeney LA. Plasma carotenoids and vitamin C concentrations and risk of urothelial cell carcinoma in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2012;96:902–910. doi: 10.3945/ajcn.111.032920. [DOI] [PubMed] [Google Scholar]
- 17.Hotaling JM, Wright JL, Pocobelli G, Bhatti P, Porter MP, White E. Long-term use of supplemental vitamins and minerals does not reduce the risk of urothelial cell carcinoma of the bladder in the VITamins And Lifestyle study. J Urol. 2011;185:1210–1215. doi: 10.1016/j.juro.2010.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkman MT, Karagas MR, Zens MS, Schned A, Reulen RC, Zeegers MP. Minerals and vitamins and the risk of bladder cancer: results from the New Hampshire Study. Cancer Causes Control. 2010;21:609–619. doi: 10.1007/s10552-009-9490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K, Tjonneland A. Micronutrient intake and risk of urothelial carcinoma in a prospective Danish cohort. Eur Urol. 2009;56:764–770. doi: 10.1016/j.eururo.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Liang D, Lin J, Grossman HB, Ma J, Wei B, Dinney CP, Wu X. Plasma vitamins E and A and risk of bladder cancer: a case-control analysis. Cancer Causes Control. 2008;19:981–992. doi: 10.1007/s10552-008-9165-2. [DOI] [PubMed] [Google Scholar]
- 21.Kellen E, Zeegers M, Buntinx F. Selenium is inversely associated with bladder cancer risk: a report from the Belgian case-control study on bladder cancer. Int J Urol. 2006;13:1180–1184. doi: 10.1111/j.1442-2042.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 22.Ozasa K, Ito Y, Suzuki K, Watanabe Y, Hayashi K, Mikami K, Nakao M, Miki T, Mori M, Sakauchi F, Washio M, Kubo T, Wakai K, Tamakoshi A. Serum carotenoids and other antioxidative substances associated with urothelial cancer risk in a nested case-control study in Japanese men. J Urol. 2005;173:1502–1506. doi: 10.1097/01.ju.0000154614.58321.e6. [DOI] [PubMed] [Google Scholar]
- 23.Castelao JE, Yuan JM, Gago-Dominguez M, Skipper PL, Tannenbaum SR, Chan KK, Watson MA, Bell DA, Coetzee GA, Ross RK, Yu MC. Carotenoids/vitamin C and smoking-related bladder cancer. Int J Cancer. 2004;110:417–423. doi: 10.1002/ijc.20104. [DOI] [PubMed] [Google Scholar]
- 24.Nomura AM, Lee J, Stemmermann GN, Franke AA. Serum vitamins and the subsequent risk of bladder cancer. J Urol. 2003;170:1146–1150. doi: 10.1097/01.ju.0000086040.24795.ad. [DOI] [PubMed] [Google Scholar]
- 25.Michaud DS, Pietinen P, Taylor PR, Virtanen M, Virtamo J, Albanes D. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. Br J Cancer. 2002;87:960–965. doi: 10.1038/sj.bjc.6600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeegers MP, Goldbohm RA, van den Brandt PA. Are retinol, vitamin C, vitamin E, folate and carotenoids intake associated with bladder cancer risk? Results from the Netherlands Cohort Study. Br J Cancer. 2001;85:977–983. doi: 10.1054/bjoc.2001.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virtamo J, Edwards BK, Virtanen M, Taylor PR, Malila N, Albanes D, Huttunen JK, Hartman AM, Hietanen P, Maenpaa H, Koss L, Nordling S, Heinonen OP. Effects of supplemental alpha-tocopherol and beta-carotene on urinary tract cancer: incidence and mortality in a controlled trial (Finland) Cancer Causes Control. 2000;11:933–939. doi: 10.1023/a:1026546803917. [DOI] [PubMed] [Google Scholar]
- 28.Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci E. Prospective study of dietary supplements, macronutrients, micronutrients, and risk of bladder cancer in US men. Am J Epidemiol. 2000;152:1145–1153. doi: 10.1093/aje/152.12.1145. [DOI] [PubMed] [Google Scholar]
- 29.Vena JE, Graham S, Freudenheim J, Marshall J, Zielezny M, Swanson M, Sufrin G. Diet in the epidemiology of bladder cancer in western New York. Nutr Cancer. 1992;18:255–264. doi: 10.1080/01635589209514226. [DOI] [PubMed] [Google Scholar]
- 30.Shibata A, Paganini-Hill A, Ross RK, Henderson BE. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br J Cancer. 1992;66:673–679. doi: 10.1038/bjc.1992.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riboli E, Gonzalez CA, Lopez-Abente G, Errezola M, Izarzugaza I, Escolar A, Nebot M, Hemon B, Agudo A. Diet and bladder cancer in Spain: a multi-centre case-control study. Int J Cancer. 1991;49:214–219. doi: 10.1002/ijc.2910490212. [DOI] [PubMed] [Google Scholar]
- 32.Nomura AM, Kolonel LN, Hankin JH, Yoshizawa CN. Dietary factors in cancer of the lower urinary tract. Int J Cancer. 1991;48:199–205. doi: 10.1002/ijc.2910480208. [DOI] [PubMed] [Google Scholar]
- 33.Comstock GW, Helzlsouer KJ, Bush TL. Prediagnostic serum levels of carotenoids and vitamin E as related to subsequent cancer in Washington County, Maryland. Am J Clin Nutr. 1991;53:260S–264S. doi: 10.1093/ajcn/53.1.260S. [DOI] [PubMed] [Google Scholar]
- 34.Helzlsouer KJ, Comstock GW, Morris JS. Selenium, lycopene, alpha-tocopherol, beta-carotene, retinol, and subsequent bladder cancer. Cancer Res. 1989;49:6144–6148. [PubMed] [Google Scholar]
- 35.Nomura AM, Stemmermann GN, Heilbrun LK, Salkeld RM, Vuilleumier JP. Serum vitamin levels and the risk of cancer of specific sites in men of Japanese ancestry in Hawaii. Cancer Res. 1985;45:2369–2372. [PubMed] [Google Scholar]
- 36.Wald NJ, Thompson SG, Densem JW, Boreham J, Bailey A. Serum vitamin E and subsequent risk of cancer. Br J Cancer. 1987;56:69–72. doi: 10.1038/bjc.1987.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Risch HA, Burch JD, Miller AB, Hill GB, Steele R, Howe GR. Dietary factors and the incidence of cancer of the urinary bladder. Am J Epidemiol. 1988;127:1179–1191. doi: 10.1093/oxfordjournals.aje.a114911. [DOI] [PubMed] [Google Scholar]
- 38.Steineck G, Hagman U, Gerhardsson M, Norell SE. Vitamin A supplements, fried foods, fat and urothelial cancer. A case-referent study in Stockholm in 1985-87. Int J Cancer. 1990;45:1006–1011. doi: 10.1002/ijc.2910450604. [DOI] [PubMed] [Google Scholar]
- 39.Knekt P, Aromaa A, Maatela J, Alfthan G, Aaran RK, Nikkari T, Hakama M, Hakulinen T, Teppo L. Serum micronutrients and risk of cancers of low incidence in Finland. Am J Epidemiol. 1991;134:356–361. doi: 10.1093/oxfordjournals.aje.a116097. [DOI] [PubMed] [Google Scholar]
- 40.Wakai K, Takashi M, Okamura K, Yuba H, Suzuki K, Murase T, Obata K, Itoh H, Kato T, Kobayashi M, Sakata T, Otani T, Ohshima S, Ohno Y. Foods and nutrients in relation to bladder cancer risk: a case-control study in Aichi Prefecture, Central Japan. Nutr Cancer. 2000;38:13–22. doi: 10.1207/S15327914NC381_3. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs EJ, Henion AK, Briggs PJ, Connell CJ, McCullough ML, Jonas CR, Rodriguez C, Calle EE, Thun MJ. Vitamin C and vitamin E supplement use and bladder cancer mortality in a large cohort of US men and women. Am J Epidemiol. 2002;156:1002–1010. doi: 10.1093/aje/kwf147. [DOI] [PubMed] [Google Scholar]
- 42.Holick CN, De Vivo I, Feskanich D, Giovannucci E, Stampfer M, Michaud DS. Intake of fruits and vegetables, carotenoids, folate, and vitamins A, C, E and risk of bladder cancer among women (United States) Cancer Causes Control. 2005;16:1135–1145. doi: 10.1007/s10552-005-0337-z. [DOI] [PubMed] [Google Scholar]
- 43.Zeegers MP, Selen RF, Kleinjans JC, Goldbohm RA, van den Brandt PA. Nitrate intake does not influence bladder cancer risk: the Netherlands cohort study. Environ Health Perspect. 2006;114:1527–1531. doi: 10.1289/ehp.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Closas R, Garcia-Closas M, Kogevinas M, Malats N, Silverman D, Serra C, Tardon A, Carrato A, Castano-Vinyals G, Dosemeci M, Moore L, Rothman N, Sinha R. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur J Cancer. 2007;43:1731–1740. doi: 10.1016/j.ejca.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Wu JW, Cross AJ, Baris D, Ward MH, Karagas MR, Johnson A, Schwenn M, Cherala S, Colt JS, Cantor KP, Rothman N, Silverman DT, Sinha R. Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States. Br J Cancer. 2012;106:1891–1898. doi: 10.1038/bjc.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myung SK, Ju W, Kim SC, Kim H. Vitamin or antioxidant intake (or serum level) and risk of cervical neoplasm: a meta-analysis. BJOG. 2011;118:1285–1291. doi: 10.1111/j.1471-0528.2011.03032.x. [DOI] [PubMed] [Google Scholar]
- 47.Xu X, Yu E, Liu L, Zhang W, Wei X, Gao X, Song N, Fu C. Dietary intake of vitamins A, C, and E and the risk of colorectal adenoma: a meta-analysis of observational studies. Eur J Cancer Prev. 2013;22:529–539. doi: 10.1097/CEJ.0b013e328364f1eb. [DOI] [PubMed] [Google Scholar]
- 48.Stebbing J, Hart CA. Antioxidants and cancer. Lancet Oncol. 2011;12:996. doi: 10.1016/S1470-2045(11)70282-0. [DOI] [PubMed] [Google Scholar]
- 49.Feiz HR, Mobarhan S. Does vitamin C intake slow the progression of gastric cancer in Helicobacter pylori-infected populations? Nutr Rev. 2002;60:34–36. doi: 10.1301/002966402760240345. [DOI] [PubMed] [Google Scholar]
- 50.Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 51.Patterson RE, White E, Kristal AR, Neuhouser ML, Potter JD. Vitamin supplements and cancer risk: the epidemiologic evidence. Cancer Causes Control. 1997;8:786–802. doi: 10.1023/a:1018443724293. [DOI] [PubMed] [Google Scholar]
- 52.Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr. 2002;76:703–716. doi: 10.1093/ajcn/76.4.703. [DOI] [PubMed] [Google Scholar]
- 53.Ricciarelli R, Zingg JM, Azzi A. Vitamin E: protective role of a Janus molecule. FASEB J. 2001;15:2314–2325. doi: 10.1096/fj.01-0258rev. [DOI] [PubMed] [Google Scholar]
- 54.Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, Virtanen MJ, Albanes D, Taylor PR, Albert P. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein SJ, Wright ME, Lawson KA, Snyder K, Mannisto S, Taylor PR, Virtamo J, Albanes D. Serum and dietary vitamin E in relation to prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:1253–1259. doi: 10.1158/1055-9965.EPI-06-1084. [DOI] [PubMed] [Google Scholar]


